Spatiotemporal Regulation of Vibrio Exotoxins by HlyU and Other Transcriptional Regulators
Abstract
1. Introduction
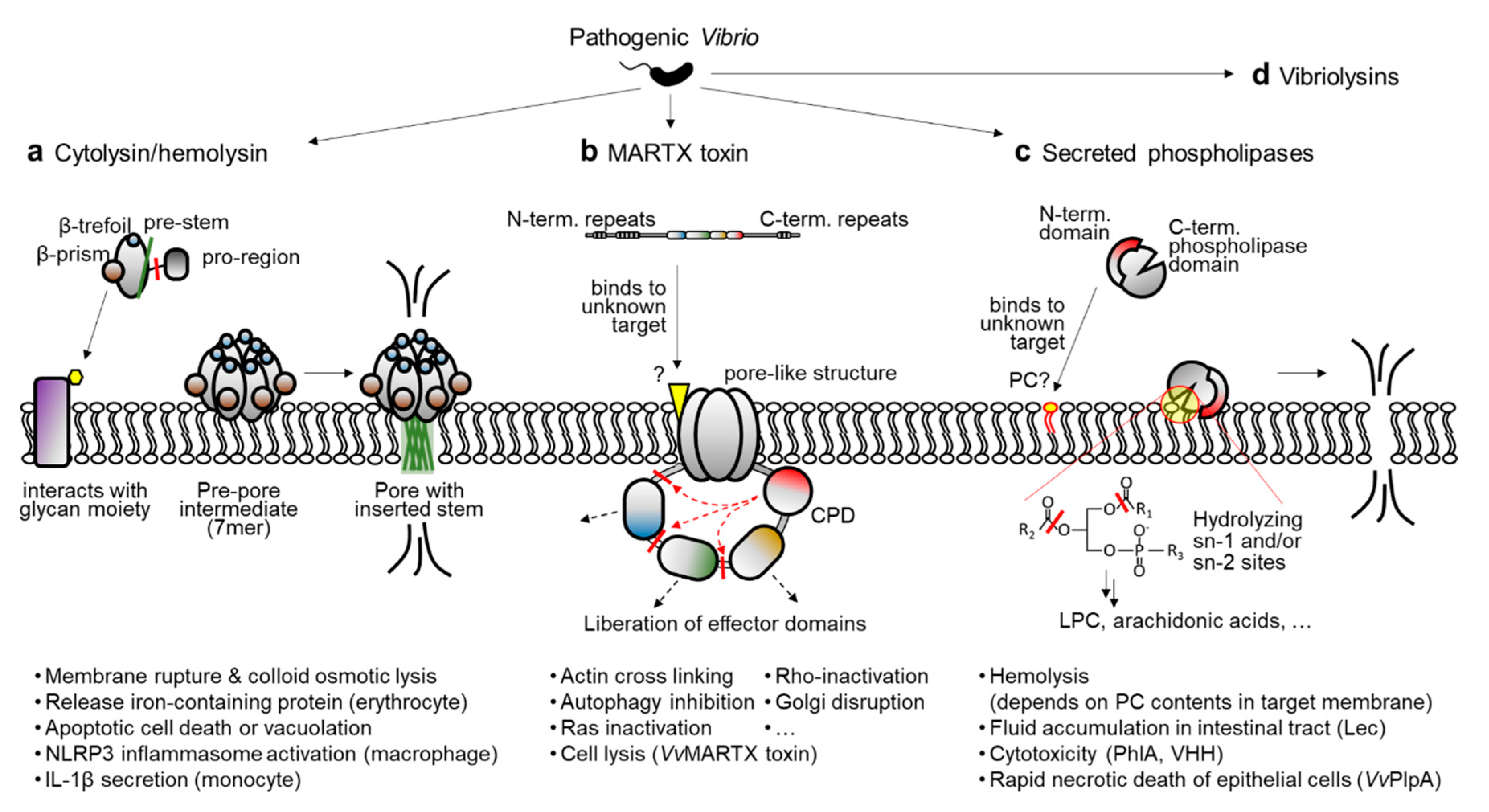
2. Major Exotoxins Produced by Pathogenic Vibrio Species
2.1. Cytolysin/Hemolysin
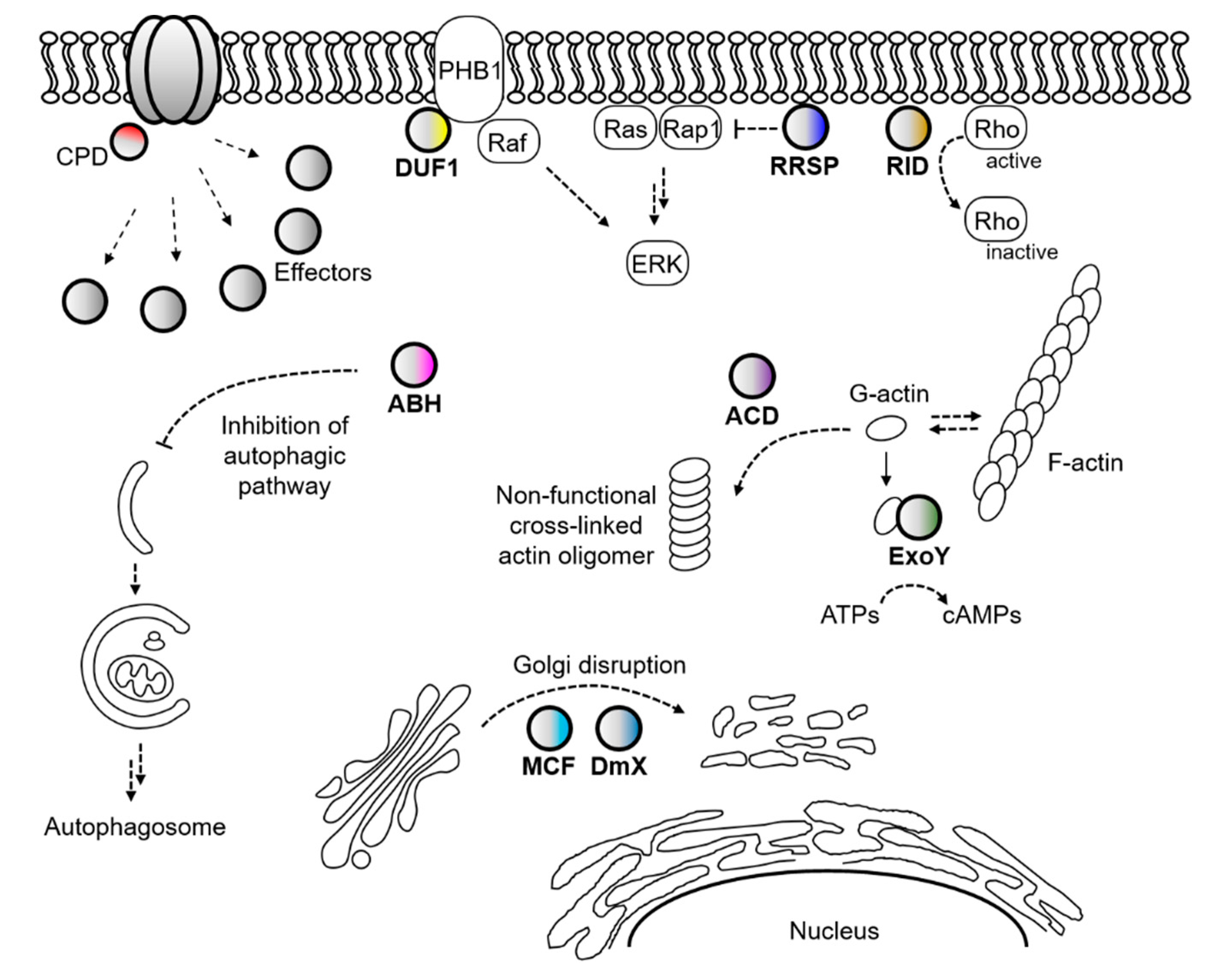
2.2. MARTX Toxin
2.3. Secreted Phospholipases
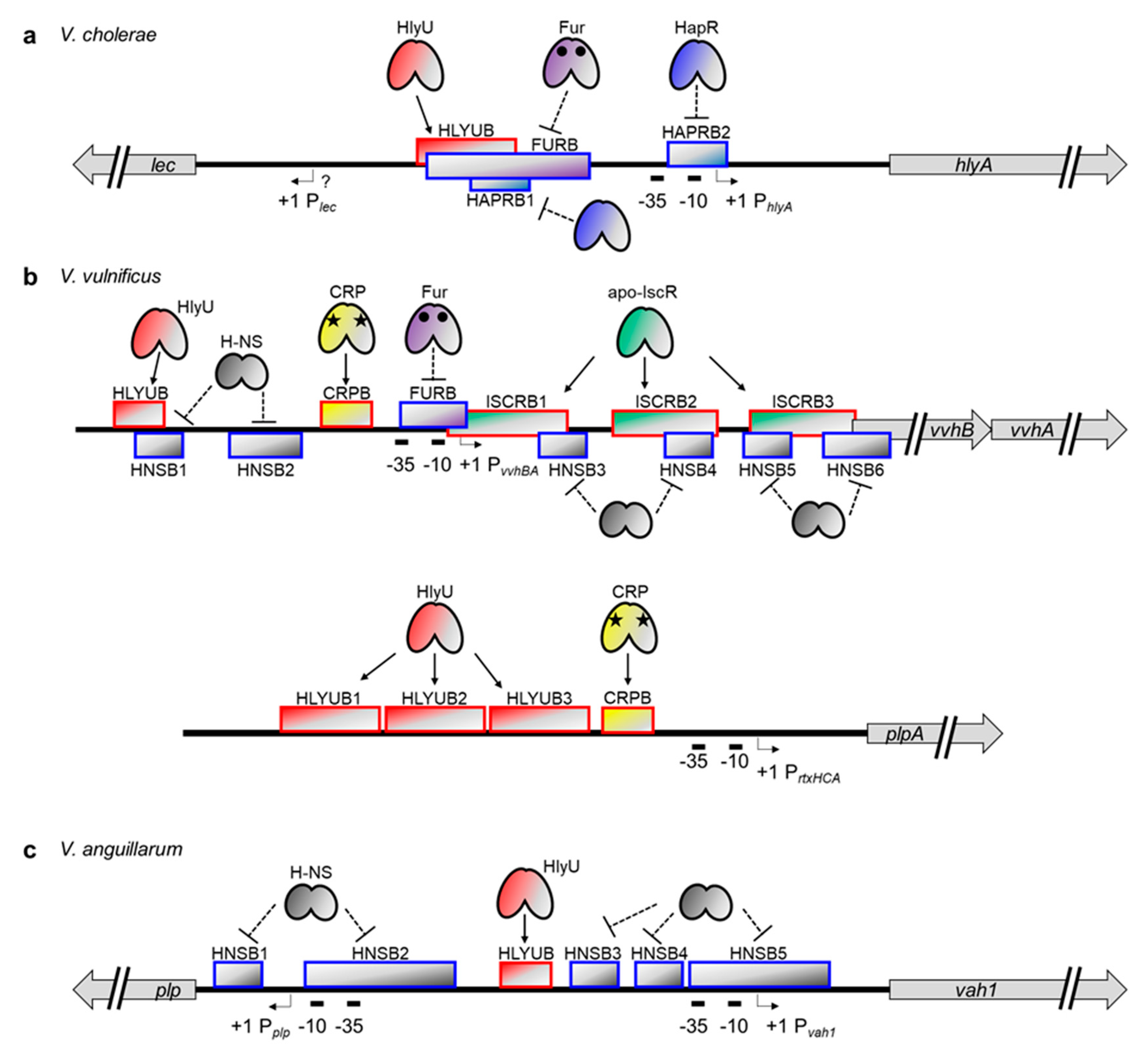
3. Coordinated but Distinct Regulation of Exotoxin Genes in Vibrio Species
3.1. HlyU, a Common Transcriptional Regulator of Major Exotoxin Genes in Vibrio Species
3.2. Transcriptional Regulation of Cytolysin/Hemolysin Genes in Vibrio Species
3.3. Transcriptional Regulation of the MARTX Toxin Genes in Vibrio Species
3.4. Transcriptional Regulation of Secreted Phospholipase Genes in Vibrio Species
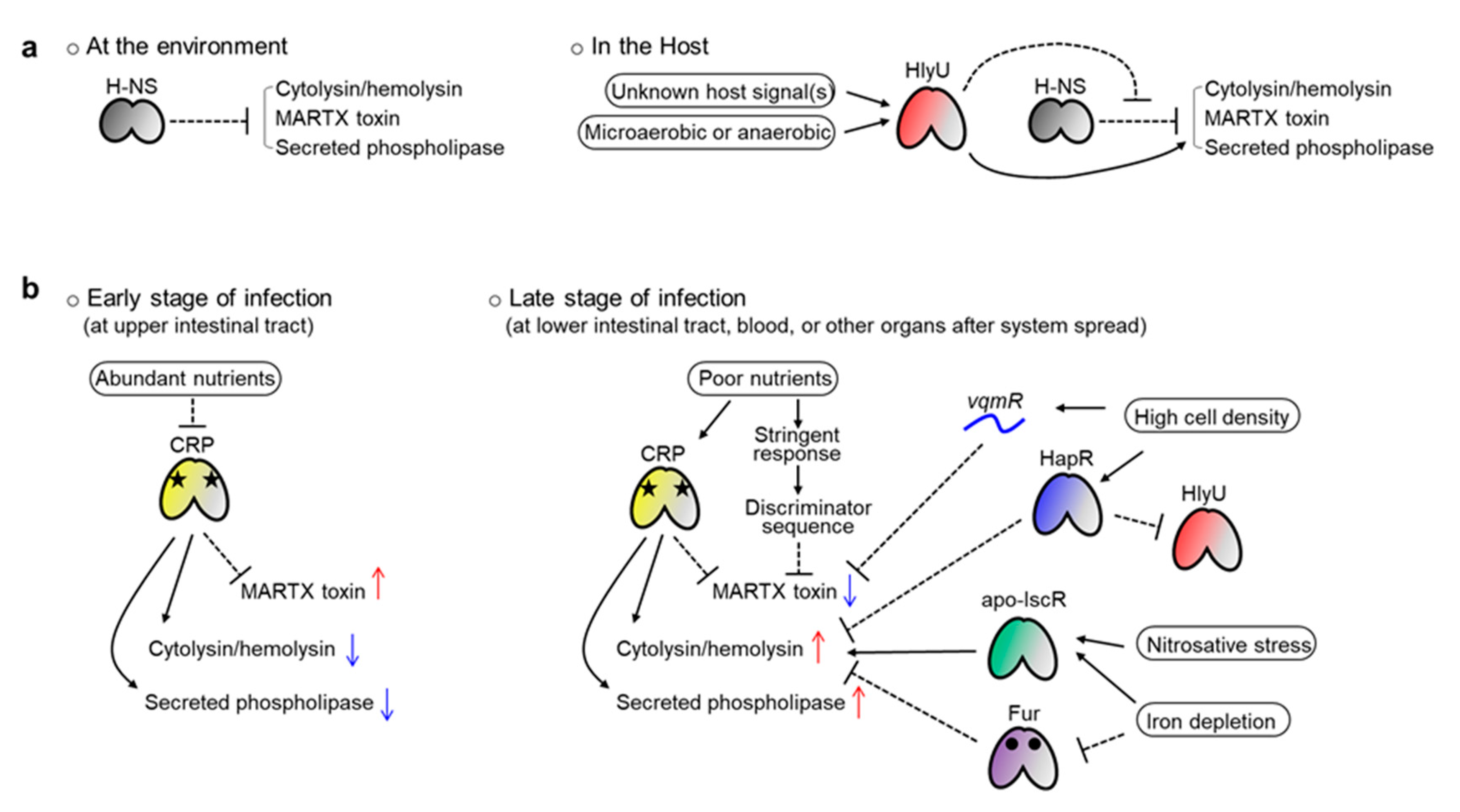
3.5. Spatiotemporal Regulation of Exotoxin Genes for Successful Pathogenesis
4. Anti-Virulence Strategy Targeting HlyU and Future Directions
Funding
Conflicts of Interest
References
- Thompson, F.L.; Iida, T.; Swings, J. Biodiversity of vibrios. Microbiol. Mol. Biol. Rev. 2004, 68, 403–431. [Google Scholar] [CrossRef] [PubMed]
- Boyd, E.F.; Carpenter, M.R.; Chowdhury, N.; Cohen, A.L.; Haines-Menges, B.L.; Kalburge, S.S.; Kingston, J.J.; Lubin, J.B.; Ongagna-Yhombi, S.Y.; Whitaker, W.B. Post-Genomic Analysis of Members of the Family Vibrionaceae. Microbiol. Spectr. 2015, 3, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Rivera, D.; Prieto-Davó, A.; Rodríguez-Fuentes, G.; Escalante-Herrera, K.S.; Gaxiola, G. A vibriosis outbreak in the Pacific white shrimp, Litopenaeus vannamei reared in biofloc and clear seawater. J. Invertebr. Pathol. 2019, 167. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhang, J.; Ma, L.; Li, L.; Zhang, W.; Li, J. Identification of fish source Vibrio alginolyticus and evaluation of its bacterial ghosts vaccine immune effects. MicrobiologyOpen 2018, 7, e00576. [Google Scholar] [CrossRef] [PubMed]
- Tison, D.L.; Nishibuchi, M.; Greenwood, J.D.; Seidler, R.J. Vibrio vulnificus biogroup 2: New biogroup pathogenic for eels. Appl. Environ. Microbiol. 1982, 44, 640–646. [Google Scholar] [CrossRef]
- Biosca, E.G.; Oliver, J.D.; Amaro, C. Phenotypic characterization of Vibrio vulnificus biotype 2, a lipopolysaccharide-based homogeneous O serogroup within Vibrio vulnificus. Appl. Environ. Microbiol. 1996, 62, 918–927. [Google Scholar] [CrossRef]
- Bruto, M.; James, A.; Petton, B.; Labreuche, Y.; Chenivesse, S.; Alunno-Bruscia, M.; Polz, M.F.; Le Roux, F. Vibrio crassostreae, a benign oyster colonizer turned into a pathogen after plasmid acquisition. ISME J. 2017, 11, 1043–1052. [Google Scholar] [CrossRef]
- Egidius, E. Vibriosis: Pathogenicity and pathology. A review. Aquaculture 1987, 67, 15–28. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Primers 2018, 4, 8. [Google Scholar] [CrossRef]
- Krebs, S.J.; Taylor, R.K. Protection and attachment of Vibrio cholerae mediated by the toxin-coregulated pilus in the infant mouse model. J. Bacteriol. 2011, 193, 5260–5270. [Google Scholar] [CrossRef]
- Kirn, T.J.; Lafferty, M.J.; Sandoe, C.M.; Taylor, R.K. Delineation of pilin domains required for bacterial association into microcolonies and intestinal colonization by Vibrio cholerae. Mol. Microbiol. 2000, 35, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.K.; Miller, V.L.; Furlong, D.B.; Mekalanos, J.J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. USA 1987, 84, 2833–2837. [Google Scholar] [CrossRef] [PubMed]
- Arezes, J.; Jung, G.; Gabayan, V.; Valore, E.; Ruchala, P.; Gulig, P.A.; Ganz, T.; Nemeth, E.; Bulut, Y. Hepcidin-Induced Hypoferremia Is a Critical Host Defense Mechanism against the Siderophilic Bacterium Vibrio vulnificus. Cell Host Microbe 2015, 17, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Litwin, C.M.; Rayback, T.W.; Skinner, J. Role of catechol siderophore synthesis in Vibrio vulnificus virulence. Infect. Immun. 1996, 64, 2834–2838. [Google Scholar] [CrossRef]
- Webster, A.C.; Litwin, C.M. Cloning and characterization of vuuA, a gene encoding the Vibrio vulnificus ferric vulnibactin receptor. Infect. Immun. 2000, 68, 526–534. [Google Scholar] [CrossRef]
- Kim, I.H.; Shim, J.I.; Lee, K.E.; Hwang, W.; Kim, I.J.; Choi, S.H.; Kim, K.S. Nonribosomal peptide synthase is responsible for the biosynthesis of siderophore in Vibrio vulnificus MO6-24/O. J. Microbiol. Biotechnol. 2008, 18, 35–42. [Google Scholar]
- Tan, W.; Verma, V.; Jeong, K.; Kim, S.; Jung, C.-H.; Lee, S.; Rhee, J. Molecular characterization of vulnibactin biosynthesis in Vibrio vulnificus indicates the existence of an alternative siderophore. Front. Microbiol. 2014, 5, 1. [Google Scholar] [CrossRef]
- Yoshida, S.; Ogawa, M.; Mizuguchi, Y. Relation of capsular materials and colony opacity to virulence of Vibrio vulnificus. Infect. Immun. 1985, 47, 446–451. [Google Scholar] [CrossRef]
- Jones, M.K.; Oliver, J.D. Vibrio vulnificus: Disease and pathogenesis. Infect. Immun. 2009, 77, 1723–1733. [Google Scholar] [CrossRef]
- Brown, S.A.; Palmer, K.L.; Whiteley, M. Revisiting the host as a growth medium. Nat. Rev. Microbiol. 2008, 6, 657–666. [Google Scholar] [CrossRef]
- Almagro-Moreno, S.; Boyd, E.F. Sialic Acid Catabolism Confers a Competitive Advantage to Pathogenic Vibrio cholerae in the Mouse Intestine. Infect. Immun. 2009, 77, 3807–3816. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.G.; Oh, M.H.; Kim, B.S.; Lee, M.Y.; Han, H.J.; Choi, S.H. The capability of catabolic utilization of N-acetylneuraminic acid, a sialic acid, is essential for Vibrio vulnificus pathogenesis. Infect. Immun. 2009, 77, 3209–3217. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Hwang, J.; Kim, M.H.; Choi, S.H. Cooperative regulation of the Vibrio vulnificus nan gene cluster by NanR protein, cAMP receptor protein, and N-acetylmannosamine 6-phosphate. J. Biol. Chem. 2011, 286, 40889–40899. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Kim, B.S.; Jang, S.Y.; Lim, J.G.; You, D.J.; Jung, H.S.; Oh, T.K.; Lee, J.O.; Choi, S.H.; Kim, M.H. Structural insights into the regulation of sialic acid catabolism by the Vibrio vulnificus transcriptional repressor NanR. Proc. Natl. Acad. Sci. USA 2013, 110, E2829–E2837. [Google Scholar] [CrossRef] [PubMed]
- Do Vale, A.; Cabanes, D.; Sousa, S. Bacterial Toxins as Pathogen Weapons Against Phagocytes. Front. Microbiol. 2016, 7, 42. [Google Scholar] [CrossRef]
- Bhavsar, A.P.; Guttman, J.A.; Finlay, B.B. Manipulation of host-cell pathways by bacterial pathogens. Nature 2007, 449, 827–834. [Google Scholar] [CrossRef]
- Olivier, V.; Haines, G.K., 3rd; Tan, Y.; Satchell, K.J. Hemolysin and the multifunctional autoprocessing RTX toxin are virulence factors during intestinal infection of mice with Vibrio cholerae El Tor O1 strains. Infect. Immun. 2007, 75, 5035–5042. [Google Scholar] [CrossRef]
- Tsou, A.M.; Zhu, J. Quorum sensing negatively regulates hemolysin transcriptionally and posttranslationally in Vibrio cholerae. Infect. Immun. 2010, 78, 461–467. [Google Scholar] [CrossRef]
- Boardman, B.K.; Meehan, B.M.; Fullner Satchell, K.J. Growth phase regulation of Vibrio cholerae RTX toxin export. J. Bacteriol. 2007, 189, 1827–1835. [Google Scholar] [CrossRef]
- Gray, L.D.; Kreger, A.S. Purification and characterization of an extracellular cytolysin produced by Vibrio vulnificus. Infect. Immun. 1985, 48, 62–72. [Google Scholar] [CrossRef]
- Choi, H.K.; Park, N.Y.; Kim, D.-i.; Chung, H.J.; Ryu, S.; Choi, S.H. Promoter Analysis and Regulatory Characteristics of vvhBA Encoding Cytolytic Hemolysin of Vibrio vulnificus. J. Biol. Chem. 2002, 277, 47292–47299. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Jang, K.K.; Lim, J.G.; Lee, Z.-W.; Im, H.; Choi, S.H. The transcriptional regulator IscR integrates host-derived nitrosative stress and iron starvation in activation of the vvhBA operon in Vibrio vulnificus. J. Biol. Chem. 2020, 295, 5350–5361. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mou, X.; Nelson, D.R. HlyU is a positive regulator of hemolysin expression in Vibrio anguillarum. J. Bacteriol. 2011, 193, 4779–4789. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, J.; Kim, S.M.; Jeong, H.G.; Choi, S.H. Regulatory characteristics of the Vibrio vulnificus rtxHCA operon encoding a MARTX toxin. J. Microbiol. 2012, 50, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Naka, H.; Crosa, J.H. HlyU acts as an H-NS antirepressor in the regulation of the RTX toxin gene essential for the virulence of the human pathogen Vibrio vulnificus CMCP6. Mol. Microbiol. 2009, 72, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.K.; Lee, Z.W.; Kim, B.; Jung, Y.H.; Han, H.J.; Kim, M.H.; Kim, B.S.; Choi, S.H. Identification and characterization of Vibrio vulnificus plpA encoding a phospholipase A2 essential for pathogenesis. J. Biol. Chem. 2017, 292, 17129–17143. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.B.; Lee, S.E.; Rhee, J.H.; Choi, S.H. Evidence that Expression of the Vibrio vulnificus Hemolysin Gene Is Dependent on Cyclic AMP and Cyclic AMP Receptor Protein. J. Biol. Chem. 1999, 181, 7639–7642. [Google Scholar] [CrossRef]
- Miyoshi, S.-i.; Okamoto, K.; Takahashi, E. Vibriolysin. In Handbook of Proteolytic Enzymes; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 579–582. [Google Scholar]
- Miyoshi, S.; Wakae, H.; Tomochika, K.; Shinoda, S. Functional domains of a zinc metalloprotease from Vibrio vulnificus. J. Bacteriol. 1997, 179, 7606–7609. [Google Scholar] [CrossRef][Green Version]
- Jeong, H.G.; Satchell, K.J. Additive function of Vibrio vulnificus MARTX (Vv) and VvhA cytolysins promotes rapid growth and epithelial tissue necrosis during intestinal infection. PLoS Pathog. 2012, 8, e1002581. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, M.W.; Kim, B.S.; Kim, S.M.; Lee, B.C.; Kim, T.S.; Choi, S.H. Identification and characterization of the Vibrio vulnificus rtxA essential for cytotoxicity in vitro and virulence in mice. J. Microbiol. 2007, 45, 146–152. [Google Scholar]
- Liu, M.; Alice, A.F.; Naka, H.; Crosa, J.H. The HlyU protein is a positive regulator of rtxA1, a gene responsible for cytotoxicity and virulence in the human pathogen Vibrio vulnificus. Infect. Immun. 2007, 75, 3282–3289. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Xu, J.; Lu, X.; Li, J.; Lou, J.; Zhao, H.; Diao, B.; Shi, Q.; Zhang, Y.; Kan, B. Expression of Hemolysin Is Regulated Under the Collective Actions of HapR, Fur, and HlyU in Vibrio cholerae El Tor Serogroup O1. Front. Microbiol. 2018, 9, 1310. [Google Scholar] [CrossRef]
- Miyoshi, S. Extracellular proteolytic enzymes produced by human pathogenic vibrio species. Front. Microbiol. 2013, 4, 339. [Google Scholar] [CrossRef] [PubMed]
- Benitez, J.A.; Silva, A.J. Vibrio cholerae hemagglutinin (HA)/protease: An extracellular metalloprotease with multiple pathogenic activities. Toxicon 2016, 115, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Elgaml, A.; Miyoshi, S.I. Regulation systems of protease and hemolysin production in Vibrio vulnificus. Microbiol. Immunol. 2017, 61, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Iida, T.; Honda, T. Hemolysins Produced by Vibrios. J. Toxicol. Toxin Rev. 1997, 16, 215–227. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Austin, B. Haemolysins in Vibrio species. J. Appl. Microbiol. 2005, 98, 1011–1019. [Google Scholar] [CrossRef]
- Skaar, E.P. The Battle for Iron between Bacterial Pathogens and Their Vertebrate Hosts. PLoS Pathog. 2010, 6, e1000949. [Google Scholar] [CrossRef]
- Zughaier, S.M.; Cornelis, P. Editorial: Role of Iron in Bacterial Pathogenesis. Front. Cell. Infect. Microbiol. 2018, 8, 344. [Google Scholar] [CrossRef]
- Joseph, S.W.; Colwell, R.R.; Kaper, J.B. Vibrio parahaemolyticus and related halophilic Vibrios. Crit. Rev. Microbiol. 1982, 10, 77–124. [Google Scholar] [CrossRef]
- Takeda, Y. Thermostable direct hemolysin of Vibrio parahaemolyticus. Pharmacol. Ther. 1982, 19, 123–146. [Google Scholar] [CrossRef]
- Honda, T.; Ni, Y.X.; Miwatani, T. Purification and characterization of a hemolysin produced by a clinical isolate of Kanagawa phenomenon-negative Vibrio parahaemolyticus and related to the thermostable direct hemolysin. Infect. Immun. 1988, 56, 961–965. [Google Scholar] [CrossRef]
- Ichinose, Y.; Yamamoto, K.; Nakasone, N.; Tanabe, M.J.; Takeda, T.; Miwatani, T.; Iwanaga, M. Enterotoxicity of El Tor-like hemolysin of non-O1 Vibrio cholerae. Infect. Immun. 1987, 55, 1090–1093. [Google Scholar] [CrossRef] [PubMed]
- Saka, H.A.; Bidinost, C.; Sola, C.; Carranza, P.; Collino, C.; Ortiz, S.; Echenique, J.R.; Bocco, J.L. Vibrio cholerae cytolysin is essential for high enterotoxicity and apoptosis induction produced by a cholera toxin gene-negative V. cholerae non-O1, non-O139 strain. Microb. Pathog. 2008, 44, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Olson, R.; Gouaux, E. Vibrio cholerae cytolysin is composed of an alpha-hemolysin-like core. Protein Sci. 2003, 12, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Wright, A.C.; Kaper, J.B.; Morris, J.G., Jr. The cytolysin gene of Vibrio vulnificus: Sequence and relationship to the Vibrio cholerae E1 Tor hemolysin gene. Infect. Immun. 1990, 58, 2706–2709. [Google Scholar] [CrossRef]
- Hirono, I.; Masuda, T.; Aoki, T. Cloning and detection of the hemolysin gene of Vibrio anguillarum. Microb. Pathog. 1996, 21, 173–182. [Google Scholar] [CrossRef]
- Kim, G.T.; Lee, J.Y.; Huh, S.H.; Yu, J.H.; Kong, I.S. Nucleotide sequence of the vmhA gene encoding hemolysin from Vibrio mimicus. Biochim. Biophys. Acta 1997, 1360, 102–104. [Google Scholar] [CrossRef]
- Parker, M.W.; Buckley, J.T.; Postma, J.P.; Tucker, A.D.; Leonard, K.; Pattus, F.; Tsernoglou, D. Structure of the Aeromonas toxin proaerolysin in its water-soluble and membrane-channel states. Nature 1994, 367, 292–295. [Google Scholar] [CrossRef]
- Dal Peraro, M.; van der Goot, F.G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. Microbiol. 2016, 14, 77–92. [Google Scholar] [CrossRef]
- Bruggisser, J.; Tarek, B.; Wyder, M.; Muller, P.; von Ballmoos, C.; Witz, G.; Enzmann, G.; Deutsch, U.; Engelhardt, B.; Posthaus, H. CD31 (PECAM-1) Serves as the Endothelial Cell-Specific Receptor of Clostridium perfringens beta-Toxin. Cell Host Microbe 2020, 28, 69–78.e6. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Ichinose, Y.; Shinagawa, H.; Makino, K.; Nakata, A.; Iwanaga, M.; Honda, T.; Miwatani, T. Two-step processing for activation of the cytolysin/hemolysin of Vibrio cholerae O1 biotype El Tor: Nucleotide sequence of the structural gene (hlyA) and characterization of the processed products. Infect. Immun. 1990, 58, 4106–4116. [Google Scholar] [CrossRef] [PubMed]
- Nagamune, K.; Yamamoto, K.; Naka, A.; Matsuyama, J.; Miwatani, T.; Honda, T. In vitro proteolytic processing and activation of the recombinant precursor of El Tor cytolysin/hemolysin (pro-HlyA) of Vibrio cholerae by soluble hemagglutinin/protease of V. cholerae, trypsin, and other proteases. Infect. Immun. 1996, 64, 4655–4658. [Google Scholar] [CrossRef] [PubMed]
- Valeva, A.; Walev, I.; Weis, S.; Boukhallouk, F.; Wassenaar, T.M.; Endres, K.; Fahrenholz, F.; Bhakdi, S.; Zitzer, A. A cellular metalloproteinase activates Vibrio cholerae pro-cytolysin. J. Biol. Chem. 2004, 279, 25143–25148. [Google Scholar] [CrossRef]
- Nagamune, K.; Yamamoto, K.; Honda, T. Intramolecular chaperone activity of the pro-region of Vibrio cholerae El Tor cytolysin. J. Biol. Chem. 1997, 272, 1338–1343. [Google Scholar] [CrossRef]
- De, S.; Kaus, K.; Sinclair, S.; Case, B.C.; Olson, R. Structural basis of mammalian glycan targeting by Vibrio cholerae cytolysin and biofilm proteins. PLoS Pathog. 2018, 14, e1006841. [Google Scholar] [CrossRef]
- Levan, S.; De, S.; Olson, R. Vibrio cholerae cytolysin recognizes the heptasaccharide core of complex N-glycans with nanomolar affinity. J. Mol. Biol. 2013, 425, 944–957. [Google Scholar] [CrossRef]
- Rai, A.K.; Paul, K.; Chattopadhyay, K. Functional mapping of the lectin activity site on the beta-prism domain of Vibrio cholerae cytolysin: Implications for the membrane pore-formation mechanism of the toxin. J. Biol. Chem. 2013, 288, 1665–1673. [Google Scholar] [CrossRef]
- Khilwani, B.; Chattopadhyay, K. Signaling beyond Punching Holes: Modulation of Cellular Responses by Vibrio cholerae Cytolysin. Toxins 2015, 7, 3344–3358. [Google Scholar] [CrossRef]
- Toma, C.; Higa, N.; Koizumi, Y.; Nakasone, N.; Ogura, Y.; McCoy, A.J.; Franchi, L.; Uematsu, S.; Sagara, J.; Taniguchi, S.; et al. Pathogenic Vibrio activate NLRP3 inflammasome via cytotoxins and TLR/nucleotide-binding oligomerization domain-mediated NF-kappa B signaling. J. Immunol. 2010, 184, 5287–5297. [Google Scholar] [CrossRef]
- Queen, J.; Agarwal, S.; Dolores, J.S.; Stehlik, C.; Satchell, K.J. Mechanisms of inflammasome activation by Vibrio cholerae secreted toxins vary with strain biotype. Infect. Immun. 2015, 83, 2496–2506. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Fullner, K.J.; Clayton, R.; Sexton, J.A.; Rogers, M.B.; Calia, K.E.; Calderwood, S.B.; Fraser, C.; Mekalanos, J.J. Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc. Natl. Acad. Sci. USA 1999, 96, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Fullner, K.J.; Mekalanos, J.J. In vivo covalent cross-linking of cellular actin by the Vibrio cholerae RTX toxin. EMBO J. 2000, 19, 5315–5323. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, K.L.; Satchell, K.J. Inactivation of small Rho GTPases by the multifunctional RTX toxin from Vibrio cholerae. Cell Microbiol. 2007, 9, 1324–1335. [Google Scholar] [CrossRef] [PubMed]
- Cordero, C.L.; Kudryashov, D.S.; Reisler, E.; Satchell, K.J. The Actin cross-linking domain of the Vibrio cholerae RTX toxin directly catalyzes the covalent cross-linking of actin. J. Biol. Chem. 2006, 281, 32366–32374. [Google Scholar] [CrossRef] [PubMed]
- Satchell, K.J. MARTX, multifunctional autoprocessing repeats-in-toxin toxins. Infect. Immun. 2007, 75, 5079–5084. [Google Scholar] [CrossRef]
- Satchell, K.J.F. Multifunctional-autoprocessing repeats-in-toxin (MARTX) Toxins of Vibrios. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef]
- Sheahan, K.L.; Cordero, C.L.; Satchell, K.J. Autoprocessing of the Vibrio cholerae RTX toxin by the cysteine protease domain. EMBO J. 2007, 26, 2552–2561. [Google Scholar] [CrossRef]
- Kim, B.S. The Modes of Action of MARTX Toxin Effector Domains. Toxins 2018, 10, 507. [Google Scholar] [CrossRef]
- Fullner, K.J.; Lencer, W.I.; Mekalanos, J.J. Vibrio cholerae-induced cellular responses of polarized T84 intestinal epithelial cells are dependent on production of cholera toxin and the RTX toxin. Infect. Immun. 2001, 69, 6310–6317. [Google Scholar] [CrossRef]
- Kim, Y.R.; Lee, S.E.; Kook, H.; Yeom, J.A.; Na, H.S.; Kim, S.Y.; Chung, S.S.; Choy, H.E.; Rhee, J.H. Vibrio vulnificus RTX toxin kills host cells only after contact of the bacteria with host cells. Cell Microbiol. 2008, 10, 848–862. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Rock, J.L.; Nelson, D.R. Identification and characterization of a repeat-in-toxin gene cluster in Vibrio anguillarum. Infect. Immun. 2008, 76, 2620–2632. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kim, J.-H.; Choi, S.; Park, S.; Lee, E.-Y.; Koh, S.; Ryu, C.-M.; Kim, S.-Y.; Kim, M.H. MARTX Toxin-stimulated interplay between human cells and Vibrio vulnificus. mSphere 2020, 5, e00659-20. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Gavin, H.E.; Satchell, K.J. Distinct roles of the repeat-containing regions and effector domains of the Vibrio vulnificus multifunctional-autoprocessing repeats-in-toxin (MARTX) toxin. mBio 2015, 6, e00324-15. [Google Scholar] [CrossRef]
- Dolores, J.S.; Agarwal, S.; Egerer, M.; Satchell, K.J. Vibrio cholerae MARTX toxin heterologous translocation of beta-lactamase and roles of individual effector domains on cytoskeleton dynamics. Mol. Microbiol. 2014, 95, 590–604. [Google Scholar] [CrossRef]
- Agarwal, S.; Kim, H.; Chan, R.B.; Agarwal, S.; Williamson, R.; Cho, W.; Paolo, G.D.; Satchell, K.J. Autophagy and endosomal trafficking inhibition by Vibrio cholerae MARTX toxin phosphatidylinositol-3-phosphate-specific phospholipase A1 activity. Nat. Commun. 2015, 6, 8745. [Google Scholar] [CrossRef]
- Kim, B.A.; Lim, J.Y.; Rhee, J.H.; Kim, Y.R. Characterization of Prohibitin 1 as a Host Partner of Vibrio vulnificus RtxA1 Toxin. J. Infect. Dis. 2016, 213, 131–138. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, B.S.; Choi, S.; Lee, E.-Y.; Park, S.; Hwang, J.; Kwon, Y.; Hyun, J.; Lee, C.; Kim, J.F.; et al. Makes caterpillars floppy-like effector-containing MARTX toxins require host ADP-ribosylation factor (ARF) proteins for systemic pathogenicity. Proc. Natl. Acad. Sci. USA 2019, 116, 18031–18040. [Google Scholar] [CrossRef]
- Herrera, A.; Muroski, J.; Sengupta, R.; Nguyen, H.H.; Agarwal, S.; Ogorzalek Loo, R.R.; Mattoo, S.; Loo, J.A.; Satchell, K.J.F. N-terminal autoprocessing and acetylation of multifunctional-autoprocessing repeats-in-toxins (MARTX) Makes Caterpillars Floppy-like effector is stimulated by adenosine diphosphate (ADP)-Ribosylation Factor 1 in advance of Golgi fragmentation. Cell Microbiol. 2020, 22, e13133. [Google Scholar] [CrossRef]
- Kim, B.S.; Satchell, K.J. MARTX effector cross kingdom activation by Golgi-associated ADP-ribosylation factors. Cell Microbiol. 2016, 18, 1078–1093. [Google Scholar] [CrossRef]
- Antic, I.; Biancucci, M.; Zhu, Y.; Gius, D.R.; Satchell, K.J. Site-specific processing of Ras and Rap1 Switch I by a MARTX toxin effector domain. Nat. Commun. 2015, 6, 7396. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.Y.; Hwang, J.; Kim, B.S.; Lee, E.Y.; Oh, B.H.; Kim, M.H. Structural basis of inactivation of Ras and Rap1 small GTPases by Ras/Rap1-specific endopeptidase from the sepsis-causing pathogen Vibrio vulnificus. J. Biol. Chem. 2018, 293, 18110–18122. [Google Scholar] [CrossRef] [PubMed]
- Biancucci, M.; Minasov, G.; Banerjee, A.; Herrera, A.; Woida, P.J.; Kieffer, M.B.; Bindu, L.; Abreu-Blanco, M.; Anderson, W.F.; Gaponenko, V.; et al. The bacterial Ras/Rap1 site-specific endopeptidase RRSP cleaves Ras through an atypical mechanism to disrupt Ras-ERK signaling. Sci. Signal. 2018, 11. [Google Scholar] [CrossRef]
- Ziolo, K.J.; Jeong, H.G.; Kwak, J.S.; Yang, S.; Lavker, R.M.; Satchell, K.J. Vibrio vulnificus biotype 3 multifunctional autoprocessing RTX toxin is an adenylate cyclase toxin essential for virulence in mice. Infect. Immun. 2014, 82, 2148–2157. [Google Scholar] [CrossRef] [PubMed]
- Belyy, A.; Raoux-Barbot, D.; Saveanu, C.; Namane, A.; Ogryzko, V.; Worpenberg, L.; David, V.; Henriot, V.; Fellous, S.; Merrifield, C.; et al. Actin activates Pseudomonas aeruginosa ExoY nucleotidyl cyclase toxin and ExoY-like effector domains from MARTX toxins. Nat. Commun. 2016, 7, 13582. [Google Scholar] [CrossRef] [PubMed]
- Gavin, H.E.; Satchell, K.J. MARTX toxins as effector delivery platforms. Pathog. Dis. 2015, 73, ftv092. [Google Scholar] [CrossRef] [PubMed]
- Woida, P.J.; Satchell, K.J.F. Coordinated delivery and function of bacterial MARTX toxin effectors. Mol. Microbiol. 2018, 107, 133–141. [Google Scholar] [CrossRef]
- Woida, P.J.; Satchell, K.J.F. The Vibrio cholerae MARTX toxin silences the inflammatory response to cytoskeletal damage before inducing actin cytoskeleton collapse. Sci. Signal. 2020, 13, eaaw9447. [Google Scholar] [CrossRef]
- Schmiel, D.H.; Miller, V.L. Bacterial phospholipases and pathogenesis. Microbes Infect. 1999, 1, 1103–1112. [Google Scholar] [CrossRef]
- Flores-Diaz, M.; Monturiol-Gross, L.; Naylor, C.; Alape-Giron, A.; Flieger, A. Bacterial Sphingomyelinases and Phospholipases as Virulence Factors. Microbiol. Mol. Biol. Rev. 2016, 80, 597–628. [Google Scholar] [CrossRef]
- Diener, M.; Egleme, C.; Rummel, W. Phospholipase C-induced anion secretion and its interaction with carbachol in the rat colonic mucosa. Eur. J. Pharmacol. 1991, 200, 267–276. [Google Scholar] [CrossRef]
- Sato, H.; Frank, D.W.; Hillard, C.J.; Feix, J.B.; Pankhaniya, R.R.; Moriyama, K.; Finck-Barbancon, V.; Buchaklian, A.; Lei, M.; Long, R.M.; et al. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO J. 2003, 22, 2959–2969. [Google Scholar] [CrossRef] [PubMed]
- Testa, J.; Daniel, L.W.; Kreger, A.S. Extracellular phospholipase A2 and lysophospholipase produced by Vibrio vulnificus. Infect. Immun. 1984, 45, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.E.; Michalski, J.M.; Russell, R.G.; Sears, C.L.; Kaper, J.B. Cloning, characterization, and chromosomal mapping of a phospholipase (lecithinase) produced by Vibrio cholerae. Infect. Immun. 1997, 65, 3112–3117. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ahn, S.H.; Kim, S.H.; Choi, Y.H.; Park, K.J.; Kong, I.S. Characterization of Vibrio mimicus phospholipase A (PhlA) and cytotoxicity on fish cell. Biochem. Biophys. Res. Commun. 2002, 298, 269–276. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, X.H.; Chen, J.; Chi, Z.; Sun, B.; Li, Y.; Austin, B. Overexpression, purification, characterization, and pathogenicity of Vibrio harveyi hemolysin VHH. Infect. Immun. 2006, 74, 6001–6005. [Google Scholar] [CrossRef]
- Li, L.; Mou, X.; Nelson, D.R. Characterization of Plp, a phosphatidylcholine-specific phospholipase and hemolysin of Vibrio anguillarum. BMC Microbiol. 2013, 13, 271. [Google Scholar] [CrossRef]
- Taniguchi, H.; Ohta, H.; Ogawa, M.; Mizuguchi, Y. Cloning and expression in Escherichia coli of Vibrio parahaemolyticus thermostable direct hemolysin and thermolabile hemolysin genes. J. Bacteriol. 1985, 162, 510–515. [Google Scholar] [CrossRef]
- Taniguchi, H.; Hirano, H.; Kubomura, S.; Higashi, K.; Mizuguchi, Y. Comparison of the nucleotide sequences of the genes for the thermostable direct hemolysin and the thermolabile hemolysin from Vibrio parahaemolyticus. Microb. Pathog. 1986, 1, 425–432. [Google Scholar] [CrossRef]
- Shinoda, S.; Matsuoka, H.; Tsuchie, T.; Miyoshi, S.; Yamamoto, S.; Taniguchi, H.; Mizuguchi, Y. Purification and characterization of a lecithin-dependent haemolysin from Escherichia coli transformed by a Vibrio parahaemolyticus gene. J. Gen. Microbiol. 1991, 137, 2705–2711. [Google Scholar] [CrossRef][Green Version]
- Wan, Y.; Liu, C.; Ma, Q. Structural analysis of a Vibrio phospholipase reveals an unusual Ser-His-chloride catalytic triad. J. Biol. Chem. 2019, 294, 11391–11401. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; Raynard, R.S.; Ellis, A.E. The phospholipid composition of Atlantic salmon, Salmo solar L., erythrocyte membranes. J. Fish Biol. 1989, 35, 313–314. [Google Scholar] [CrossRef]
- Rock, J.L.; Nelson, D.R. Identification and characterization of a hemolysin gene cluster in Vibrio anguillarum. Infect. Immun. 2006, 74, 2777–2786. [Google Scholar] [CrossRef][Green Version]
- Williams, S.G.; Manning, P.A. Transcription of the Vibrio cholerae haemolysin gene, hlyA, and cloning of a positive regulatory locus, hlyU. Mol. Microbiol. 1991, 5, 2031–2038. [Google Scholar] [CrossRef]
- Williams, S.G.; Attridge, S.R.; Manning, P.A. The transcriptional activator HlyU of Vibrio cholerae: Nucleotide sequence and role in virulence gene expression. Mol. Microbiol. 1993, 9, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Mou, X.; Spinard, E.J.; Driscoll, M.V.; Zhao, W.; Nelson, D.R. H-NS is a negative regulator of the two hemolysin/cytotoxin gene clusters in Vibrio anguillarum. Infect. Immun. 2013, 81, 3566–3576. [Google Scholar] [CrossRef]
- Nishi, K.; Lee, H.J.; Park, S.Y.; Bae, S.J.; Lee, S.E.; Adams, P.D.; Rhee, J.H.; Kim, J.S. Crystal structure of the transcriptional activator HlyU from Vibrio vulnificus CMCP6. FEBS Lett. 2010, 584, 1097–1102. [Google Scholar] [CrossRef]
- Mukherjee, D.; Datta, A.B.; Chakrabarti, P. Crystal structure of HlyU, the hemolysin gene transcription activator, from Vibrio cholerae N16961 and functional implications. Biochim. Biophys. Acta 2014, 1844, 2346–2354. [Google Scholar] [CrossRef]
- Brennan, R.G. The winged-helix DNA-binding motif: Another helix-turn-helix takeoff. Cell 1993, 74, 773–776. [Google Scholar] [CrossRef]
- Saha, R.P.; Chakrabarti, P. Molecular modeling and characterization of Vibrio cholerae transcription regulator HlyU. BMC Struct. Biol. 2006, 6, 24. [Google Scholar] [CrossRef][Green Version]
- Mukherjee, D.; Pal, A.; Chakravarty, D.; Chakrabarti, P. Identification of the target DNA sequence and characterization of DNA binding features of HlyU, and suggestion of a redox switch for hlyA expression in the human pathogen Vibrio cholerae from in silico studies. Nucleic Acids Res. 2015, 43, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Dong, J.; Scott, R.A.; Ksenzenko, M.Y.; Rosen, B.P. The role of arsenic-thiol interactions in metalloregulation of the ars operon. J. Biol. Chem. 1996, 271, 9291–9297. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, H.H.; Ghosh, A.; Paul, K.; Chowdhury, R. Effect of anaerobiosis on expression of virulence factors in Vibrio cholerae. Infect. Immun. 2004, 72, 3961–3967. [Google Scholar] [CrossRef] [PubMed]
- Stoebner, J.A.; Payne, S.M. Iron-regulated hemolysin production and utilization of heme and hemoglobin by Vibrio cholerae. Infect. Immun. 1988, 56, 2891–2895. [Google Scholar] [CrossRef] [PubMed]
- Elgaml, A.; Miyoshi, S. Role of the Histone-Like Nucleoid Structuring Protein (H-NS) in the Regulation of Virulence Factor Expression and Stress Response in Vibrio vulnificus. Biocontrol. Sci. 2015, 20, 263–274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shao, C.P.; Lo, H.R.; Lin, J.H.; Hor, L.I. Regulation of cytotoxicity by quorum-sensing signaling in Vibrio vulnificus is mediated by SmcR, a repressor of hlyU. J. Bacteriol. 2011, 193, 2557–2565. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, J.-A.; Lee, M.-A.; Park, S.-J.; Lee, K.-H. Regulation of haemolysin (VvhA) production by ferric uptake regulator (Fur) in Vibrio vulnificus: Repression of vvhA transcription by Fur and proteolysis of VvhA by Fur-repressive exoproteases. Mol. Microbiol. 2013, 88, 813–826. [Google Scholar] [CrossRef]
- Kim, C.M.; Chung, Y.Y.; Shin, S.H. Iron differentially regulates gene expression and extracellular secretion of Vibrio vulnificus cytolysin-hemolysin. J. Infect. Dis. 2009, 200, 582–589. [Google Scholar] [CrossRef]
- Browning, D.F.; Busby, S.J.W. Local and global regulation of transcription initiation in bacteria. Nat. Rev. Microbiol. 2016, 14, 638. [Google Scholar] [CrossRef]
- Boardman, B.K.; Satchell, K.J. Vibrio cholerae strains with mutations in an atypical type I secretion system accumulate RTX toxin intracellularly. J. Bacteriol. 2004, 186, 8137–8143. [Google Scholar] [CrossRef]
- Lee, B.C.; Lee, J.H.; Kim, M.W.; Kim, B.S.; Oh, M.H.; Kim, K.S.; Kim, T.S.; Choi, S.H. Vibrio vulnificus rtxE is important for virulence, and its expression is induced by exposure to host cells. Infect. Immun. 2008, 76, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vazquez, P.; Dewey, C.N.; Kitten, N.; Ross, W.; Gourse, R.L. Genome-wide effects on Escherichia coli transcription from ppGpp binding to its two sites on RNA polymerase. Proc. Natl. Acad. Sci. USA 2019, 116, 8310–8319. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Lee, S.E.; Kim, C.M.; Kim, S.Y.; Shin, E.K.; Shin, D.H.; Chung, S.S.; Choy, H.E.; Progulske-Fox, A.; Hillman, J.D.; et al. Characterization and pathogenic significance of Vibrio vulnificus antigens preferentially expressed in septicemic patients. Infect. Immun. 2003, 71, 5461–5471. [Google Scholar] [CrossRef] [PubMed]
- Papenfort, K.; Forstner, K.U.; Cong, J.P.; Sharma, C.M.; Bassler, B.L. Differential RNA-seq of Vibrio cholerae identifies the VqmR small RNA as a regulator of biofilm formation. Proc. Natl. Acad. Sci. USA 2015, 112, E766–E775. [Google Scholar] [CrossRef]
- Herzog, R.; Peschek, N.; Frohlich, K.S.; Schumacher, K.; Papenfort, K. Three autoinducer molecules act in concert to control virulence gene expression in Vibrio cholerae. Nucleic Acids Res. 2019, 47, 3171–3183. [Google Scholar] [CrossRef]
- Lee, Z.W.; Hwang, S.H.; Choi, G.; Jang, K.K.; Lee, T.H.; Chung, K.M.; Kim, B.S.; Choi, S.H. A MARTX toxin rtxA gene is controlled by host environmental signals through a CRP-coordinated regulatory network in Vibrio vulnificus. mBio 2020, 11, e00723-20. [Google Scholar] [CrossRef]
- Manneh-Roussel, J.; Haycocks, J.R.J.; Magan, A.; Perez-Soto, N.; Voelz, K.; Camilli, A.; Krachler, A.M.; Grainger, D.C. cAMP Receptor Protein Controls Vibrio cholerae Gene Expression in Response to Host Colonization. mBio 2018, 9, e00966-18. [Google Scholar] [CrossRef]
- Fang, F.C. Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat. Rev. Microbiol. 2004, 2, 820–832. [Google Scholar] [CrossRef]
- Giel, J.L.; Nesbit, A.D.; Mettert, E.L.; Fleischhacker, A.S.; Wanta, B.T.; Kiley, P.J. Regulation of iron-sulphur cluster homeostasis through transcriptional control of the Isc pathway by [2Fe-2S]-IscR in Escherichia coli. Mol. Microbiol. 2013, 87, 478–492. [Google Scholar] [CrossRef]
- Roope, L.S.J.; Smith, R.D.; Pouwels, K.B.; Buchanan, J.; Abel, L.; Eibich, P.; Butler, C.C.; Tan, P.S.; Walker, A.S.; Robotham, J.V.; et al. The challenge of antimicrobial resistance: What economics can contribute. Science 2019, 364, eaau4679. [Google Scholar] [CrossRef]
- Romero, J.; Feijoo, C.G.; Navarrete, P. Antibiotics in Aquaculture—Use, Abuse and Alternatives. In Health and Environment in Aquaculture; Carvalho, E.D., David, G.S., Silva, R.J., Eds.; INTECH Open Access Publisher: London, UK, 2012. [Google Scholar] [CrossRef]
- Baker-Austin, C. Antimicrobial Resistance in Vibrio Species. In Antimicrobial Resistance and Food Safety; Chen, C.-Y., Yan, X., Jackson, C.R., Eds.; Academic Press: San Diego, CA, USA, 2015; Chapter 6; pp. 105–118. [Google Scholar] [CrossRef]
- Clatworthy, A.E.; Pierson, E.; Hung, D.T. Targeting virulence: A new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007, 3, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Rasko, D.A.; Sperandio, V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 2010, 9, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Getz, L.J.; Thomas, N.A. The Transcriptional Regulator HlyU Positively Regulates Expression of exsA, Leading to Type III Secretion System 1 Activation in Vibrio parahaemolyticus. J. Bacteriol. 2018, 200. [Google Scholar] [CrossRef] [PubMed]
- Imdad, S.; Chaurasia, A.K.; Kim, K.K. Identification and Validation of an Antivirulence Agent Targeting HlyU-Regulated Virulence in Vibrio vulnificus. Front. Cell. Infect. Microbiol. 2018, 8. [Google Scholar] [CrossRef]
- Imdad, S.; Batool, N.; Pradhan, S.; Chaurasia, A.; Kim, K. Identification of 2′,4′-Dihydroxychalcone as an Antivirulence Agent Targeting HlyU, a Master Virulence Regulator in Vibrio vulnificus. Molecules 2018, 23, 1492. [Google Scholar] [CrossRef]
- Lee, Z.-W.; Kim, B.S.; Jang, K.K.; Bang, Y.-J.; Kim, S.; Ha, N.-C.; Jung, Y.H.; Lee, H.J.; Han, H.J.; Kim, J.-S.; et al. Small-molecule inhibitor of HlyU attenuates virulence of Vibrio species. Sci. Rep. 2019, 9, 4346. [Google Scholar] [CrossRef]
- Ng, W.L.; Perez, L.; Cong, J.; Semmelhack, M.F.; Bassler, B.L. Broad spectrum pro-quorum-sensing molecules as inhibitors of virulence in vibrios. PLoS Pathog. 2012, 8, e1002767. [Google Scholar] [CrossRef]
- Kim, B.S.; Jang, S.Y.; Bang, Y.J.; Hwang, J.; Koo, Y.; Jang, K.K.; Lim, D.; Kim, M.H.; Choi, S.H. QStatin, a Selective Inhibitor of Quorum Sensing in Vibrio Species. mBio 2018, 9, e02262-17. [Google Scholar] [CrossRef]

© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.S. Spatiotemporal Regulation of Vibrio Exotoxins by HlyU and Other Transcriptional Regulators. Toxins 2020, 12, 544. https://doi.org/10.3390/toxins12090544
Kim BS. Spatiotemporal Regulation of Vibrio Exotoxins by HlyU and Other Transcriptional Regulators. Toxins. 2020; 12(9):544. https://doi.org/10.3390/toxins12090544
Chicago/Turabian StyleKim, Byoung Sik. 2020. "Spatiotemporal Regulation of Vibrio Exotoxins by HlyU and Other Transcriptional Regulators" Toxins 12, no. 9: 544. https://doi.org/10.3390/toxins12090544
APA StyleKim, B. S. (2020). Spatiotemporal Regulation of Vibrio Exotoxins by HlyU and Other Transcriptional Regulators. Toxins, 12(9), 544. https://doi.org/10.3390/toxins12090544



