Probing the Interactions of Ochratoxin B, Ochratoxin C, Patulin, Deoxynivalenol, and T-2 Toxin with Human Serum Albumin
Abstract
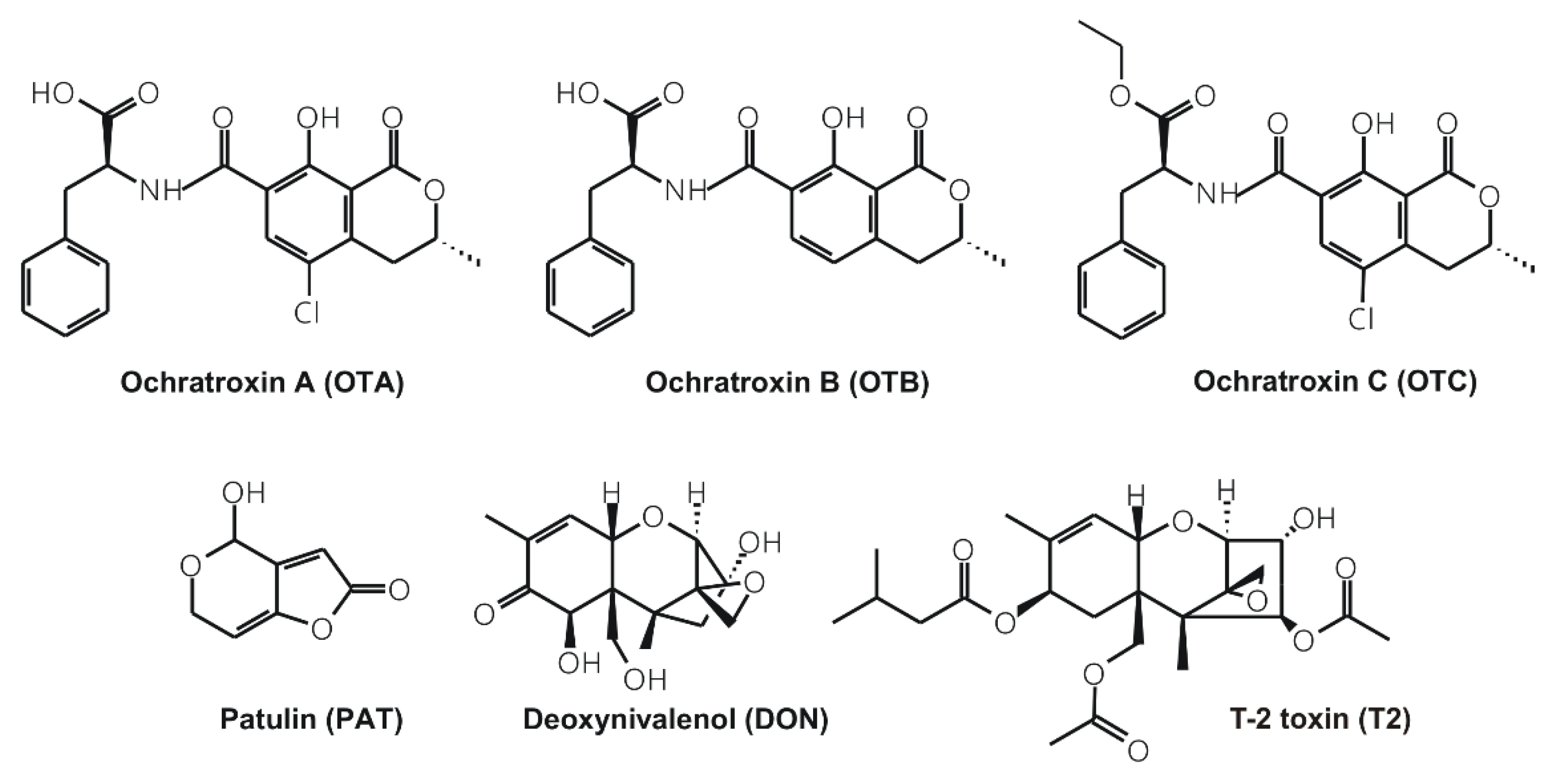
1. Introduction
2. Results and Discussion
2.1. Fluorescence Spectroscopic Investigation of the Interactions of OTB and OTC with HSA
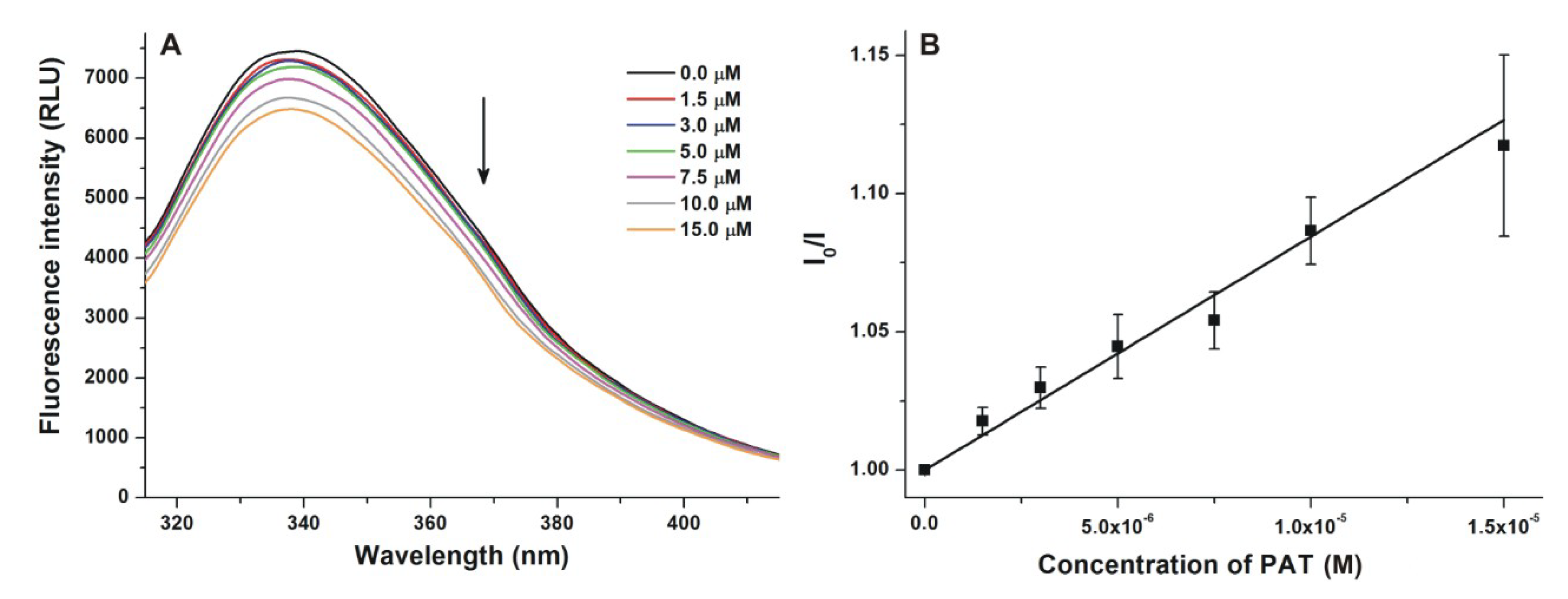
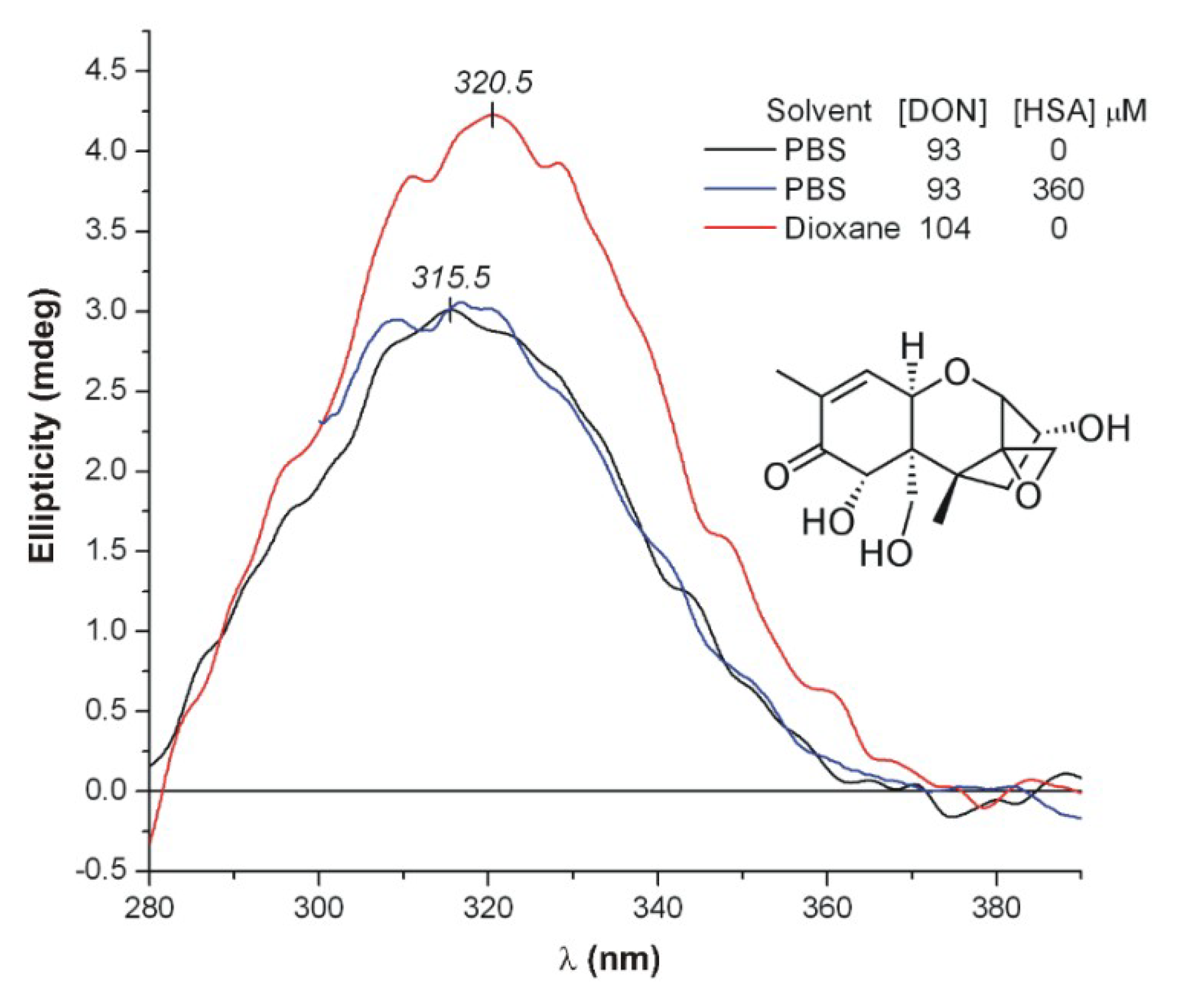
2.2. Spectroscopic Investigation of the Interactions of PAT, DON, and T2 with HSA
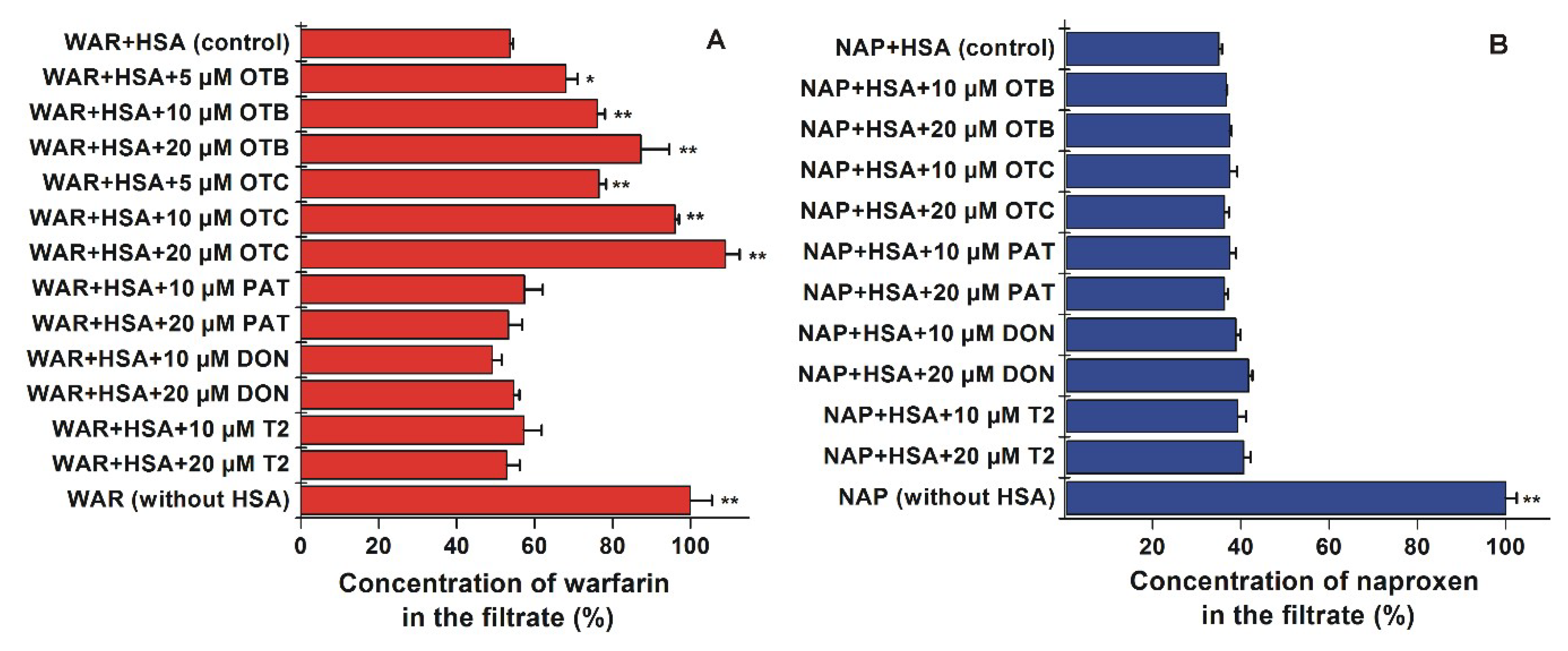
2.3. Effects of Mycotoxins on the Albumin Binding of Site I and II Markers Based on Ultrafiltration
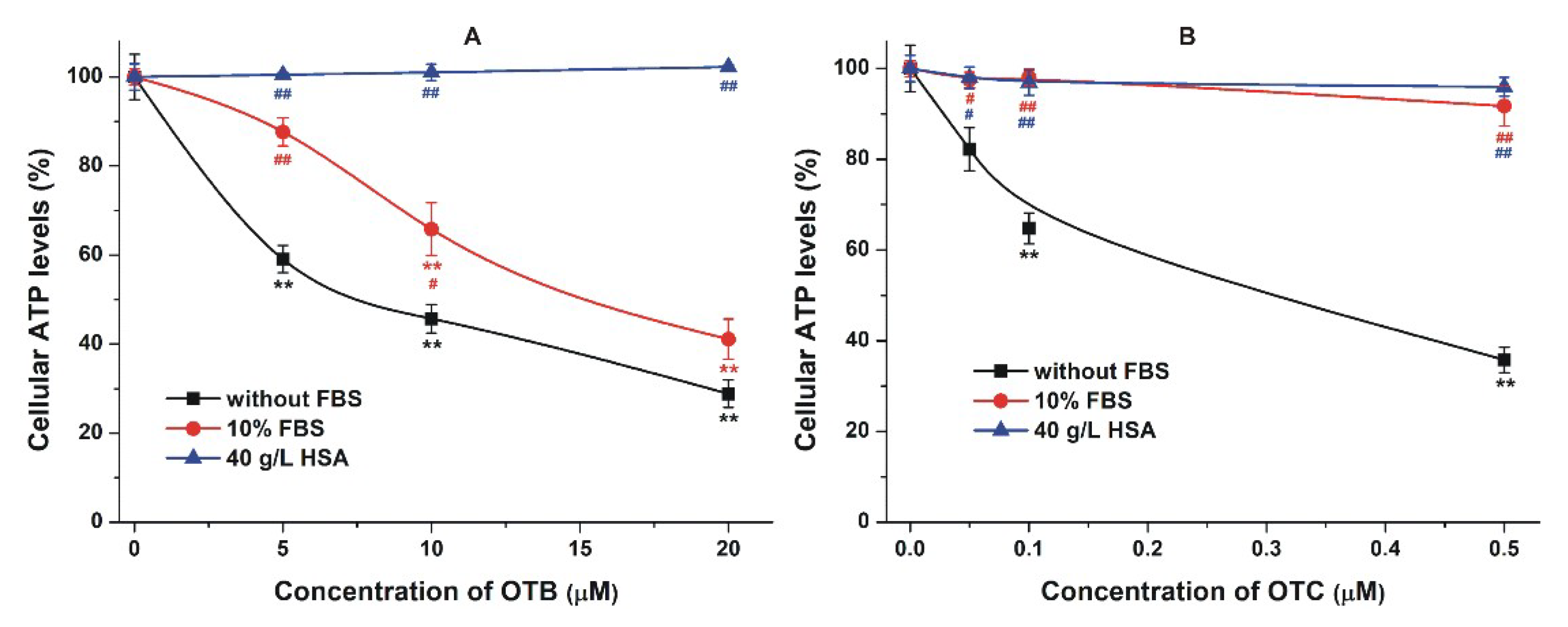
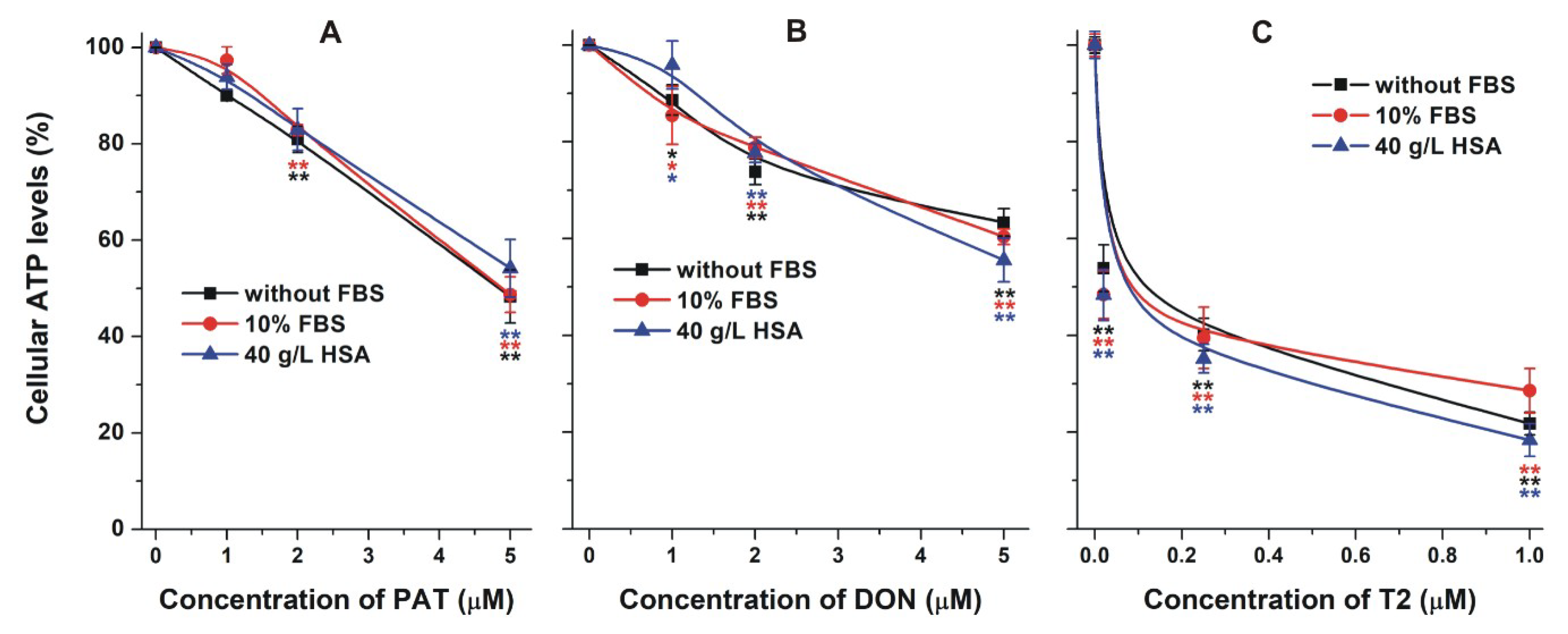
2.4. Effect of Albumin on the Acute Cellular Toxicity of Mycotoxins
3. Conclusions
4. Materials and Methods
4.1. Reagents
4.2. Fluorescence Spectroscopic Measurements
4.3. Circular Dichroism (CD) and Absorption Spectroscopic Measurements
4.4. Ultrafiltration
4.5. Cell Culturing and Viability Assay
4.6. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Da Rocha, M.E.B.; Freire, F.C.O.; Maia, F.E.F.; Guedes, M.I.F.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 59–165. [Google Scholar] [CrossRef]
- Chu, F.S. Studies on ochratoxins. CRC Crit. Rev. Toxicol. 1974, 2, 499–524. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] related to ochratoxin A in food. EFSA J. 2006, 365, 1–56. [Google Scholar] [CrossRef]
- Heussner, A.H.; Bingle, L.E.H. Comparative ochratoxin toxicity: A review of the available data. Toxins 2015, 7, 4253–4282. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Puel, O.; Galtier, P.; Oswald, I.P. Biosynthesis and toxicological effects of patulin. Toxins 2010, 2, 613–631. [Google Scholar] [CrossRef]
- WHO Joint FAO/WHO Expert Committee on Food additives (JECFA). Position paper on patulin. In Proceedings of the 30th Session, The Hague, The Netherlands, 9–13 March 1998. [Google Scholar]
- Rotter, B.A.; Prelusky, D.B.; Pestka, J.J. Toxicology of deoxynivalenol (vomitoxin). J. Toxicol. Environ. Health 1996, 48, 1–34. [Google Scholar] [CrossRef]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdisc. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef]
- Miller, K.; Atkinson, H.A.C. The in vitro effects of trichothecenes on the immune system. Food Chem. Toxicol. 1986, 24, 545–549. [Google Scholar] [CrossRef]
- Lutsky, I.; Mor, N.; Yagen, B.; Joffe, A.Z. The role of T-2 toxin in experimental alimentary toxic aleukia: A toxicity study in cats. Toxicol. Appl. Pharmacol. 1978, 43, 111–124. [Google Scholar] [CrossRef]
- Hayes, A.W. Mycotoxin: A review of biological effects and their role in human disease. Clin. Toxicol. 1980, 17, 45–83. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.P. Immunotoxicity of mycotoxins. J. Dairy Sci. 1993, 76, 892–897. [Google Scholar] [CrossRef]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Chuang, V.T.; Maruyama, T.; Otagiri, M. Albumin-drug interaction and its clinical implication. Biochim. Biophys. Acta 2013, 1830, 5435–5443. [Google Scholar] [CrossRef] [PubMed]
- Zsila, F. Subdomain IB is the third major drug binding region of human serum albumin: Toward the three-sites model. Mol. Pharm. 2013, 10, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Il’ichev, Y.V.; Perry, J.L.; Simon, J.D. Interaction of ochratoxin A with human serum albumin. Preferential binding of the dianion and pH effects. J. Phys. Chem. B 2002, 106, 452–459. [Google Scholar] [CrossRef]
- Poór, M.; Lemli, B.; Bálint, M.; Hetényi, C.; Sali, N.; Kőszegi, T.; Kunsági-Máté, S. Interaction of citrinin with human serum albumin. Toxins 2015, 7, 5155–5166. [Google Scholar] [CrossRef] [PubMed]
- Faisal, Z.; Lemli, B.; Szerencsés, D.; Kunsági-Máté, S.; Bálint, M.; Hetényi, C.; Kuzma, M.; Mayer, M.; Poór, M. Interactions of zearalenone and its reduced metabolites α-zearalenol and β-zearalenol with serum albumins: Species differences, binding sites, and thermodynamics. Mycotoxin Res. 2018, 34, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Fliszár-Nyúl, E.; Lemli, B.; Kunsági-Máté, S.; Dellafiora, L.; Dall’Asta, C.; Cruciani, G.; Pethő, G.; Poór, M. Interaction of mycotoxin alternariol with serum albumin. Int. J. Mol. Sci. 2019, 20, 2352. [Google Scholar] [CrossRef]
- Kőszegi, T.; Poór, M. Ochratoxin A: Molecular interactions, mechanisms of toxicity and prevention at the molecular level. Toxins 2016, 8, 111. [Google Scholar] [CrossRef]
- Perry, J.L.; Il’ichev, Y.V.; Kempf, V.R.; McClendon, J.; Park, G.; Manderville, R.A.; Rüker, F.; Dockal, M.; Simon, J.D. Binding of ochratoxin A derivatives to human serum albumin. J. Phys. Chem. B 2003, 107, 6644–6647. [Google Scholar] [CrossRef]
- Sueck, F.; Poór, M.; Faisal, Z.; Gertzen, C.G.W.; Cramer, B.; Lemli, B.; Kunsági-Máté, S.; Gohlke, H.; Humpf, H.-U. Interaction of ochratoxin A and its thermal degradation product 2’R-ochratoxin A with human serum albumin. Toxins 2018, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Yuqin, L.; Hao, W.; Baoxiu, J.; Caihong, L.; Ke, L.; Yongxiu, Q.; Zhide, H. Study of the interaction of deoxynivalenol with human serum albumin by spectroscopic technique and molecular modelling. Food Addit. Contam. Part A Chem. 2013, 30, 356–364. [Google Scholar] [CrossRef]
- Yuqin, L.; Guirong, Y.; Zhen, Y.; Caihong, L.; Baoxiu, J.; Jiao, C.; Yurong, G. Investigation of the interaction between patulin and human serum albumin by a spectroscopic method, atomic force microscopy, and molecular modeling. Biomed. Res. Int. 2014, 2014, 734850. [Google Scholar] [CrossRef]
- Fruhauf, S.; Novak, B.; Nagl, V.; Hackl, M.; Hartinger, D.; Rainer, V.; Labudová, S.; Adam, G.; Aleschko, M.; Moll, W.-D.; et al. Biotransformation of the mycotoxin zearalenone to its metabolites hydrolyzed zearalenone (HZEN) and decarboxylated hydrolyzed zearalenone (DHZEN) diminishes its estrogenicity in vitro and in vivo. Toxins 2019, 11, 481. [Google Scholar] [CrossRef]
- Poór, M.; Bálint, M.; Hetényi, C.; Gődér, B.; Kunsági-Máté, S.; Kőszegi, T.; Lemli, B. Investigation of non-covalent interactions of aflatoxins (B1, B2, G1, G2, and M1) with serum albumin. Toxins 2017, 9, 339. [Google Scholar] [CrossRef]
- Bagheri, M.; Fatemi, M.H. Fluorescence spectroscopy, molecular docking and molecular dynamic simulation studies of HSA-Aflatoxin B1 and G1 interactions. J. Lumin. 2018, 202, 345–353. [Google Scholar] [CrossRef]
- Tan, H.; Chen, L.; Ma, L.; Liu, S.; Zhou, H.; Zhang, Y.; Guo, T.; Liu, W.; Dai, H.; Yu, Y. Fluorescence spectroscopic investigation of competitive interactions between quercetin and aflatoxin B₁ for binding to human serum albumin. Toxins 2019, 11, 214. [Google Scholar] [CrossRef]
- Il’ichev, Y.V.; Perry, J.L.; Rüker, F.; Dockal, M.; Simon, J.D. Interaction of ochratoxin A with human serum albumin. Binding sites localized by competitive interactions with the native protein and its recombinant fragments. Chem. Biol. Interact. 2002, 141, 275–293. [Google Scholar] [CrossRef]
- Il’ichev, Y.V.; Perry, J.L.; Simon, J.D. Interaction of ochratoxin A with human serum albumin. A common binding site of ochratoxin A and warfarin in subdomain IIA. J. Phys. Chem. B 2002, 106, 460–465. [Google Scholar] [CrossRef]
- Dobretsov, G.E.; Syrejschikova, T.I.; Smolina, N.V. On mechanisms of fluorescence quenching by water. Biophysics 2014, 59, 183–188. [Google Scholar] [CrossRef]
- Gradinaru, C.C.; Marushchak, D.O.; Samim, M.; Krull, U.J. Fluorescence anisotropy: From single molecules to live cells. Analyst 2010, 135, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Faisal, Z.; Derdák, D.; Lemli, B.; Kunsági-Máté, S.; Bálint, M.; Hetényi, C.; Csepregi, R.; Kőszegi, T.; Sueck, F.; Humpf, H.-U.; et al. Interaction of 2′R-ochratoxin A with serum albumins: Binding site, effects of site markers, thermodynamics, species differences of albumin-binding, and influence of albumin on its toxicity in MDCK cells. Toxins 2018, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Park, G.; Perry, J.L.; Il’ichev, Y.V.; Bow, D.A.J.; Pritchard, J.B.; Faucet, V.; Pfohl-Leszkowicz, A.; Manderville, R.A.; Simon, J.D. Molecular aspects of the transport and toxicity of ochratoxin A. Acc. Chem. Res. 2004, 37, 874–881. [Google Scholar] [CrossRef]
- Perry, J.L.; Goldsmith, M.R.; Peterson, M.A.; Beratan, D.N. Structure of the ochratoxin A binding site within human serum albumin. J. Phys. Chem. B 2004, 108, 16960–16964. [Google Scholar] [CrossRef]
- Wybranowski, T.; Ziomkowska, B.; Cwynar, A.; Kruszewski, S. The influence of displacement compounds on the binding of ochratoxin A to human serum albumin examined with fluorescence anisotropy methods. Opt. Appl. 2014, 44, 357–364. [Google Scholar] [CrossRef]
- Li, Y.; Czibulya, Z.; Poór, M.; Lecomte, S.; Kiss, L.; Harte, E.; Kőszegi, T.; Kunsági-Máté, S. Thermodynamic study of the effects of ethanol on the interaction of ochratoxin A with human serum albumin. J. Lumin. 2014, 148, 18–25. [Google Scholar] [CrossRef]
- Poór, M.; Kunsági-Máté, S.; Bencsik, T.; Petrik, J.; Vladimir-Knežević, S.; Kőszegi, T. Flavonoid aglycones can compete with Ochratoxin A for human serum albumin: A new possible mode of action. Int. J. Biol. Macromol. 2012, 51, 279–283. [Google Scholar] [CrossRef]
- Poór, M.; Li, Y.; Matisz, G.; Kiss, L.; Kunsági-Máté, S.; Kőszegi, T. Quantitation of species differences in albumin-ligand interactions for bovine, human and rat serum albumins using fluorescence spectroscopy: A test case with some Sudlow’s site I ligands. J. Lumin. 2014, 145, 767–773. [Google Scholar] [CrossRef]
- Perry, J.L.; Christensen, T.; Goldsmith, M.R.; Toone, E.J.; Beratan, D.N.; Simon, J.D. Binding of ochratoxin A to human serum albumin stabilized by a protein-ligand ion pair. J. Phys. Chem. B 2003, 107, 7884–7888. [Google Scholar] [CrossRef]
- Galtier, P. Etude toxicologique et pharmacocinétique d’une mycotoxine, l’ochratoxin A. Ph.D. Thesis, Université Paul Sabatier, Toulouse, France, 1979. [Google Scholar]
- Galtier, P.; Alvinerie, M.; Charpenteau, J.L. The pharmacokinetic profiles of ochratoxin A in pigs, rabbits and chickens. Food Cosmet. Toxicol. 1981, 19, 735–738. [Google Scholar] [CrossRef]
- Hagelberg, S.; Hult, K.; Fuchs, R. Toxicokinetics of ochratoxin A in several species and its plasma-binding properties. J. Appl. Toxicol. 1989, 9, 91–96. [Google Scholar] [CrossRef]
- Horváth, E.; Kálmán, N.; Pesti, M.; Iwata, K.; Kunsági-Máté, S. Thermodynamic and kinetic processes during the unfolding of BSA in the presence of the mycotoxin patulin. Acta Biol. Hung. 2012, 63, 389–398. [Google Scholar] [CrossRef]
- Zsila, F.; Bikádi, Z.; Fitos, I.; Simonyi, M. Probing protein binding sites by circular dichroism spectroscopy. Curr. Drug Discov. Technol. 2004, 1, 133–153. [Google Scholar] [CrossRef] [PubMed]
- Zsila, F.; Beke-Somfai, T. Dimeric binding of plant alkaloid ellipticine to human serum proteins. RSC Adv. 2016, 6, 44096–44105. [Google Scholar] [CrossRef]
- Kasha, M.; Rawls, H.R.; El-Bayoumi, M.A. The exciton model in molecular spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar] [CrossRef]
- Boiadjiev, S.E.; Lightner, D.A. Exciton chirality. (A) Origins of and (B) applications from strongly fluorescent dipyrrinone chromophores. Mon. Chem. 2005, 136, 489–508. [Google Scholar] [CrossRef]
- Berova, N.; Di Bari, L.; Pescitelli, G. Application of electronic circular dichroism in configurational and conformational analysis of organic compounds. Chem. Soc. Rev. 2007, 36, 914–931. [Google Scholar] [CrossRef] [PubMed]
- Zsila, F.; Bikadi, Z.; Simonyi, M. Induced chirality upon crocetin binding to human serum albumin: Origin and nature. Tetrahedron Asymmetry 2001, 12, 3125–3137. [Google Scholar] [CrossRef]
- Fitos, I.; Visy, J.; Zsila, F.; Bikadi, Z.; Mády, G.; Simonyi, M. Specific ligand binding on genetic variants of human α1-acid glycoprotein studied by circular dichroism spectroscopy. Biochem. Pharmacol. 2004, 67, 679–688. [Google Scholar] [CrossRef]
- Zsila, F.; Fitos, I.; Bencze, G.; Kéri, G.; Őrfi, L. Determination of human serum α1-acid glycoprotein and albumin binding of various marketed and preclinical kinase inhibitors. Curr. Med. Chem. 2009, 16, 1964–1977. [Google Scholar] [CrossRef] [PubMed]
- Kirk, D.N.; Klyne, W.; Wallis, S.R. Optical rotatory dispersion. Part LXIV. Influence of solvents upon the cotton effects of some ketones. J. Chem. Soc. C 1970, 2, 350–360. [Google Scholar] [CrossRef]
- Catalan, J.; Catalan, J.P. On the solvatochromism of the n ↔ π* electronic transitions in ketones. Phys. Chem. Chem. Phys. 2011, 13, 4072–4082. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C.; Welton, T. Solvent effects on the absorption spectra of organic compounds. In Solvents and Solvent Effects in Organic Chemistry, 1st ed.; Wiley-VCH Verlag: Weinheim, Germany, 2011; pp. 359–424. [Google Scholar] [CrossRef]
- Zsila, F. Circular dichroism spectroscopic detection of ligand binding induced subdomain IB specific structural adjustment of human serum albumin. J. Phys. Chem. B 2013, 117, 10798–10806. [Google Scholar] [CrossRef] [PubMed]
- Mohos, V.; Fliszár-Nyúl, E.; Schilli, G.; Hetényi, C.; Lemli, B.; Kunsági-Máté, S.; Bognár, B.; Poór, M. Interaction of chrysin and its main conjugated metabolites chrysin-7-sulfate and chrysin-7-glucuronide with serum albumin. Int. J. Mol. Sci. 2018, 19, 4073. [Google Scholar] [CrossRef] [PubMed]
- Fliszár-Nyúl, E.; Mohos, V.; Bencsik, T.; Lemli, B.; Kunsági-Máté, S.; Poór, M. Interactions of 7,8-dihydroxyflavone with serum albumin as well as with CYP2C9, CYP2C19, CYP3A4, and xanthine oxidase biotransformation enzymes. Biomolecules 2019, 9, 655. [Google Scholar] [CrossRef]
- Gekle, M.; Pollock, C.A.; Silbernagl, S. Time- and concentration-dependent biphasic effect of ochratoxin A on growth of proximal tubular cells in primary culture. J. Pharmacol. Exp. Ther. 1995, 275, 397–404. [Google Scholar]
- Xiao, H.; Madhyastha, S.; Marquardt, R.R.; Li, S.; Vodela, J.K.; Frohlich, A.A.; Kemppainen, B.W. Toxicity of ochratoxin A, its opened lactone form and several of its analogs: Structure-activity relationships. Toxicol. Appl. Pharmacol. 1996, 137, 182–192. [Google Scholar] [CrossRef]
- Visconti, A.; Minervini, F.; Lucivero, G.; Gambatesa, V. Cytotoxic and immunotoxic effects of Fusarium mycotoxins using a rapid colorimetric bioassay. Mycopathologia 1991, 113, 181–186. [Google Scholar] [CrossRef]
- Hu, T.; Liu, Y. Probing the interaction of cefodizime with human serum albumin using multi-spectroscopic and molecular docking techniques. J. Pharm. Biomed. Anal. 2015, 107, 325–332. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibriumconstants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739. [Google Scholar] [CrossRef]
- Csepregi, R.; Temesfői, V.; Poór, M.; Faust, Z.; Kőszegi, T. Green fluorescent protein-based viability assay in a multiparametric configuration. Molecules 2018, 23, 1575. [Google Scholar] [CrossRef] [PubMed]
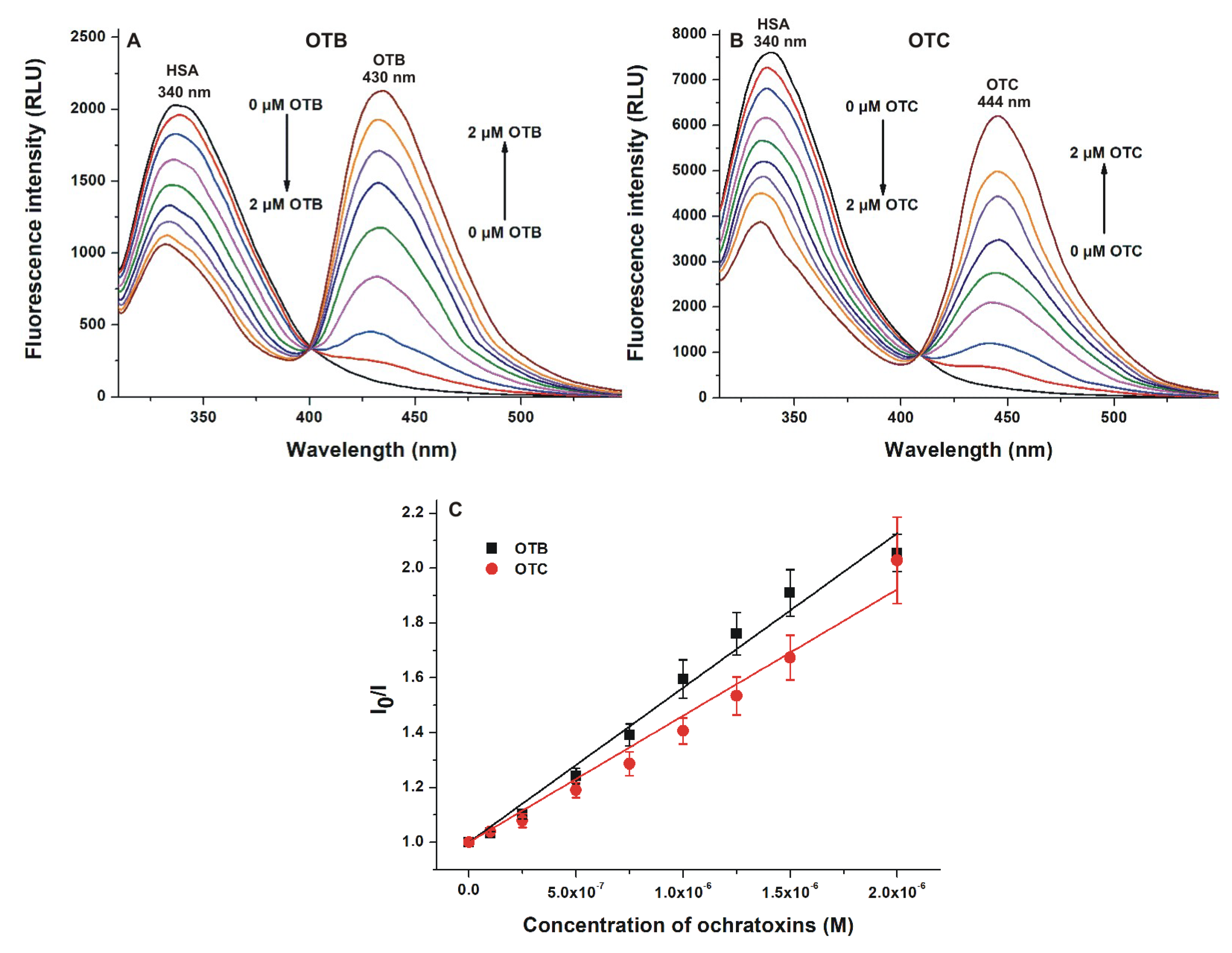
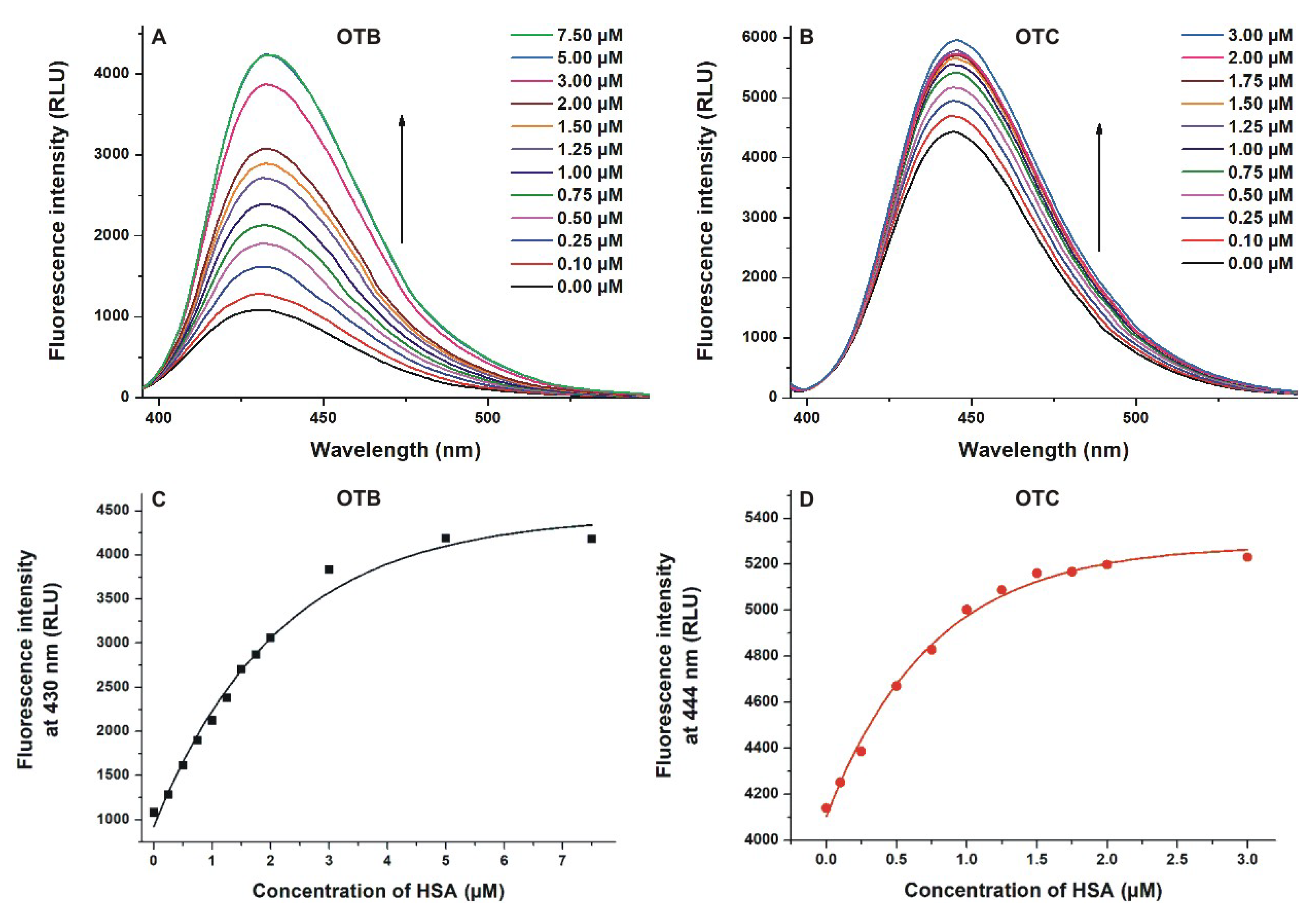

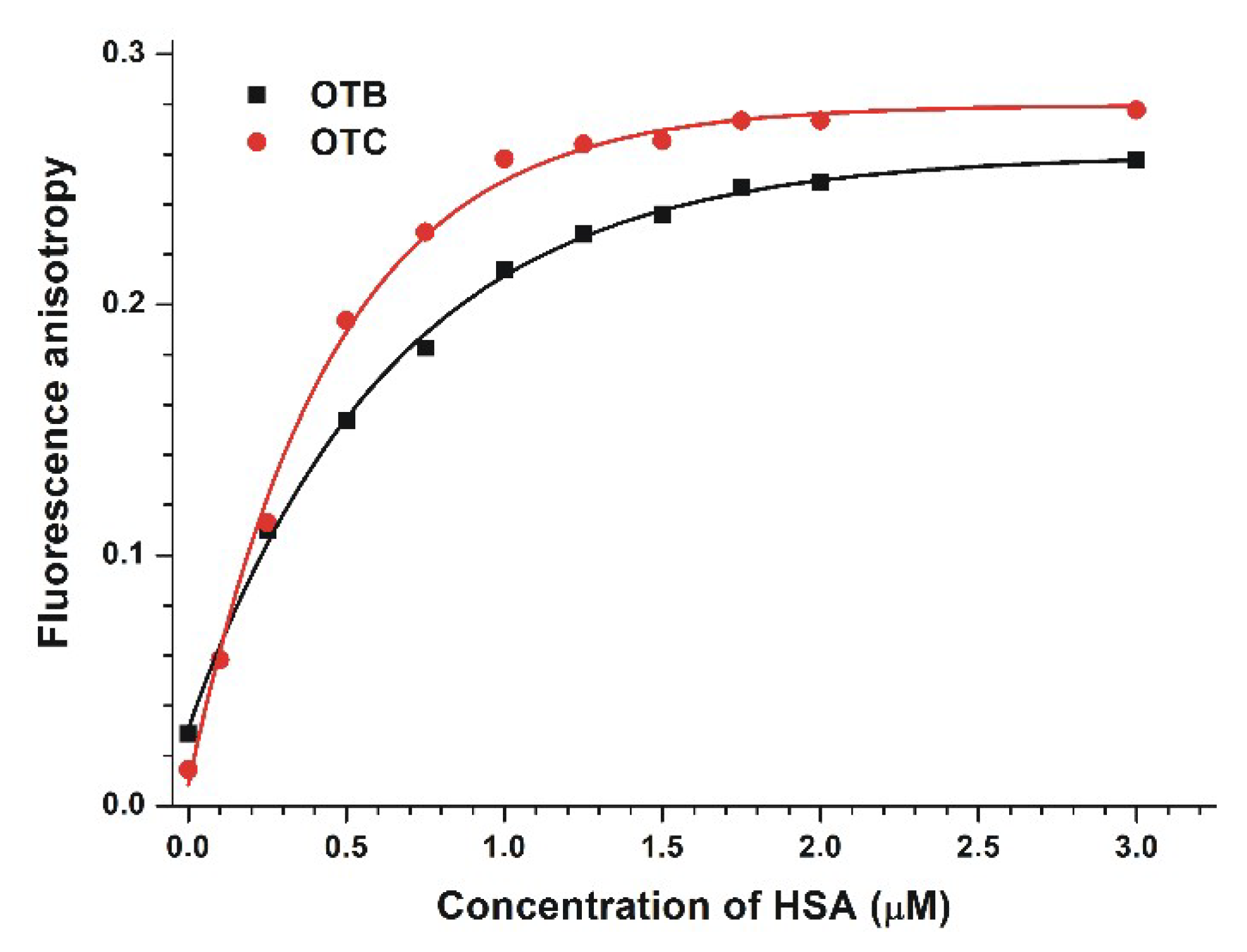

| Complex | KSV (±SEM) [×106 L/mol] (Stern–Volmer Plot, Figure 2) | Ka (±SEM) [×106 L/mol] (Hyperquad, Figure 2) | Ka (±SEM) [×106 L/mol] (Hyperquad, Figure 3) | Ka (±SEM) [×106 L/mol] (Hyperquad, Figure 4) | Ka (±SEM) [×106 L/mol] (Anisotropy, Figure 5) |
|---|---|---|---|---|---|
| OTB–HSA | 0.57 ± 0.05 | 0.83 ± 0.08 | 1.38 ± 0.21 | 1.02± 0.13 | 0.92 ± 0.04 |
| OTC–HSA | 0.46 ± 0.06 | 0.77 ± 0.11 | 16.02 ± 4.7 | 9.36 ± 2.00 | 6.82 ± 0.24 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faisal, Z.; Vörös, V.; Fliszár-Nyúl, E.; Lemli, B.; Kunsági-Máté, S.; Csepregi, R.; Kőszegi, T.; Zsila, F.; Poór, M. Probing the Interactions of Ochratoxin B, Ochratoxin C, Patulin, Deoxynivalenol, and T-2 Toxin with Human Serum Albumin. Toxins 2020, 12, 392. https://doi.org/10.3390/toxins12060392
Faisal Z, Vörös V, Fliszár-Nyúl E, Lemli B, Kunsági-Máté S, Csepregi R, Kőszegi T, Zsila F, Poór M. Probing the Interactions of Ochratoxin B, Ochratoxin C, Patulin, Deoxynivalenol, and T-2 Toxin with Human Serum Albumin. Toxins. 2020; 12(6):392. https://doi.org/10.3390/toxins12060392
Chicago/Turabian StyleFaisal, Zelma, Virág Vörös, Eszter Fliszár-Nyúl, Beáta Lemli, Sándor Kunsági-Máté, Rita Csepregi, Tamás Kőszegi, Ferenc Zsila, and Miklós Poór. 2020. "Probing the Interactions of Ochratoxin B, Ochratoxin C, Patulin, Deoxynivalenol, and T-2 Toxin with Human Serum Albumin" Toxins 12, no. 6: 392. https://doi.org/10.3390/toxins12060392
APA StyleFaisal, Z., Vörös, V., Fliszár-Nyúl, E., Lemli, B., Kunsági-Máté, S., Csepregi, R., Kőszegi, T., Zsila, F., & Poór, M. (2020). Probing the Interactions of Ochratoxin B, Ochratoxin C, Patulin, Deoxynivalenol, and T-2 Toxin with Human Serum Albumin. Toxins, 12(6), 392. https://doi.org/10.3390/toxins12060392






