Dandelion Extract Alleviated Lipopolysaccharide-Induced Oxidative Stress through the Nrf2 Pathway in Bovine Mammary Epithelial Cells
Abstract
1. Introduction
2. Results
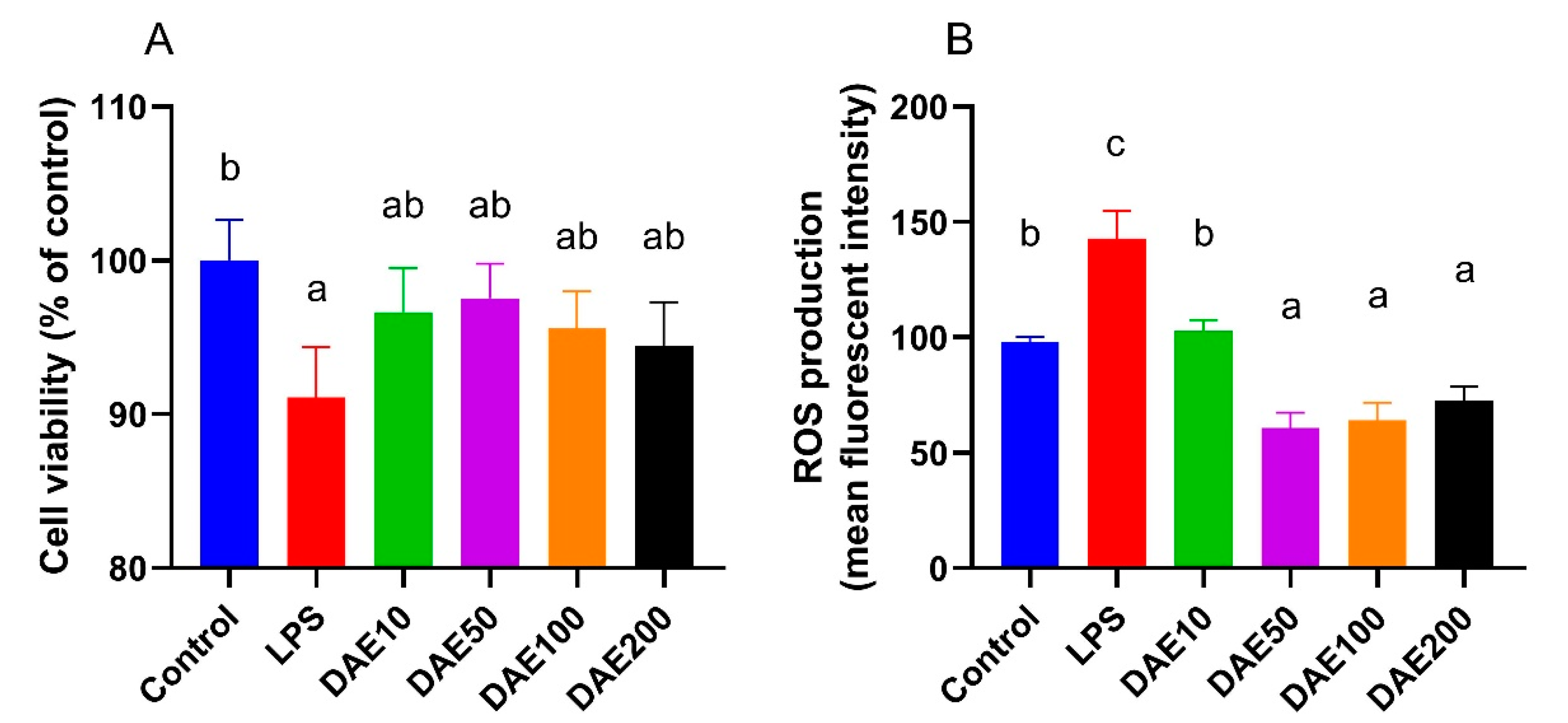
2.1. Effects of LPS and DAE on Cell Viability and ROS Production
2.2. Effects of LPS and DAE on the Concentration of Oxidative Damage Markers
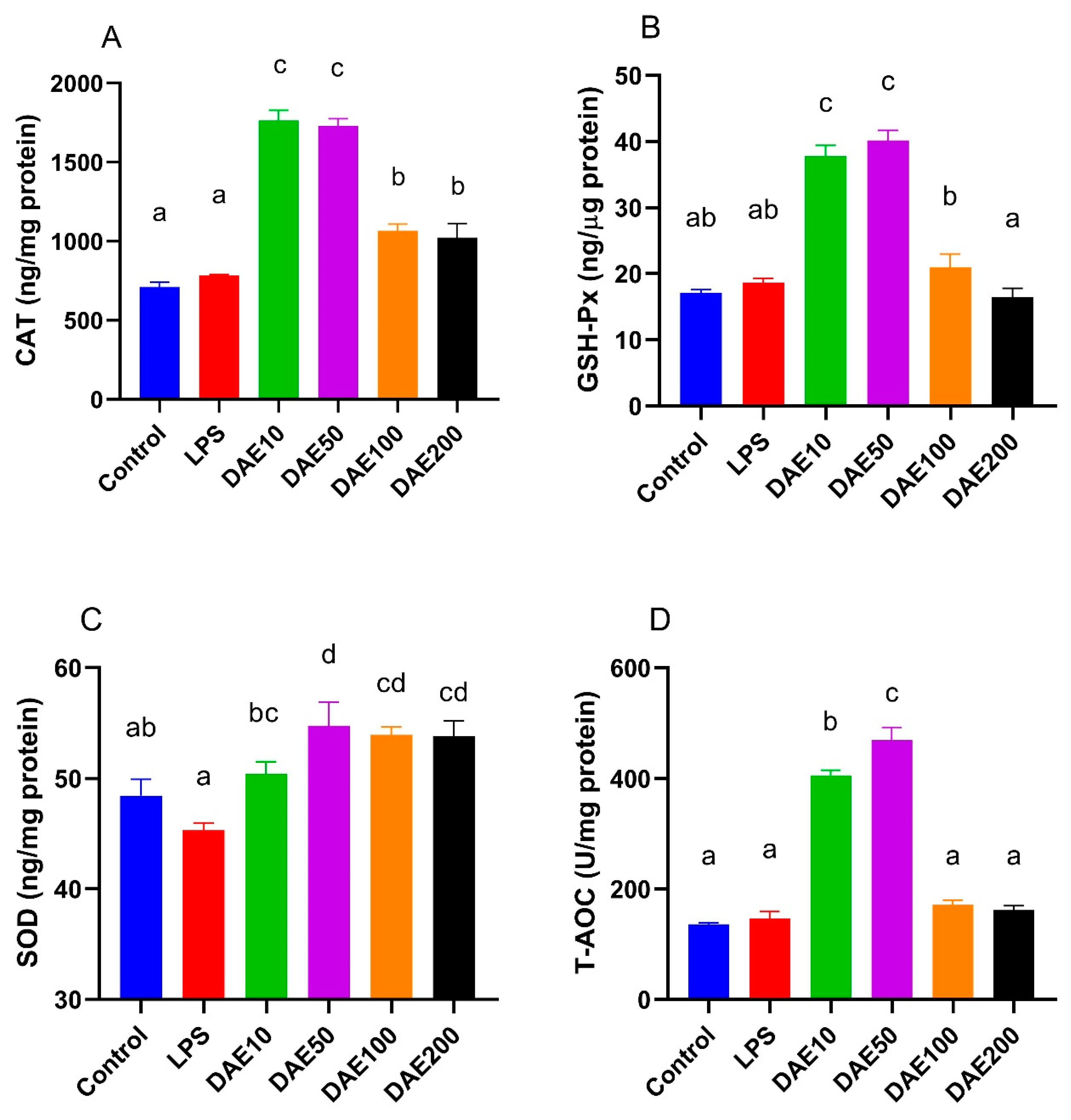
2.3. Effects of LPS and DAE on Antioxidant Enzyme Activity
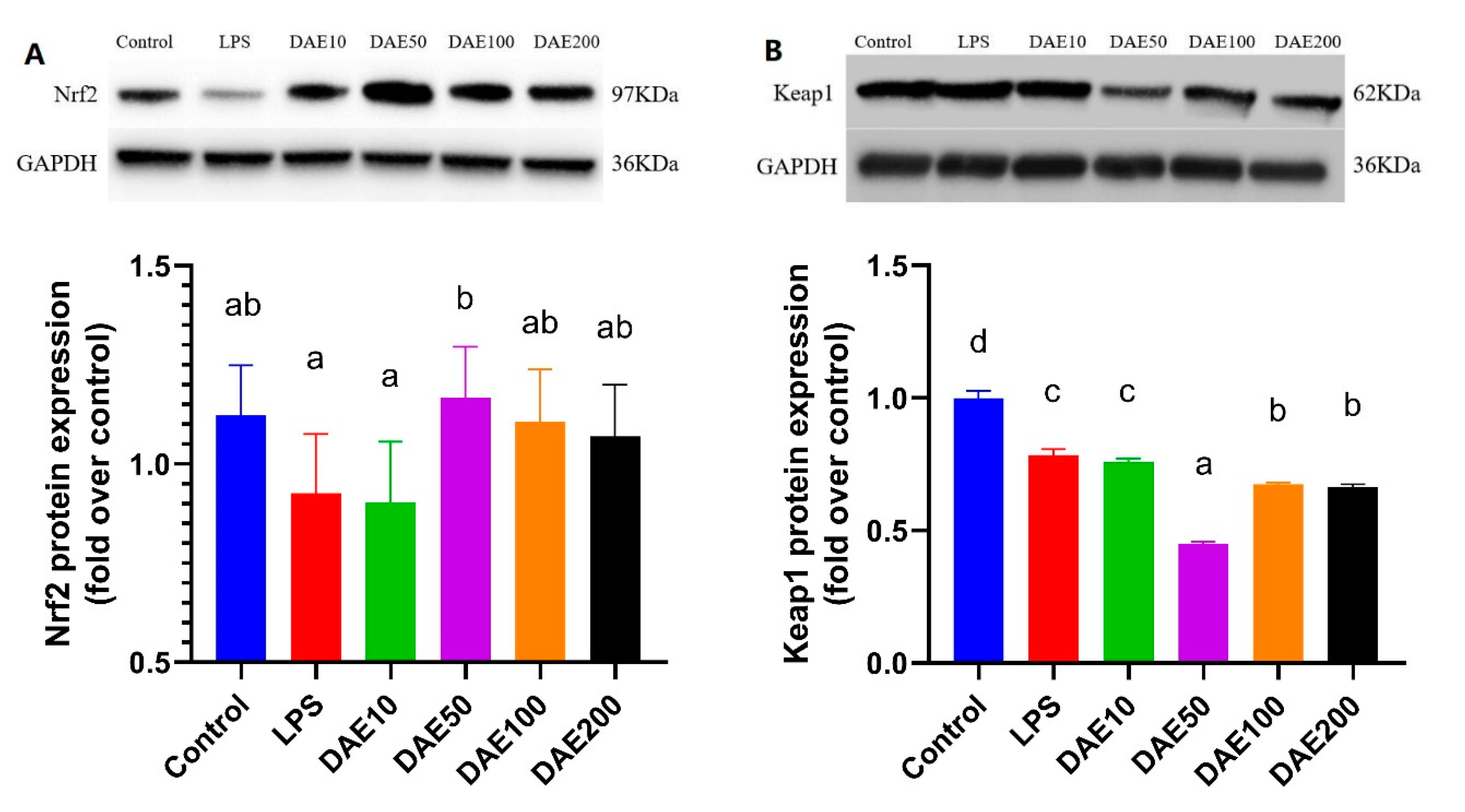
2.4. Effects of LPS and DAE on the Protein Expression of Nrf2 and Keap1
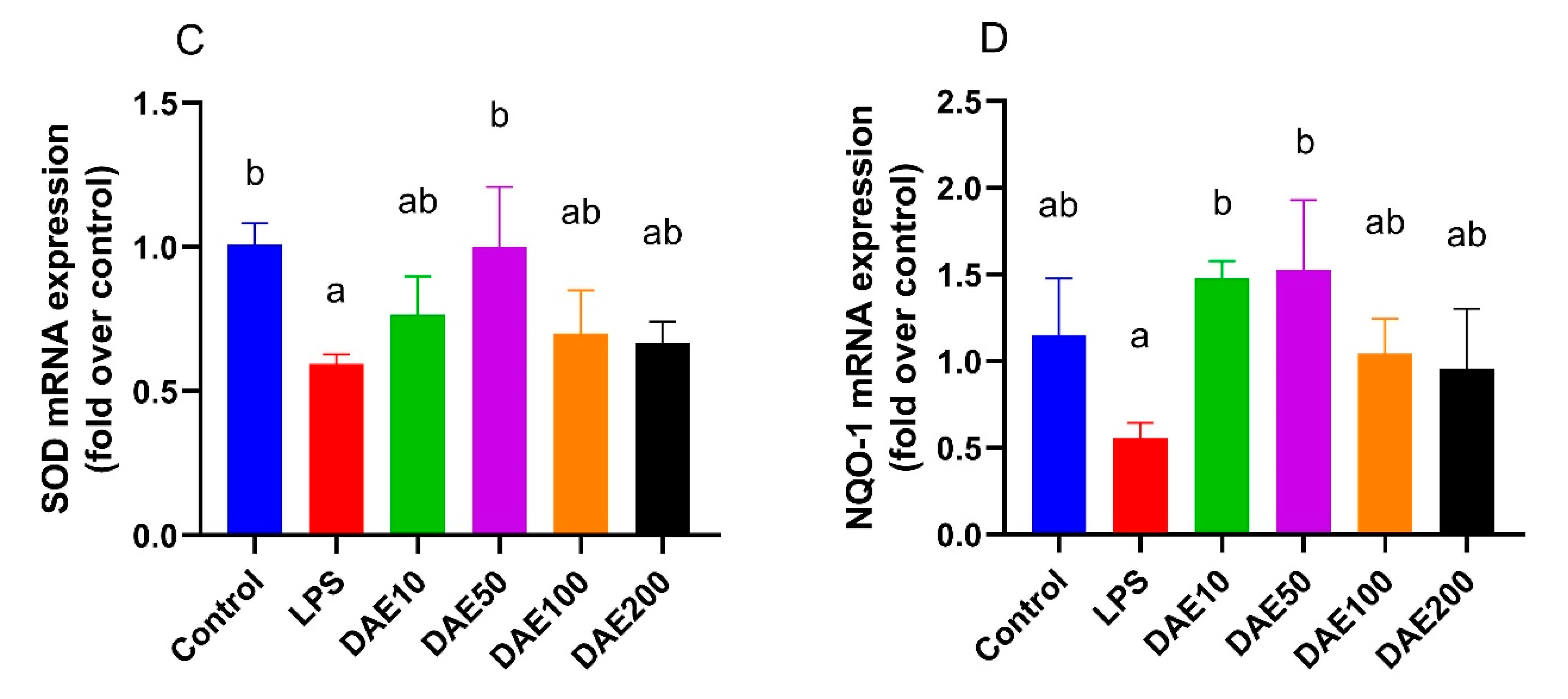
2.5. Effects of LPS and DAE on the mRNA Expression of Antioxidative Genes
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Preparation of DAE
5.2. Cell Culture and Treatments
5.3. Cell Viability Assay
5.4. Reactive Oxygen Species Detection
5.5. Assay for Oxidative Damage Markers and Antioxidant Enzyme Activity
5.6. RNA Isolation, cDNA Synthesis, and Quantitative Real-Time PCR (qPCR)
5.7. Western Blot Analysis
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dröge, W. Free radicals in the radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Zanichelli, F.; Capasso, S.; Di Bernardo, G.; Cipollaro, M.; Pagnotta, E.; Cartenì, M.; Casale, F.; Iori, R.; Giordano, A.; Galderisi, U. Low concentrations of isothiocyanates protect mesenchymal stem cells from oxidative injuries, while hi concentrations exacerbate DNA damage. Apoptosis 2012, 17, 964–974. [Google Scholar] [CrossRef]
- Abaker, J.A.; Xu, T.L.; Jin, D.; Chang, G.J.; Zhang, K.; Shen, X.Z. Lipopolysaccharide derived from the digestive tract provokes oxidative stress in the liver of dairy cows fed a high-grain diet. J. Dairy Sci. 2017, 100, 666–678. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef]
- Jin, D.; Chang, G.; Zhang, K.; Guo, J.; Xu, T.; Shen, X. Rumen-derived lipopolysaccharide enhances the expression of lingual antimicrobial peptide in mammary glands of dairy cows fed a high-concentrate diet. BMC Vet. Res. 2016, 12, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, H.H.; Nie, X.T.; Jiang, W.R.; Zhang, Y.S. Sodium butyrate ameliorates lipopolysaccharide-induced cow mammary epithelial cells from oxidative stress damage and apoptosis. J. Cell. Biochem. 2019, 120, 2370–2381. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, N.; Sun, J.; Gong, Q.; Ma, H.; Kan, X.; Cao, Y.; Wang, J.; Fu, S. Butyrate alleviates oxidative stress by regulating NRF2 nuclear accumulation and H3K9/14 acetylation via GPR109A in bovine mammary epithelial cells and mammary glands. Free Radic. Biol. Med. 2020, 152, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Carle, R.; Schieber, A. Taraxacum: A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef]
- González-Castejón, M.; Visioli, F.; Rodriguez-Casado, A. Diverse biological activities of dandelion. Nutr. Rev. 2012, 70, 534–547. [Google Scholar] [CrossRef]
- Estabrook, R.W.; Shet, M.S.; Fisher, C.W.; Jenkins, C.M.; Waterman, M.R. The interaction of NADPH-P450 reductase with P450: An electrochemical study of the role of the flavin mononucleotide-binding domain. Arch. Biochem. Biophys. 1996, 333, 308–315. [Google Scholar] [CrossRef]
- Hu, C.; Kitts, D.D. Antioxidant, prooxidant, and cytotoxic activities of solvent fractionated dandelion (Taraxacum officinale) flower extracts in vitro. J. Agric. Food Chem. 2003, 51, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Kitts, D.D. Dandelion (Taraxacum officinale) flower extract suppresses both reactive oxygen species and nitric oxide and prevents lipid oxidation in vitro. Phytomedicine 2005, 12, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Lis, B.; Rolnik, A.; Jedrejek, D.; Soluch, A.; Stochmal, A.; Olas, B. Dandelion (Taraxacum officinale L.) root components exhibit anti-oxidative and antiplatelet action in an in vitro study. J. Funct. Foods 2019, 59, 16–24. [Google Scholar] [CrossRef]
- Park, C.M.; Park, J.Y.; Noh, K.H.; Shin, J.H.; Song, Y.S. Taraxacum officinale Weber extracts inhibit LPS-induced oxidative stress and nitric oxide production via the NF-κB modulation in RAW 264.7 cells. J. Ethnopharmacol. 2011, 133, 834–842. [Google Scholar] [CrossRef]
- Choi, U.K.; Lee, O.H.; Yim, J.H.; Cho, C.W.; Rhee, Y.K.; Lim, S.I.; Kim, Y.C. Hypolipidemic and antioxidant effects of dandelion (Taraxacum officinale) root and leaf on cholesterol-fed rabbits. Int. J. Mol. Sci. 2010, 11, 67–78. [Google Scholar] [CrossRef]
- Sumanth, M.; Rana, A.C. Invivo antioxidant activity of hydroalcoholic extract of Taraxacum officinale roots in rats. Res. Lett. 2006, 38, 54–55. [Google Scholar] [CrossRef]
- Tan, X.; Sun, Z.; Zhou, C.; Huang, Z.; Tan, L.; Xun, P.; Huang, Q.; Lin, H.; Ye, C.; Wang, A. Effects of dietary dandelion extract on intestinal morphology, antioxidant status, immune function and physical barrier function of juvenile golden pompano Trachinotus ovatus. Fish Shellfish Immun. 2018, 73, 197–206. [Google Scholar] [CrossRef]
- Hochmuth, C.E.; Biteau, B.; Bohmann, D.; Jasper, H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell. 2011, 8, 188–199. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Xiao, D.; Yuan, D.; Tan, B.; Wang, J.; Liu, Y.; Tan, B. The role of Nrf2 signaling pathway in Eucommia ulmoides flavones regulating oxidative stress in the intestine of piglets. Oxid. Med. Cell. Longev. 2019, 2019, 9719618. [Google Scholar] [CrossRef]
- Ludwig, S.; Pleschka, S.; Planz, O.; Wollff, T. Ringing the alarm bells: Signaling and apoptosis in influenza virus infected cells. Cell. Microbiol. 2006, 8, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Shafiee-Kermani, F.; Grusak, M.A.; Gustafson, S.J.; Lila, M.A.; Niculescu, M.D. Lower Concentrations of blueberry polyphenolic-rich extract differentially alter HepG2 cell proliferation and expression of genes related to cell-cycle, oxidation and epigenetic machinery. J. Nutr. Disord. Ther. 2012, 3, 120. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Aitken, S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 2009, 128, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.M.; Kubota, H.; Okita, M.; Maeda, T. The anti-inflammatory and antioxidant effects of melatonin on LPS-stimulated bovine mammary epithelial cells. PLoS ONE 2017, 12, e0178525. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, Y.; Yang, S.; Guo, X.; Zhao, Y.; Shi, B. The protective effect of selenium on the lipopolysaccharide-induced oxidative stress and depressed gene expression related to milk protein synthesis in bovine mammary epithelial cells. Biol. Trace Elem. Res. 2019, 197, 141–148. [Google Scholar] [CrossRef]
- Hsu, D.Z.; Li, Y.H.; Chu, P.Y.; Chien, S.P.; Chuang, Y.C.; Li, M.Y. Attenuation of endotoxin-induced oxidative stress and multiple organ injury by 3,4-methylenedioxyphenol in rats. Shock 2006, 25, 300–305. [Google Scholar] [CrossRef][Green Version]
- Moriya, S.; Yokoyama, H.; Fukuda, M.; Okamura, Y.; Kamegaya, Y.; Mizukami, T.; Ohgo, H.; Ishii, H. Glutathione depletion enhances the formation of superoxide anion released into hepatic sinusoids after lipopolysaccharide challenge. Alcohol. Clin. Exp. Res. 2000, 24, 59S–63S. [Google Scholar] [CrossRef]
- Cho, H.Y.; Reddy, S.P.; Debiase, A.; Yamamoto, M.; Kleeberger, S.R. Gene expression profiling of Nrf2-mediated protection against oxidative injury. Free Radic. Biol. Med. 2005, 38, 325–343. [Google Scholar] [CrossRef]
- Jin, X.; Wang, K.; Liu, H.; Hu, F.; Zhao, F.; Liu, J. Protection of bovine mammary epithelial cells from hydrogen peroxide-induced oxidative cell damage by resveratrol. Oxid. Med. Cell. Longev. 2016, 2016, 2572175. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Wen, Z.S.; Xue, R.; Du, M.; Tang, Z.; Xiang, X.W.; Zheng, B.; Qu, Y.L. Hemp seed polysaccharides protect intestinal epithelial cells from hydrogen peroxide-induced oxidative stress. Int. J. Biol. Macromol. 2019, 135, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.Y.; Yan, S.M.; Guo, Y.M.; Zhang, B.Q.; Guo, X.Y.; Shi, B.L. Vitamin A pretreatment protects NO-induced bovine mammary epithelial cells from oxidative stress by modulating Nrf2 and NF-κB signaling pathways. J. Anim. Sci. 2018, 96, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Gao, C.; Luo, M.; Wang, W.; Zhao, C.; Zu, Y.; Efferth, T.; Fu, Y. Dihydroquercetin (DHQ) induced HO-1 and NQO-1 expression against oxidative stress through the Nrf2-dependent antioxidant pathway. J. Agric. Food Chem. 2013, 61, 2755–2761. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Sato, H.; Kuriyama-Matsumura, K.; Sato, K.; Maebara, K.; Wang, H.; Tamba, M.; Itoh, K.; Yamamoto, M.; Bannai, S. Electrophile response element-mediated induction of the cyctine/glutamate exchange transporter gene expression. J. Biol. Chem. 2002, 227, 44765–44771. [Google Scholar] [CrossRef] [PubMed]
- Nioi, P.; McMahon, M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: Reassessement of the ARE consensus sequence. Biochem. J. 2003, 374, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.S.; Zheng, Y.; Duan, H.; Lei, L.; Deng, X.; Liu, X.Q.; Mei, Z.N.; Deng, X.K. Dandelion polyphenols protect against acetaminophen-induced hepatotoxicity in mice via activation of the Nrf2/Ho-1 pathway and inhibition of the JNK signaling pathway. Chin. J. Nat. Med. 2020, 18, 103–113. [Google Scholar] [CrossRef]
- Hu, G.; Wang, J.; Hong, D.; Zhang, T.; Duan, H.; Mu, X.; Yang, Z. Effects of aqueous extracts of Taraxacum Officinale on expression of tumor necrosis factor-alpha and intracellular adhesion molecule 1 in LPS-stimulated RMMVECs. BMC Complement. Altern. Med. 2017, 17, 38–46. [Google Scholar] [CrossRef]


| Gene | Primer Sequence | Product Size (bp) | GenBank Accession No. |
|---|---|---|---|
| HO-1 | F: GGCAGCAAGGTGCAAGA | 221 | NM_001014912.1 |
| R: GAAGGAAGCCAGCCAAGAG | |||
| SOD | F: GAGGCAAAGGGAGATACAGTC | 197 | NM_174615.2 |
| R: GTCACATTGCCCAGGTCTC | |||
| NQO-1 | F: GGTGCTCATAGGGGAGTTCG | 235 | NM_001034535.1 |
| R: GGGAGTGTGCCCAATGCTAT | |||
| XCT | F: GATACAAACGCCCAGATATGC | 136 | XM_002694373.2 |
| R: ATGATGAAGCCAATCCCTGTA | |||
| GAPDH | F: GGGTCATCATCTCTGCACCT | 177 | NM_001034034.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wu, Y.; Wang, Z.; Chen, J.; Yang, Y.; Dong, G. Dandelion Extract Alleviated Lipopolysaccharide-Induced Oxidative Stress through the Nrf2 Pathway in Bovine Mammary Epithelial Cells. Toxins 2020, 12, 496. https://doi.org/10.3390/toxins12080496
Sun Y, Wu Y, Wang Z, Chen J, Yang Y, Dong G. Dandelion Extract Alleviated Lipopolysaccharide-Induced Oxidative Stress through the Nrf2 Pathway in Bovine Mammary Epithelial Cells. Toxins. 2020; 12(8):496. https://doi.org/10.3390/toxins12080496
Chicago/Turabian StyleSun, Yawang, Yongjiang Wu, Zili Wang, Juncai Chen, You Yang, and Guozhong Dong. 2020. "Dandelion Extract Alleviated Lipopolysaccharide-Induced Oxidative Stress through the Nrf2 Pathway in Bovine Mammary Epithelial Cells" Toxins 12, no. 8: 496. https://doi.org/10.3390/toxins12080496
APA StyleSun, Y., Wu, Y., Wang, Z., Chen, J., Yang, Y., & Dong, G. (2020). Dandelion Extract Alleviated Lipopolysaccharide-Induced Oxidative Stress through the Nrf2 Pathway in Bovine Mammary Epithelial Cells. Toxins, 12(8), 496. https://doi.org/10.3390/toxins12080496





