Effective Zearalenone Degradation in Model Solutions and Infected Wheat Grain Using a Novel Heterologous Lactonohydrolase Secreted by Recombinant Penicillium canescens
Abstract
1. Introduction
2. Results
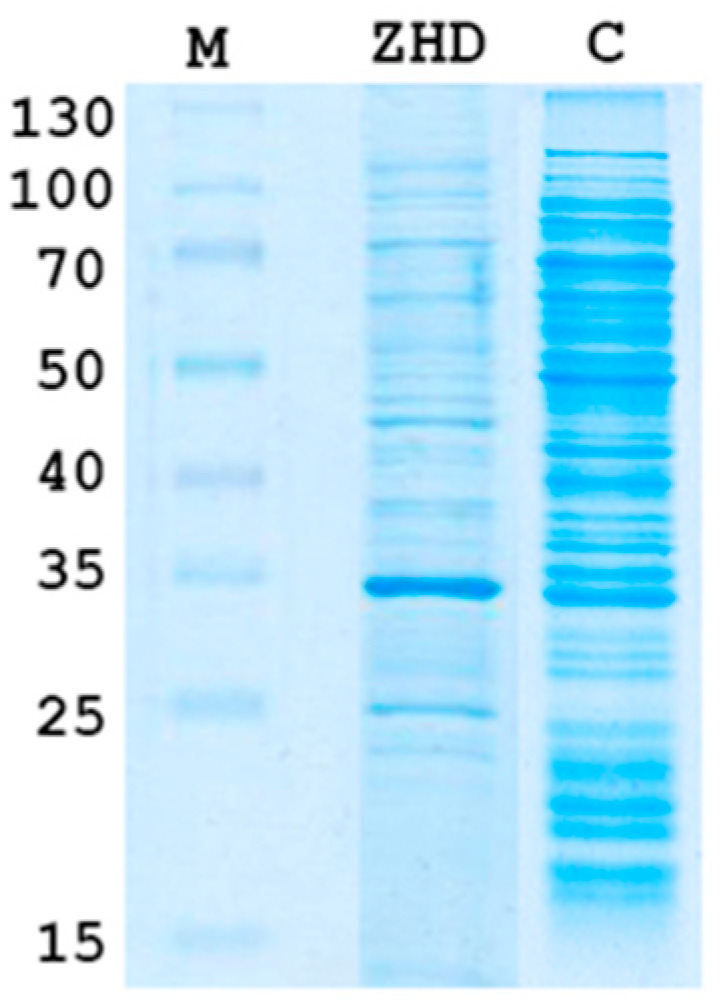
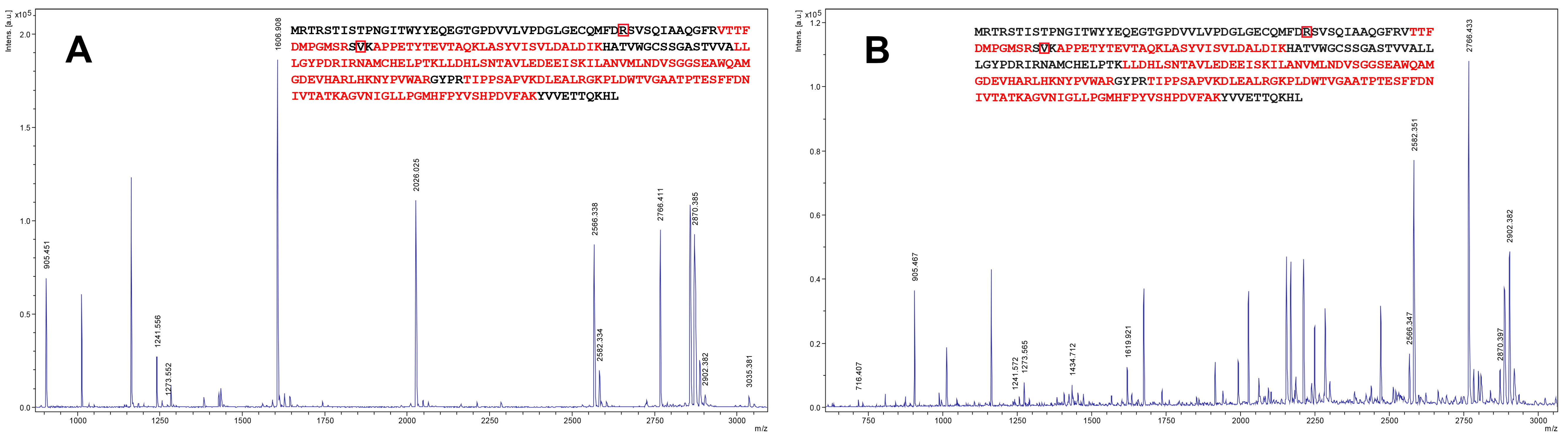
2.1. Cloning and Expression of Zhd101 Gene from C. rosea GRZ7 in Heterologous Hosts
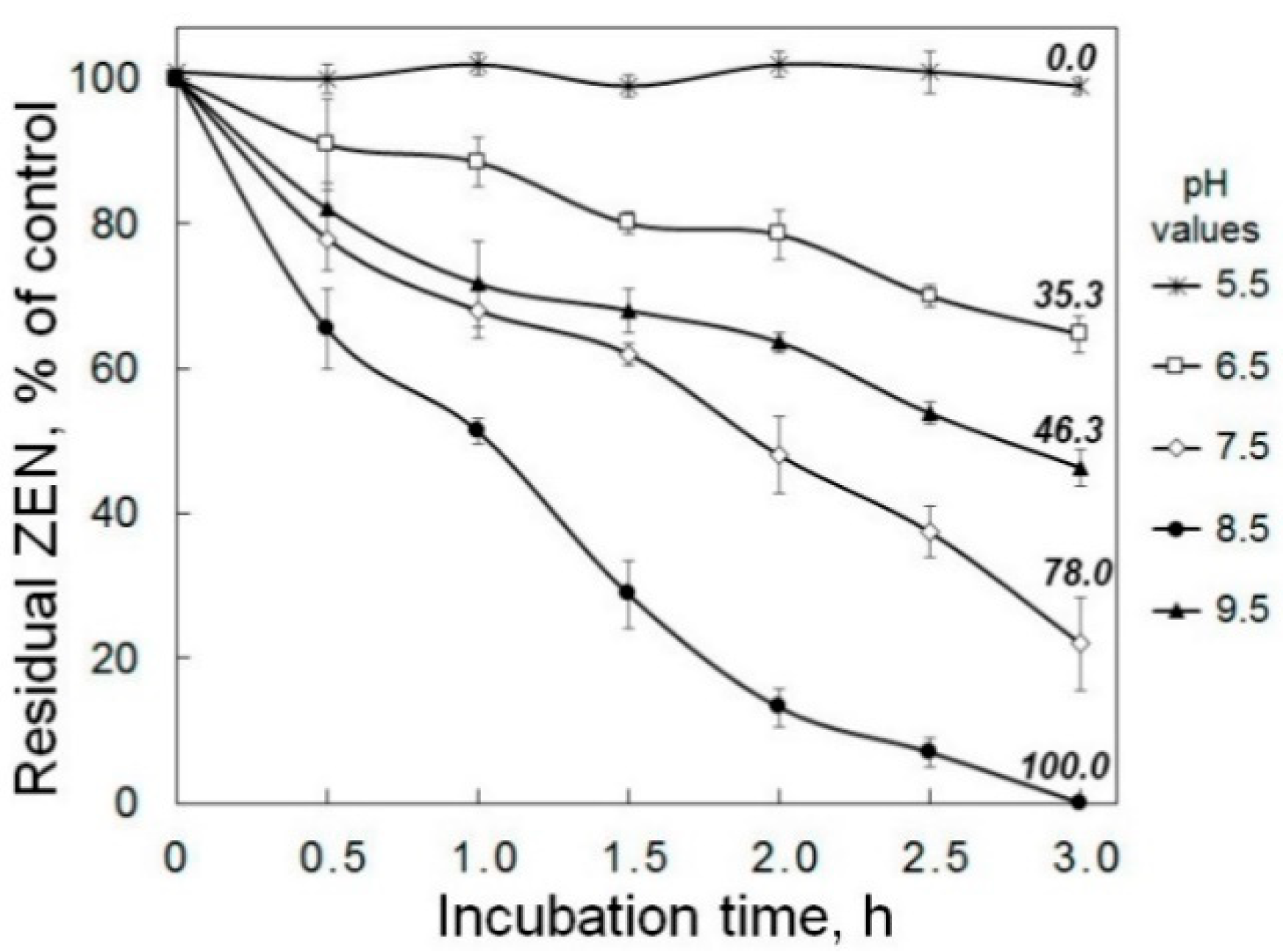
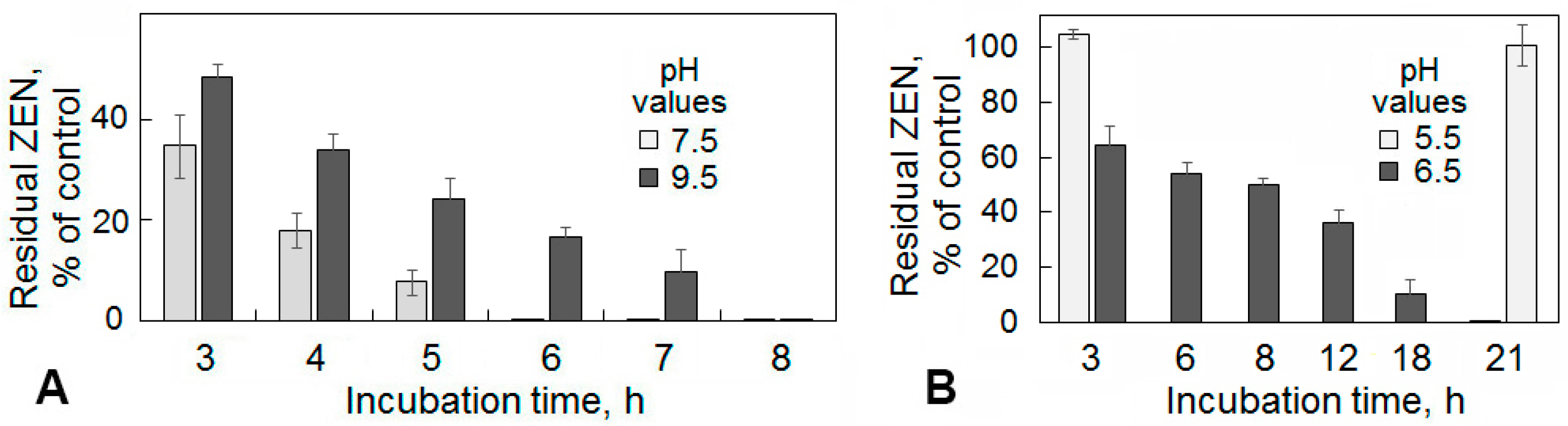
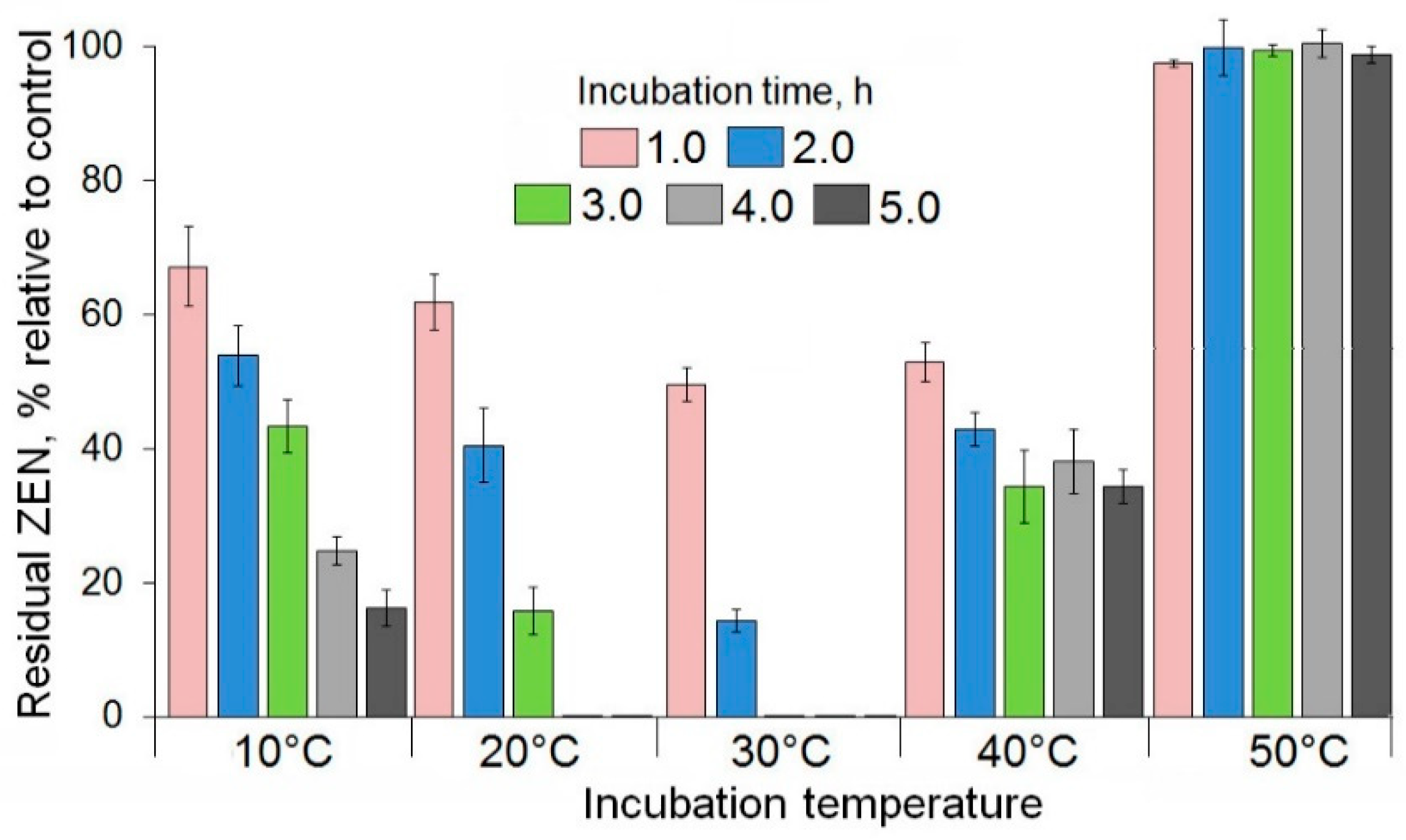
2.2. Enzymatic Degradation of Zearalenone in Model Solutions and Wheat Grain Infected with a Toxigenic F. culmorum
3. Discussion
4. Materials and Methods
4.1. Microbial Strains and Cultivation Media
4.2. Expression of Zhd101 Gene in Heterologous Hosts and ZHD101 Identification
4.3. Estimation of the Zearalenone Removal from Model Solutions under Different Conditions
4.4. Decontamination Test
4.5. Quantification ZEN Residues
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manubolu, M.; Goodla, L.; Pathakoti, K.; Malmlöf, K. Enzymes as direct decontaminating agents—mycotoxins. In Enzymes in Human and Animal Nutrition: Principles and Perspectives; Nunes, C.S., Kumar, V., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 313–330. ISBN 978-0-12-805419-2. [Google Scholar] [CrossRef]
- Dai, Z.; Cui, L.; Li, J.; Wang, B.; Guo, L.; Wu, Z.; Zhu, W.; Wu, G. Fermentation techniques in feed production. In Animal Agriculture; Bazer, F.W., Lamb, G.C., Wu, G., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 407–429. ISBN 978-0-12-817052-6. [Google Scholar] [CrossRef]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Maragos, C.M. Zearalenone occurrence and human exposure. World Mycotoxin J. 2010, 3, 369–383. [Google Scholar] [CrossRef]
- Mally, A.; Solfrizzo, M.; Degen, G.H. Biomonitoring of the mycotoxin zearalenone: Current state-of-the art and application to human exposure assessment. Arch. Toxicol. 2016, 90, 1281–1292. [Google Scholar] [CrossRef]
- Burkin, A.A.; Kononenko, G.P.; Gavrilova, O.P.; Gagkaeva, T.Y. About zearalenone levels in grass fodders and toxin producing activity of Fusarium fungi. Agricul. Biol. 2015, 50, 255–262. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- Richard, J.L. Some major mycotoxins and their mycotoxicoses—An overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [CrossRef]
- Gajęcki, M.; Gajęcka, M.; Jakimiuk, E.; Zielonka, Ł.; Obremski, K. Zearalenone: Undesirable substance. In Mycotoxins in Food, Feed and Bioweapons; Rai, M., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 131–144. ISBN 978-3-642-00724-8. [Google Scholar] [CrossRef]
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Piastowska-Ciesielska, A.W. Zearalenone as an endocrine disruptor in humans. Environ. Toxicol. Pharmacol. 2016, 48, 141–149. [Google Scholar] [CrossRef]
- Mao, J.; He, B.; Zhang, L.; Li, P.; Zhang, Q.; Ding, X.; Zhang, W. A Structure identification and toxicity assessment of the degradation products of aflatoxin B1 in peanut oil under UV irradiation. Toxins 2016, 8, 332. [Google Scholar] [CrossRef]
- Li, P.; Su, R.; Yin, R.; Lai, D.; Wang, M.; Liu, Y. Detoxification of mycotoxins through biotransformation. Toxins 2020, 12, 121. [Google Scholar] [CrossRef]
- Abassi, H.; Ayed-Boussema, I.; Shirley, S.; Abid, S.; Bacha, H.; Micheau, O. The mycotoxin zearalenone enhances cell proliferation, colony formation and promotes cell migration in the human colon carcinoma cell line HCT116. Toxicol. Lett. 2016, 254, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hueza, I.M.; Raspantini, P.C.; Raspantini, L.E.; Latorre, A.O.; Górniak, S.L. Zearalenone, an estrogenic mycotoxin, is an immunotoxic compound. Toxins 2014, 6, 1080–1095. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, H. Conversion of zearalenone to zearalenone glycoside by Rhizopus sp. Appl. Environ. Microbiol. 1986, 52, 515–519. [Google Scholar] [CrossRef]
- Chen, S.-W.; Wang, H.-T.; Shih, W.-Y.; Ciou, Y.-A.; Chang, Y.-Y.; Ananda, L.; Wang, S.-Y.; Hsu, J.-T. Application of zearalenone (ZEN)-detoxifying Bacillus in animal feed decontamination through fermentation. Toxins 2019, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Kakeya, H.; Takahashi-Ando, N.; Kimura, M.; Onose, R.; Yamaguchi, I.; Osada, H. Biotransformation of the mycotoxin, zearalenone, to a non-estrogenic compound by a fungal strain of Clonostachys sp. Biosci. Biotechnol. Biochem. 2002, 66, 2723–2726. [Google Scholar] [CrossRef] [PubMed]
- Kriszt, R.; Krifaton, C.; Szoboszlay, S.; Cserháti, M.; Kriszt, B.; Kukolya, J.; Czéh, A.; Fehér-Tóth, S.; Török, L.; Szőke, Z.; et al. A new zearalenone biodegradation strategy using non-pathogenic Rhodococcus pyridinivorans K408 strain. PLoS ONE 2012, 7, e43608. [Google Scholar] [CrossRef]
- Wang, J.Q.; Yang, F.; Yang, P.L.; Liu, J.; Lv, Z.H. Microbial reduction of zearalenone by a new isolated Lysinibacillus sp. ZJ-2016-1. World Mycotoxin J. 2018, 11, 1–8. [Google Scholar] [CrossRef]
- Banu, I.; Lupu, A.; Aprodu, I. Degradation of zearalenone by laccase enzyme. Sci. Study Res. 2013, 14, 79–84. [Google Scholar]
- Paris, M.P.K.; Schweiger, W.; Hametner, C.; Stuckler, R.; Muehlbauer, G.J.; Varga, E.; Krska, R.; Berthiller, F.; Adam, G. Zearalenone-16-O-glucoside: A new masked mycotoxin. J. Agric. Food Chem. 2014, 62, 1181–1189. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, W.; Chen, C.-C.; Hu, X.; Guo, R.-T. Crystal structure of a mycoestrogen-detoxifying lactonase from Rhinocladiella mackenziei: Molecular insight into ZHD substrate selectivity. ACS Catal. 2018, 8, 4294–4298. [Google Scholar] [CrossRef]
- de Oliveira Garcia, S.; Vanessa Marimón Sibaja, K.; Vilar Nogueira, W.; Carla Penteado Feltrin, A.; Fabiano Alvares Pinheiro, D.; Barnes Rodrigues Cerqueira, M.; Badiale Furlong, E.; Garda-Buffon, J. Peroxidase as a simultaneous degradation agent of ochratoxin A and zearalenone applied to model solution and beer. Food Res. Int. 2020, 131, 109039. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Ko, T.-P.; Yang, Y.; Zheng, Y.; Chen, C.-C.; Zhu, Z.; Huang, C.; Zeng, Y.-F.; Huang, J.-W.; Wang, A.; et al. Crystal structure and substrate-binding mode of the mycoestrogen-detoxifying lactonase ZHD from Clonostachys rosea. RSC Adv. 2014, 107, 62321–62325. [Google Scholar] [CrossRef]
- Takahashi-Ando, N.; Kimura, M.; Kakeya, H.; Osada, H.; Yamaguchi, I. A novel lactonohydrolase responsible for the detoxification of zearalenone: Enzyme purification and gene cloning. Biochem. J. 2002, 365, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Liu, H.; Sun, J.; Wang, J.; Zhao, C.; Zhang, W.; Zhang, J.; Sun, C. Zearalenone removal from corn oil by an enzymatic strategy. Toxins 2020, 12, 117. [Google Scholar] [CrossRef]
- Takahashi-Ando, N.; Ohsato, S.; Shibata, T.; Hamamoto, H.; Yamaguchi, I.; Kimura, M. Metabolism of zearalenone by genetically modified organisms expressing the detoxification gene from Clonostachys rosea. Appl. Environ. Microbiol. 2004, 70, 3239–3245. [Google Scholar] [CrossRef] [PubMed]
- Vekiru, E.; Fruhauf, S.; Hametner, C.; Schatzmayr, G.; Krska, R.; Moll, W.D.; Schuhmacher, R. Isolation and characterization of enzymatic zearalenone hydrolysis reaction products. World Mycotoxin J. 2016, 9, 353–363. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, L.; Chen, J.; Wang, W.; Zhang, R.; Li, Y.; Zhang, Q.; Wang, W. Degradation mechanism for zearalenone ring-cleavage by zearalenone hydrolase RmZHD: A QM/MM study. Sci. Total Environ. 2019, 709, 135897. [Google Scholar] [CrossRef]
- Higa-Nishiyama, A.; Takahashi-Ando, N.; Shimizu, T.; Kudo, T.; Yamaguchi, I.; Kimura, M. A model transgenic cereal plant with detoxification activity for the estrogenic mycotoxin zearalenone. Transgenic Res. 2005, 14, 713–717. [Google Scholar] [CrossRef]
- Takahashi-Ando, N.; Tokai, T.; Hamamoto, H.; Yamaguchi, I.; Kimura, M. Efficient decontamination of zearalenone, the mycotoxin of cereal pathogen, by transgenic yeasts through the expression of a synthetic lactonohydrolase gene. Appl. Microbiol. Biotechnol. 2005, 67, 838–844. [Google Scholar] [CrossRef]
- Shcherbakova, L.A.; Mikityuk, O.D.; Nazarova, T.A.; Dzhavakhiya, V.G. Study of aflatoxin B1-destroying activity of Gliocladium roseum and Trichoderma viride and their antagonism toward toxigenic Aspergillus flavus. Agric. Biol. 2016, 51, 946–950. [Google Scholar] [CrossRef][Green Version]
- Mikityuk, O.D.; Statsyuk, N.V.; Nazarova, T.A.; Shcherbakova, L.A.; Dzhavakhiya, V.G. Mycotoxin degradation by microbial metabolites. In Proceedings of the Abstracts of International Scientific Conference PLAMIC2020 “Plants and Microorganisms: Biotechnology of the Future”, Saratov, Russia, 5–9 October 2020. in press. [Google Scholar]
- Sinitsyn, A.P.; Rozhkova, A.M. Penicillium canescens host as the platform for development of a new recombinant strains producers of carbohydrases. In Microorganisms in Biorefineries; Kamm, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–19. [Google Scholar] [CrossRef]
- Benevolenskaya, E.A.; Benevolensky, M.S.; Zatsepin, S.S.; Zorov, I.N.; Rozhkova, A.M.; Sinitsyn, A.P.; Sereda, A.S.; Bushina, E.V. A novel recombinant strain of filamentous fungus Penicillium canescens PEP3 and obtaining on its base a complex enzyme preparation with endo- and exoproteolytic activities. Patent RU 2616275, 13 April 2017. [Google Scholar]
- Vinetsky, Y.P.; Vavilova, E.A.; Chulkin, A.M.; Okunev, O.N.; Sokolova, L.M.; Sinitsyn, A.P.; Chernoglazov, V.M.; Sinitsyna, O.A. A way for production of a complex enzyme preparation (variations) for feed biorefinery and a Penicillium canescens strain (variants). Patent RU 2288267C2, 27 November 2006. [Google Scholar]
- Sinitsyn, A.P.; Rozhkova, A.M.; Zorov, I.N.; Bushina, E.V.; Sereda, A.S.; Zverlov, V.V.; Schwartz, V.G. A novel recombinant strain of filamentous fungus Penicillium canescens CS15, a producer of Clostridium thermocellum cellulose, and the producer culturing method. Patent RU 2612158C1, 2 March 2017. [Google Scholar]
- Vinetsky, Y.P.; Rozhkova, A.M.; Chulkin, A.M.; Satrutdinov, A.D.; Sinitsyna, O.A.; Fedorova, E.A.; Bekkarevich, A.O.; Okunev, O.N.; Sinitsyn, A.P. Regulatory activity of heterologous gene-activator xlnR of Aspergillus niger in Penicillium canescens. Biochemistry (Moscow) 2009, 74, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Volkov, P.V.; Rozhkova, A.M.; Pravilnikov, A.G.; Andrianov, R.M.; Dotsenko, G.S.; Bekkarevich, A.O.; Koshelev, A.V.; Okunev, O.N.; Zorov, I.N.; Sinitsyn, A.P. Production of enzyme preparations on the basis of Penicillium canescens recombinant strains with ability for the hydrolysis of plant materials. Appl. Biochem. Microb. (Mosc.) 2012, 48, 58–64. [Google Scholar] [CrossRef]
- Sinitsyna, O.A.; Gusakov, A.V.; Okunev, O.N.; Serebryany, V.A.; Vavilova, E.A.; Vinetsky, Y.P.; Sinitsyn, A.P. Recombinant endo-beta-1,4-xylanase from Penicillium canescens. Biochemistry (Moscow) 2003, 68, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Sinitsyna, O.A.; Fedorova, E.A.; Semenova, M.V.; Gusakov, A.V.; Sokolova, L.M.; Bubnova, T.M.; Okunev, O.N.; Chulkin, A.M.; Vavilova, E.A.; Vinetsky, Y.P.; et al. Isolation and characterization of extracellular pectinlyase from Penicillium canescnes. Biochemistry (Moscow) 2007, 72, 561–571. [Google Scholar] [CrossRef]
- Sinitsyna, O.A.; Fedorova, E.A.; Vakar, I.M.; Kondratieva, E.G.; Rozhkova, A.M.; Sokolova, L.M.; Bubnova, T.M.; Okunev, O.N.; Chulkin, A.M.; Vinetsky, Y.P.; et al. Isolation and characterization of extracellular alpha-galactosidase from Penicillium canescens. Biochemistry (Moscow) 2008, 73, 97–106. [Google Scholar] [CrossRef]
- Volkov, P.V.; Sinitsyna, O.A.; Fedorova, E.A.; Rojkova, A.M.; Satrutdinov, A.D.; Zorov, I.N.; Okunev, O.N.; Gusakov, A.V.; Sinitsyn, A.P. Isolation and properties of recombinant inulinases from Aspergillus sp. Biochemistry (Moscow) 2012, 77, 492–501. [Google Scholar] [CrossRef]
- Xiang, L.; Wang, Q.; Zhou, Y.; Yin, L.; Zhang, G.; Ma, Y. High-level expression of a ZEN-detoxifying gene by codon optimization and biobrick in Pichia pastoris. Microb. Res. 2016, 93, 48–56. [Google Scholar] [CrossRef]
- Grenier, B.; Loureiro-Bracarense, A.-P.; Leslie, J.F.; Oswald, I.P. Physical and Chemical Methods for Mycotoxin Decontamination in Maize. In Mycotoxin Reduction in Grain Chains; Leslie, J.F., Logrieco, A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2014; pp. 116–129. ISBN 978-0-813-82083-5. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, Y.; Zhu, J.; Yu, Z.; Tian, X.; Han, J.; Zhang, Z.; Han, W. Theoretical study on zearalenol compounds binding with wild type zearalenone hydrolase and V153H mutant. Int. J. Mol. Sci. 2018, 19, 2808. [Google Scholar] [CrossRef]
- Hao, X.; Chang, X.; Wu, S.; Wu, Z.; Sun, C. Study on the factors to affect the activity of zearalenone degrading enzyme ZLHY6. Sci. Technol. Cereals Oils Foods 2013, 21, 99–101. [Google Scholar]
- Juturu, V.; Wu, J.C. Microbial xylanases: Engineering, production and industrial applications. Biotechnol. Adv. 2012, 30, 1219–1227. [Google Scholar] [CrossRef]
- Dzhavakhiya, V.; Voinova, T.; Popletaeva, S.; Statsyuk, N.; Limantseva, L.; Shcherbakova, L. Effect of various compounds blocking the colony pigmentation on the aflatoxin B1 production by Aspergillus flavus. Toxins 2016, 8, 313. [Google Scholar] [CrossRef] [PubMed]
- Zhemchuzhina, N.; Shcherbakova, L.; Mikityuk, O.; Nazarova, T.; Campbell, B. Extracellular degradation of aflatoxin by certain fungi previously identified as aflatoxin B1 biodestructors. J. Plant Pathol. 2011, 93, 26. [Google Scholar] [CrossRef]
- Savitsky, P.; Bray, J.; Cooper, C.D.; Marsden, B.D.; Mahajan, P.; Burgess-Brown, N.A.; Gileadi, O. High-throughput production of human proteins for crystallization: The SGC experience. J. Struct. Biol. 2010, 172, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Aslanidis, C.; de Jong, P.J. Ligation-independent cloning of PCR products (LIC-PCR). Nucleic Acids Res. 1990, 18, 6069–6074. [Google Scholar] [CrossRef]
- Smith, B.E. Protein Sequencing Protocols; Humana Press: Totowa, NJ, USA, 1997. [Google Scholar]
- Dotsenko, A.S.; Rozhkova, A.M.; Gusakov, A.V. Properties and N-glycosylation of recombinant endoglucanase II from Penicillium verruculosum. Mosc. Univ. Chem. Bull. 2015, 70, 283–286. [Google Scholar] [CrossRef]
- Dotsenko, A.S.; Gusakov, A.V.; Volkov, P.V.; Rozhkova, A.M.; Sinitsyn, A.P. N-Linked glycosylation of recombinant cellobiohydrolase I (Cel7A) from Penicillium verruculosum and its effect on the enzyme activity. Biotechnol. Bioeng. 2016, 113, 283–291. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Chase, A.R. DNA Sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- Aleksenko, A.; Makarova, N.; Nikolaev, I.; Clutterbuck, A. Integrative and replicative transformation of Penicillium canescens with a heterologous nitrate-reductase gene. Curr. Genet. 1995, 28, 474–478. [Google Scholar] [CrossRef]
- Shcherbakova, L.; Statsyuk, N.; Mikityuk, O.; Nazarova, T.; Dzhavakhiya, V. Aflatoxin B1 degradation by metabolites of Phoma glomerata PG41 isolated from natural substrate colonized by aflatoxigenic Aspergillus flavus. Jundishapur J. Microbiol. 2015, 8, e24324. [Google Scholar] [CrossRef]
- Shcherbakova, L.A.; Nazarova, T.A.; Mikityuk, O.D.; Istomina, E.A.; Odintsova, T.I. An extract purified from the mycelium of a tomato wilt-controlling strain of Fusarium sambucinum can protect wheat against Fusarium and common root rots. Pathogens 2018, 7, 61. [Google Scholar] [CrossRef]

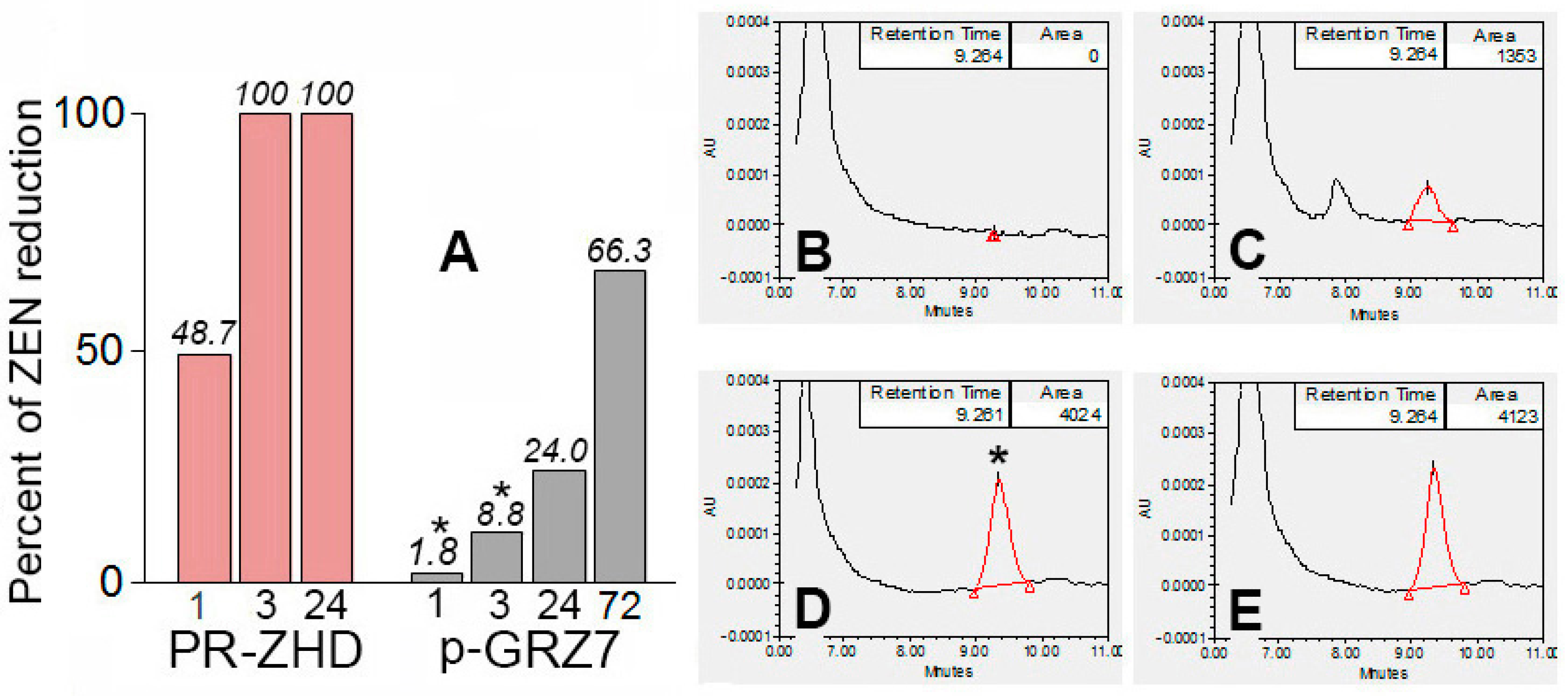
| Treatments | ZEN, µg/g (M ± SE) |
|---|---|
| Infected grain (control) | 15.58 ± 3,07 |
| Infected grain + PR-ZHD* | <0.2 ** |
| Infected grain + pPCA10 | 15.73 ± 4.35 |
| Non-infected grain + ZEN *** | 3.15 ± 0.09 *** |
| Non-infected grain + ZEN + pPCA10 | 3.17 ± 0.13 |
| Primer Name | Sequence |
|---|---|
| ZHD-LIC5 | 5′- TACTTCCAATCCATGCGCACTCGCAGCACAATCTCGAC -3′ |
| ZHD-LIC3 | 5′- TATCCACCTTTACTGTCAAAGATGCTTCTGCGTAGTTTC -3′ |
| ZHD-UpLIC | 5′- CAAACAGAAGCAACCGACACAATGCGCACTCGCAGCACAATCTCGA -3′ |
| ZHD-LowLIC | 5′- AGAGCAAGCCGAGCAGGTTCAAAGATGCTTCTGCGTAG -3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shcherbakova, L.; Rozhkova, A.; Osipov, D.; Zorov, I.; Mikityuk, O.; Statsyuk, N.; Sinitsyna, O.; Dzhavakhiya, V.; Sinitsyn, A. Effective Zearalenone Degradation in Model Solutions and Infected Wheat Grain Using a Novel Heterologous Lactonohydrolase Secreted by Recombinant Penicillium canescens. Toxins 2020, 12, 475. https://doi.org/10.3390/toxins12080475
Shcherbakova L, Rozhkova A, Osipov D, Zorov I, Mikityuk O, Statsyuk N, Sinitsyna O, Dzhavakhiya V, Sinitsyn A. Effective Zearalenone Degradation in Model Solutions and Infected Wheat Grain Using a Novel Heterologous Lactonohydrolase Secreted by Recombinant Penicillium canescens. Toxins. 2020; 12(8):475. https://doi.org/10.3390/toxins12080475
Chicago/Turabian StyleShcherbakova, Larisa, Alexandra Rozhkova, Dmitrii Osipov, Ivan Zorov, Oleg Mikityuk, Natalia Statsyuk, Olga Sinitsyna, Vitaly Dzhavakhiya, and Arkady Sinitsyn. 2020. "Effective Zearalenone Degradation in Model Solutions and Infected Wheat Grain Using a Novel Heterologous Lactonohydrolase Secreted by Recombinant Penicillium canescens" Toxins 12, no. 8: 475. https://doi.org/10.3390/toxins12080475
APA StyleShcherbakova, L., Rozhkova, A., Osipov, D., Zorov, I., Mikityuk, O., Statsyuk, N., Sinitsyna, O., Dzhavakhiya, V., & Sinitsyn, A. (2020). Effective Zearalenone Degradation in Model Solutions and Infected Wheat Grain Using a Novel Heterologous Lactonohydrolase Secreted by Recombinant Penicillium canescens. Toxins, 12(8), 475. https://doi.org/10.3390/toxins12080475





