Potential Cause of Decrease in Bloom Events of the Harmful Dinoflagellate Cochlodinium polykrikoides in Southern Korean Coastal Waters in 2016
Abstract
1. Introduction
2. Results
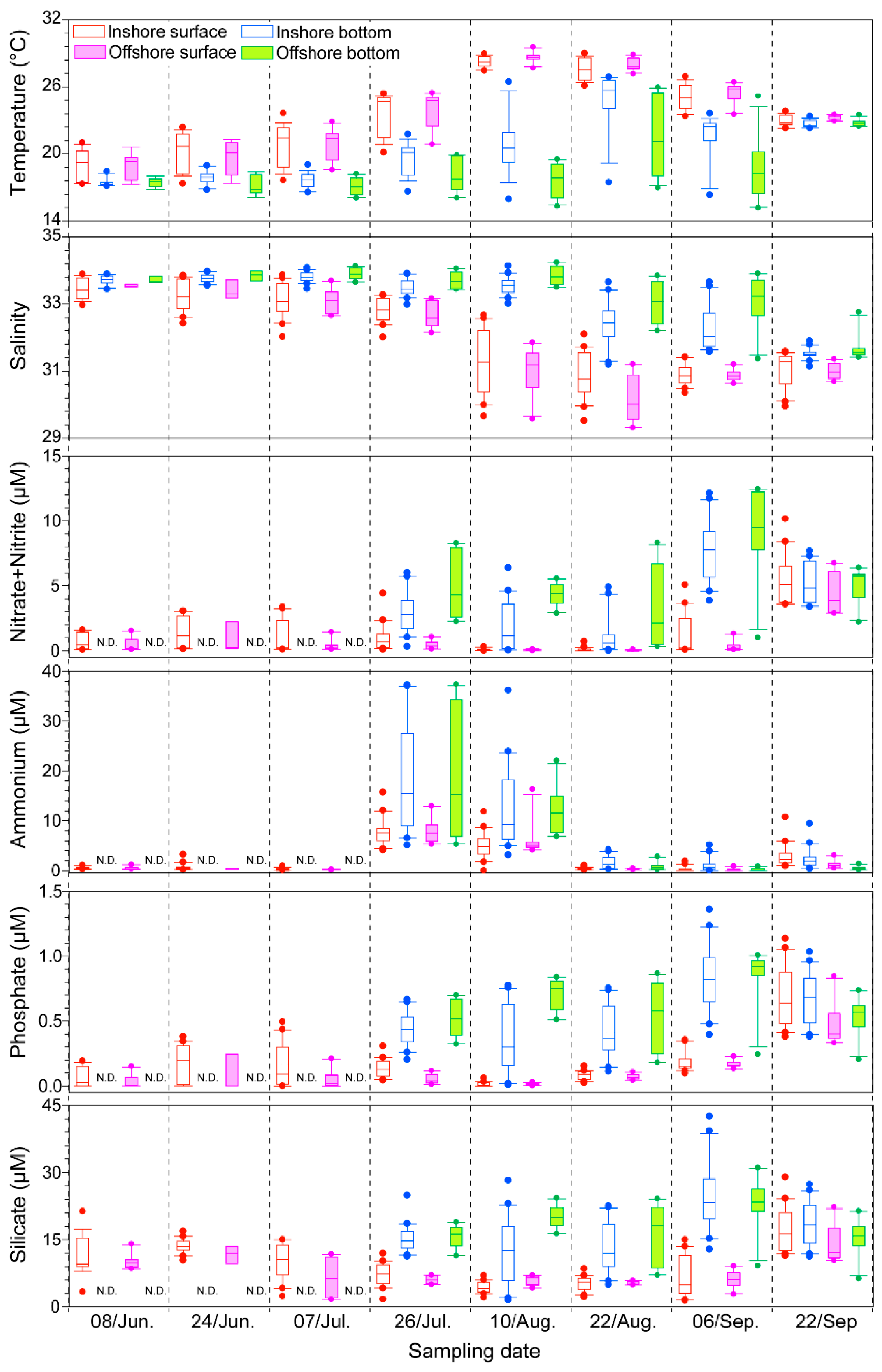
2.1. Physico-Chemical Factors along the Tongyeong Coast
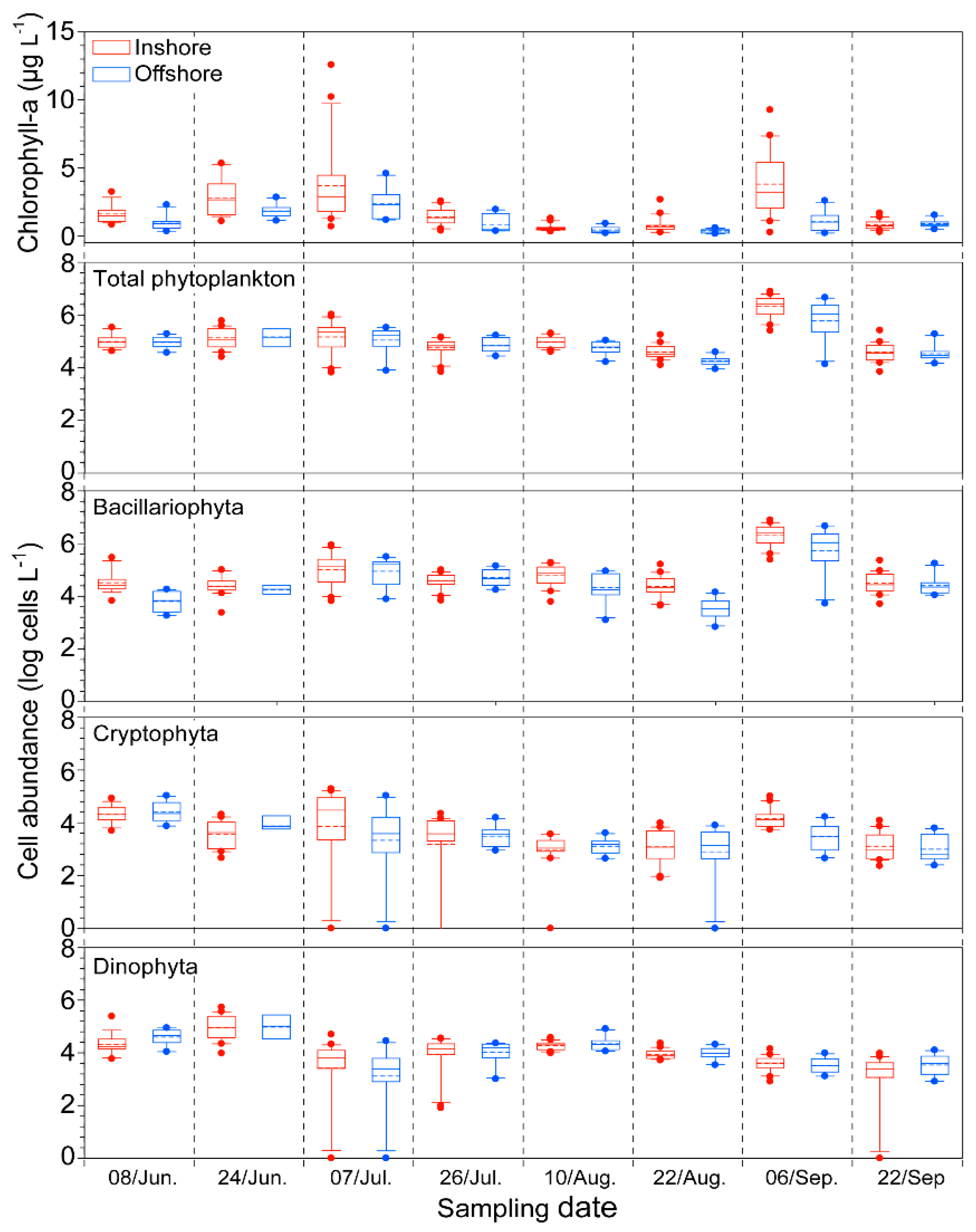
2.2. Biological Factor: Phytoplankton
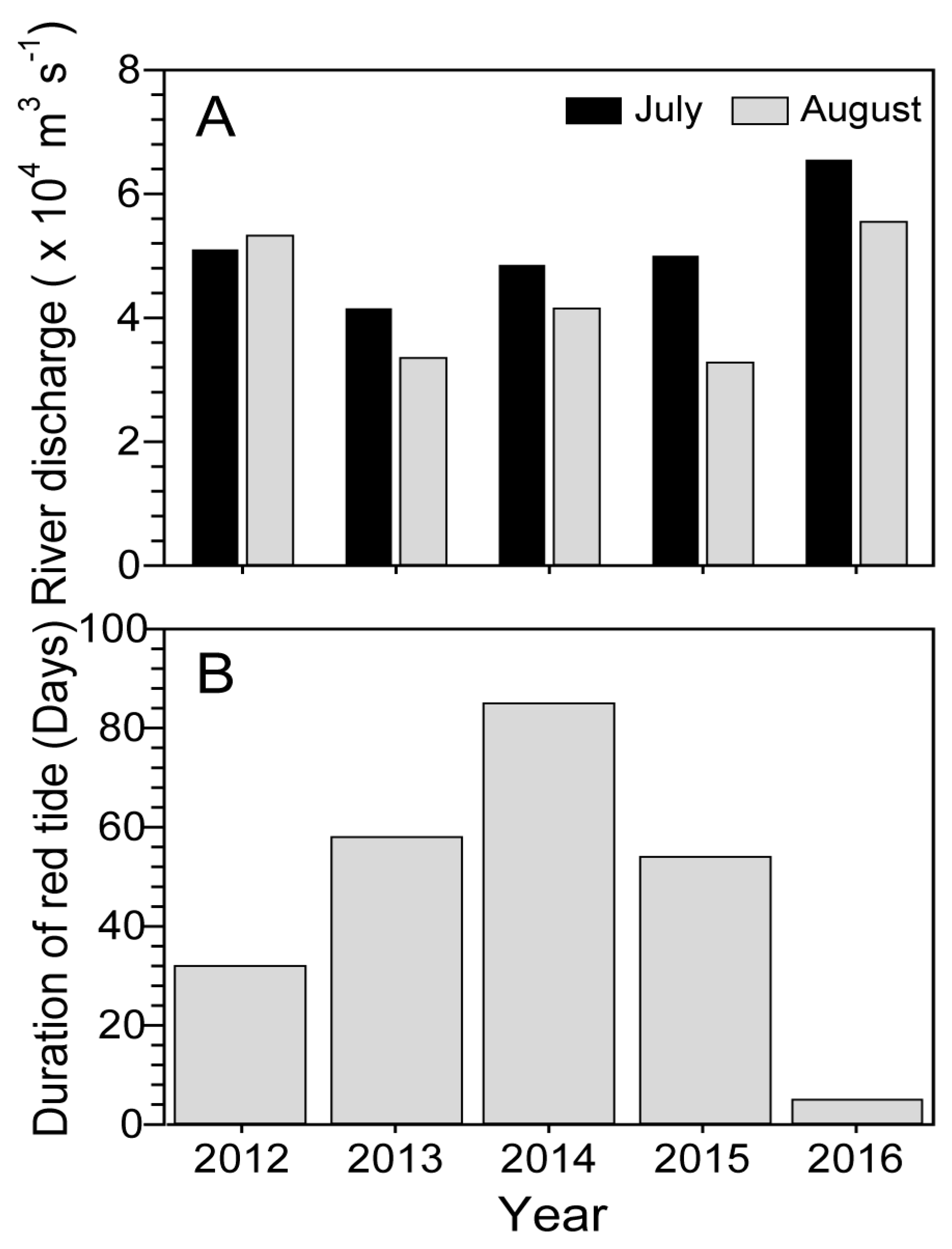
2.3. Comparisons of Water Temperature and Salinity between the Previous Four Years and 2016
2.4. Changjiang River Discharge and Duration of Blooms
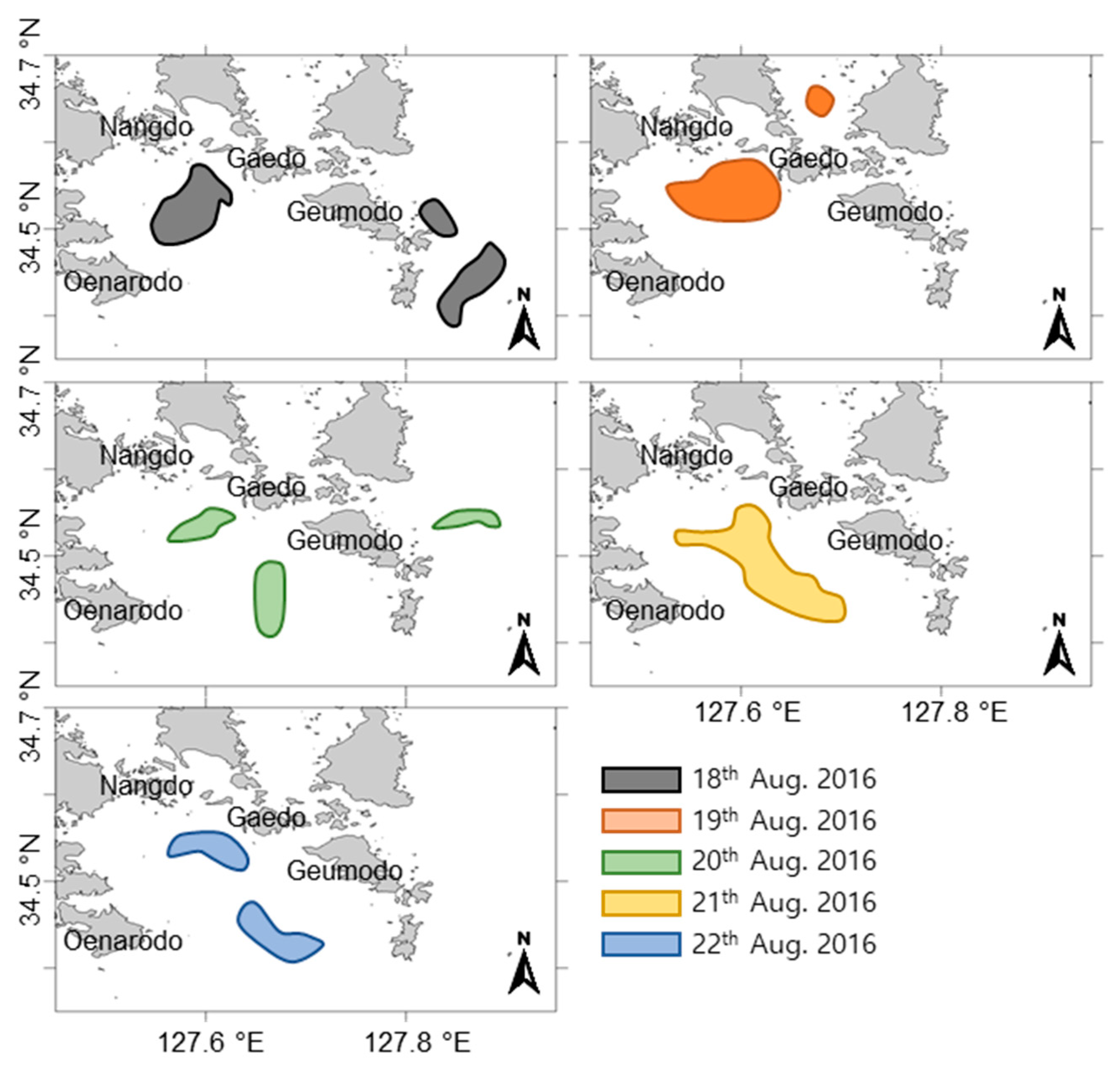
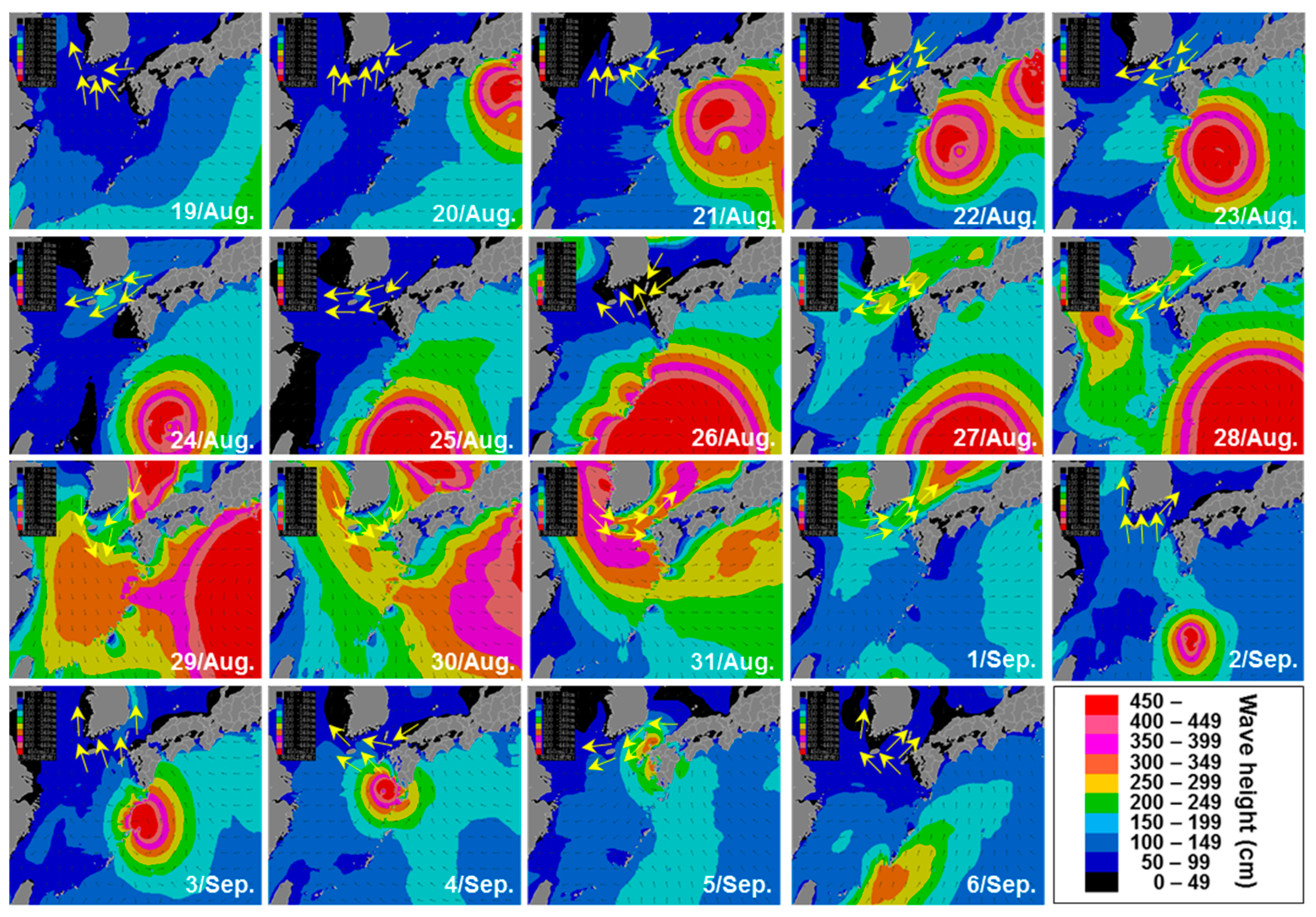
2.5. Satellite Images and Wind Direction Along the Goheung Coast
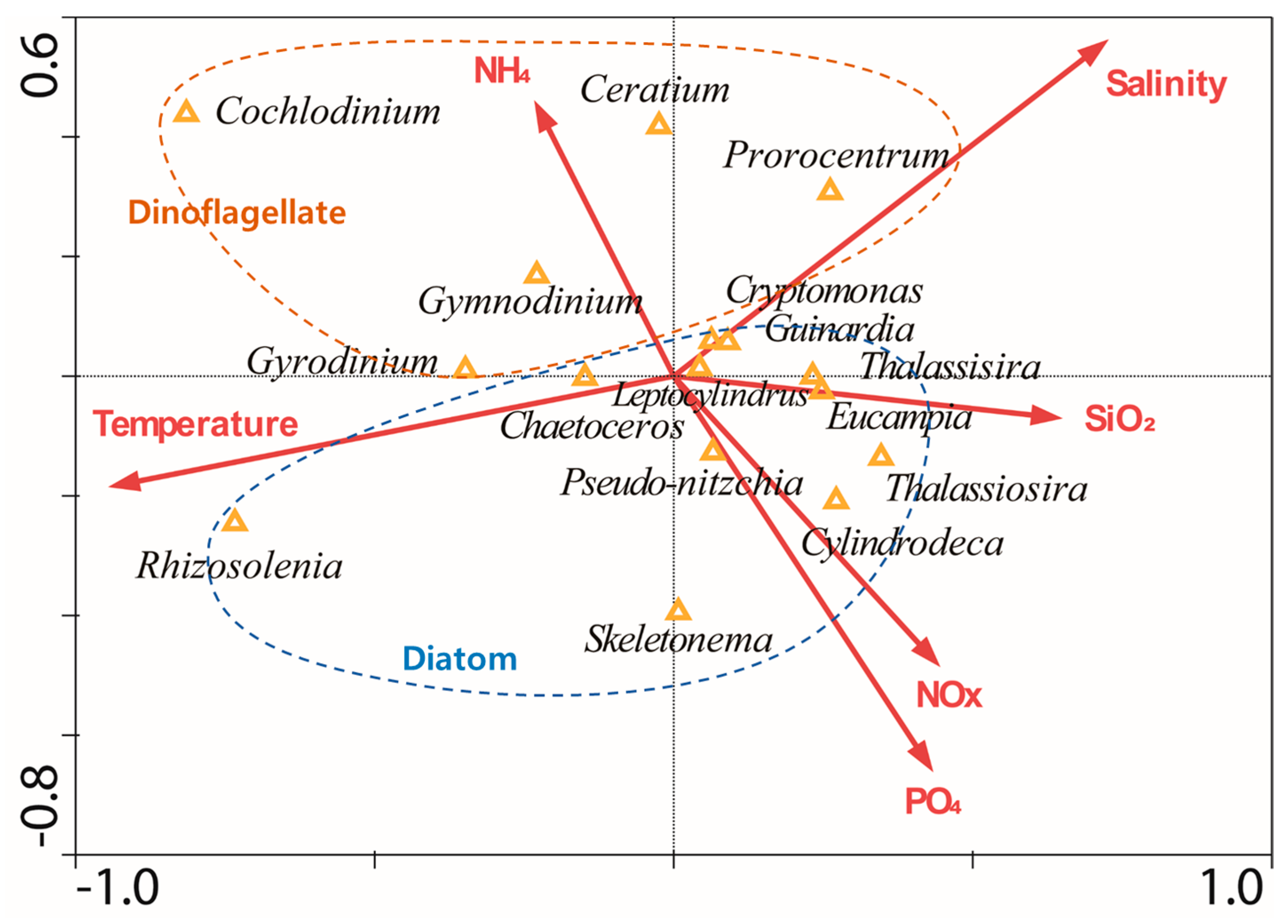
2.6. Statistical Analysis Using the Canonical Correspondence Analysis
3. Discussion
3.1. Initial Population Introduction in Southern Korean Coastal Waters
3.2. Effect of Environmental Factors on the Inhibition of Cochlodinium Bloom Formation
3.3. Extinction of the C. polykrikoides Bloom by a Typhoon Carrying Strong Winds and Heavy Rainfall
4. Conclusions
5. Materials and Methods
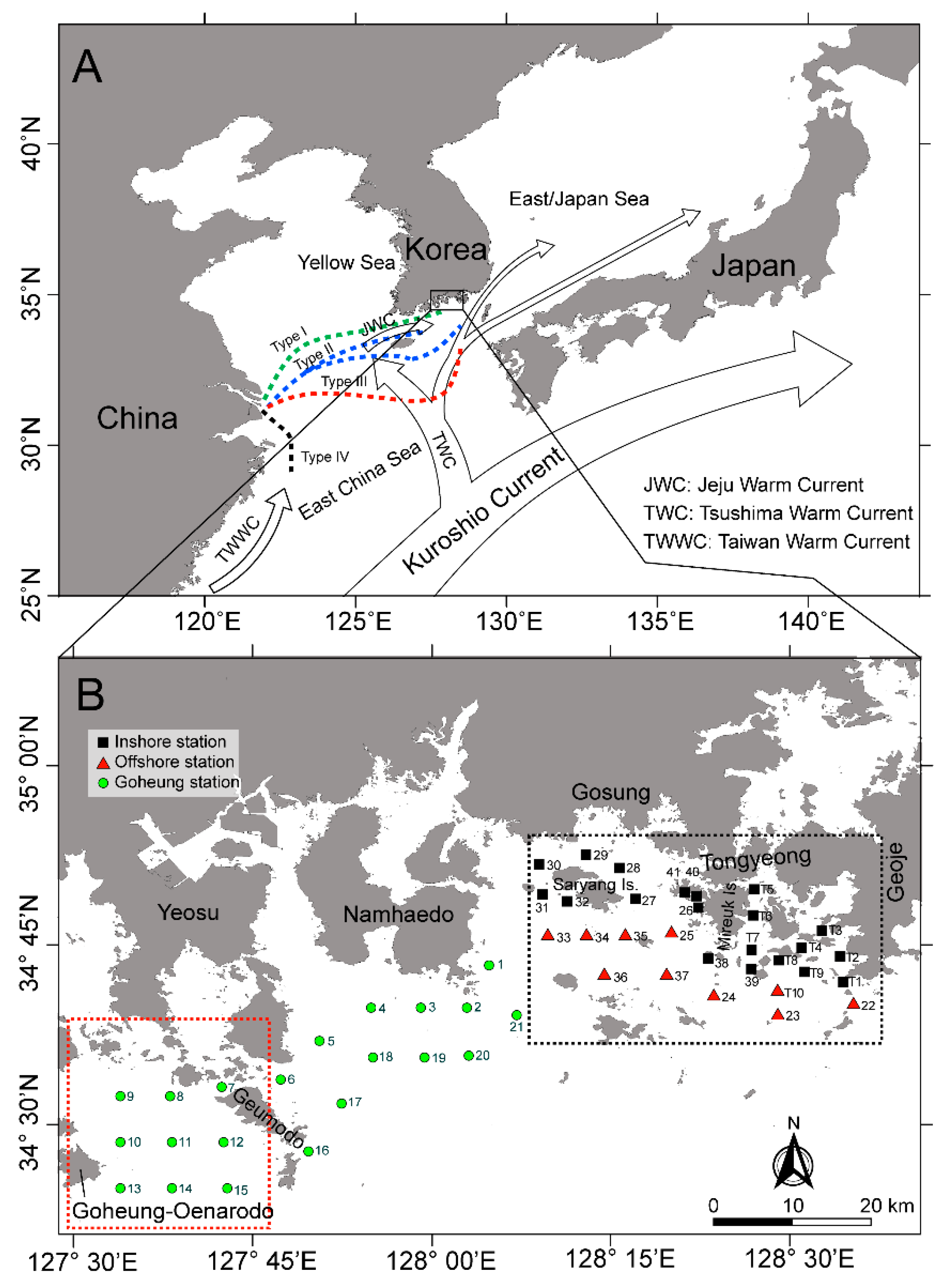
5.1. Study Area
5.2. Field Survey
5.3. In Situ Data (Meteorological Data and River Discharge Data)
5.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hallegraeff, G.M. Harmful algal blooms in the Australian region. Mar. Pollut. Bull. 1992, 25, 185–190. [Google Scholar] [CrossRef]
- Anderson, D.M. Turning back the harmful red tide. Nature 1997, 388, 513–514. [Google Scholar] [CrossRef]
- Smayda, T.J. Harmful algal blooms: Their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol. Oceanogr. 1997, 42, 1137–1153. [Google Scholar] [CrossRef]
- Lee, C.K.; Park, T.G.; Park, Y.T.; Lim, W.A. Monitoring and trends in harmful algal blooms and red tides in Korean coastal waters, with emphasis on Cochlodinium polykrikoides. Harmful Algae 2013, 30S, S3–S14. [Google Scholar] [CrossRef]
- Park, J.Y.; Jeong, H.J.; Yoo, Y.D.; Yoon, E.Y. Mixotrophic dinoflagellate red tides in Korean waters: Distribution and ecophysiology. Harmful Algae 2013, 30S, S28–S40. [Google Scholar] [CrossRef]
- Tang, Y.; Gobler, C.J. Characterization of the toxicity of Cochlodinium polykrikoides isolates from Northeast US estuaries to finfish and shellfish. Harmful Algae 2009, 8, 454–462. [Google Scholar] [CrossRef]
- Lim, A.S.; Jeong, H.J.; Jang, T.Y.; Jang, S.H.; Franks, P.J.S. Inhibition of growth rate and swimming speed of the harmful dinoflagellate Cochlodinium polykrikoides by diatom: Implications for red tide formation. Harmful Algae 2014, 37, 53–61. [Google Scholar] [CrossRef]
- Park, T.G.; Lim, W.A.; Park, Y.T.; Lee, C.K.; Jeong, H.J. Economic impact, management and mitigation of red tides in Korea. Harmful Algae 2013, 30S, S131–S143. [Google Scholar] [CrossRef]
- Park, J.G.; Jeong, M.K.; Lee, J.A.; Cho, K.J.; Kwon, O.S. Diurnal vertical migration of a harmful dinoflagellate, Cochlodinium polykrikoides (Dinophyceae) during a red tide in coastal waters of Namhae Island, Korea. Phycologia 2001, 40, 292–297. [Google Scholar] [CrossRef]
- Lee, M.O.; Kim, J.K. Characteristics of algal blooms in the southern coastal waters of Korea. Mar. Environ. Res. 2008, 65, 128–147. [Google Scholar] [CrossRef]
- Park, G.H.; Lee, K.; Koo, C.M.; Lee, H.W.; Lee, C.K.; Koo, J.S.; Lee, T.; Ahn, S.H.; Kim, H.G.; Park, B.K. A sulfur hexafluoride-based Lagrangian study on initiation and accumulation of the red tide Cochlodinium polykrikoides in southern coastal waters of Korea. Limnol. Oceanogr. 2005, 50, 578–586. [Google Scholar] [CrossRef]
- Lee, M.O.; Kim, J.K.; Kim, B.K. Factors controlling the origin of Cochlodinium polykrikoides blooms along the Goheung coast, South Korea. Mar. Pollut. Bull. 2016, 113, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Wang, P.B.; Kim, J.H.; Kim, J.H.; Gobler, C.J.; Han, M.S. Resolving the intra-specific succession within Cochlodinium polykrikoides populations in southern Korean coastal waters via use of quantitative PCR assays. Harmful Algae 2014, 37, 133–141. [Google Scholar] [CrossRef]
- Park, B.S.; Kim, J.H.; Kim, J.H.; Gobler, C.J.; Han, M.S. Dynamics of bacterial community structure during blooms of Cochlodinium polykrikoides (Gymnodiniales, Dinophyceae) in Korean coastal waters. Harmful Algae 2015, 48, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Kim, J.H.; Kim, J.H.; Baek, S.H.; Han, M.S. Intraspecific bloom succession in the harmful dinoflagellate Cochlodinium polykrikoides (Dinophyceae) extended the blooming period in Korean coastal waters in 2009. Harmful Algae 2018, 71, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Kim, S.; Kim, J.H.; Kim, J.H.; Han, M.S. Dynamics of Amoebophrya parasites during recurrent blooms of the ichthyotoxic dinoflagellate Cochlodinium polykrikoides in Korean coastal waters. Harmful Algae 2019, 84, 119–126. [Google Scholar] [CrossRef]
- Thompson, P.A.; Bonhan, P.L.; Swadline, P.I.; Swadling, K.M. Phytoplankton bloom in the Huon Estuary, Tasmania: Top-down or bottom-up control? J. Plankton Res. 2008, 30, 735–753. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, S.H. Factors affecting outbreaks of Cochlodinium polykrikoides blooms in coastal areas of Korea. Mar. Pollut. Bull. 2006, 52, 626–634. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Gobler, C.J. The toxic dinoflagellate Cochlodinium polykrikoides (Dinophyceae) produces resting cysts. Harmful Algae 2012, 20, 71–80. [Google Scholar] [CrossRef]
- Li, Z.; Han, M.S.; Matsuoka, K.; Kim, S.Y.; Shin, H.H. Identification of the resting cyst of Cochlodinium polykrikoides Margalef (Dinophyceae, Gymnodiniales) in Korean coastal sediments. J. Phycol. 2015, 51, 204–210. [Google Scholar] [CrossRef]
- Park, T.G.; Kim, J.J.; Kim, W.J.; Won, K.M. Development of real-time RT-PCR for detecting viable Cochlodinium polykrikoides (Dinophyceae) cysts in sediment. Harmful Algae 2016, 60, 36–44. [Google Scholar] [CrossRef] [PubMed]
- NIFS. Harmful Algal Blooms in Korean Coastal Waters in 2016; National Institute of Fisheries Science: Busan, Korea, 2016; Available online: http://www.nifs.go.kr/red/main.red (accessed on 7 April 2020).
- Ahn, Y.H.; Shanmugam, P.; Ryu, J.H.; Jeong, J.C. Satellite detection of harmful algal bloom occurrences in Korean waters. Harmful Algae 2005, 5, 213–231. [Google Scholar] [CrossRef]
- Kim, H.C.; Yamaguchi, H.; Yoo, S.J.; Zhus, J.R.; Okamura, K.; Kiyomoto, Y.; Tanaka, K.; Kim, S.W.; Park, T.W.; Oh, I.S.; et al. Distribution of Changjiang diluted water detected by satellite chlorophyll-a and its interannual variation during 1998-2007. J. Oceanogr. 2009, 65, 129–135. [Google Scholar] [CrossRef]
- Choi, J.K.; Min, J.E.; Noh, J.H.; Han, T.H.; Yoon, S.; Park, Y.J.; Moon, J.E.; Ahn, J.H.; Ahn, S.M.; Park, J.H. Harmful algal bloom (HAB) in the East Sea identified by the Geostationary Ocean Color Imager (GOCI). Harmful Algae 2014, 39, 295–302. [Google Scholar] [CrossRef]
- Matsuoka, K.; Iwataki, M.; Kawami, H. Morphology and taxonomy of chain-forming species of the genus Cochlodinium (Dinophyceae). Harmful Algae 2008, 7, 261–270. [Google Scholar] [CrossRef]
- Matsuoka, K.; Mizuno, A.; Iwataki, M.; Takano, Y.; Toshifumi, Y.; Yoon, Y.H.; Lee, J.B. Seed population of a harmful unarmored dinoflagellate Cochlodinium polykrikoides Margalef in the East China Sea. Harmful Algae 2010, 9, 548–556. [Google Scholar] [CrossRef]
- Nagai, S.; Nishitani, G.; Sagamoto, S.; Sugaya, T.; Lee, C.K.; Kim, C.H.; Itakura, S.; Yamaguchi, M. Genetic structuring and transfer of marine dinoflagellate Cochlodinium polykrikoides in Japanese and Korean coastal waters revealed by microsatellites. Mol. Ecol. 2009, 18, 2337–2352. [Google Scholar] [CrossRef]
- Kudela, R.M.; Gobler, C.J. Harmful dinoflagellate blooms caused by Cochlodinium sp.: Global expansion and ecological strategies facilitating bloom formation. Harmful Algae 2012, 14, 71–86. [Google Scholar] [CrossRef]
- Kim, D.I.; Matsuyama, Y.; Nagasoe, S.; Yamaguchi, M.; Yoon, Y.H.; Oshima, Y.; Imada, N.; Honjo, T. Effects of temperature, salinity, and irradiance on the growth of the harmful red tide dinoflagellate Cochlodinium polykrikoides Margalef (Dinophyceae). J. Plankton Res. 2004, 26, 61–66. [Google Scholar] [CrossRef]
- Yamatogi, T.; Sakaguchi, M.; Iwataki, M.; Matsuoka, K. Seasonal occurrence and growth characteristics of a harmful dinoflagellate Cochlodinium polykrikoides. Jpn. J. Phycol. 2005, 53, 229–235. [Google Scholar]
- Lee, D.K.; Kang, Y.H. The physical Environments and Cochlodinium polykrikoides bloom in the sea near Naro-Do. Ocean Polar Res. 2003, 25, 303–314. [Google Scholar] [CrossRef]
- Yang, S.L.; Gao, A.; Hotz, H.M.; Zhu, J.; Dai, S.B.; Li, M. Trends in annual discharge from the Yangtze River to the sea (1865–2004). Hydrol. Sci. J. 2005, 50, 825–836. [Google Scholar] [CrossRef]
- Bai, Y.; He, X.; Pan, D.; Chen, C.T.A.; Kang, Y.; Chen, X.; Cai, W.J. Summertime Changjiang River plume variation during 1998–2010. J. Geophys. Res. Oceans 2014, 119, 6238–6257. [Google Scholar] [CrossRef]
- Lie, H.J.; Cho, C.H. Seasonal circulation patterns of the Yellow and East China Seas derived from satellite-tracked drifter trajectories and hydrographic observations. Progr. Oceanogr. 2016, 146, 121–141. [Google Scholar] [CrossRef]
- Wei, H.; Luo, X.; Zhao, Y.; Zhao, L. Intraseasonal variation in the salinity of the Yellow and East China Seas in the summers of 2011, 2012, and 2013. Hydrobiologia 2015, 754, 13–28. [Google Scholar] [CrossRef]
- Elbrächter, M. On population dynamics in multi-species cultures of diatoms and dinoflagellates. Helgol. wiss. Meeresunters 1977, 30, 192–200. [Google Scholar] [CrossRef][Green Version]
- Imai, I.; Yamaguchi, M. Life cycle, physiology, ecology and red tide occurrences of the fish-killing raphidophyte Chattonella. Harmful Algae 2012, 14, 46–70. [Google Scholar] [CrossRef]
- Baek, S.H.; Kim, D.S.; Son, M.H.; Yun, S.M.; Kim, Y.O. Seasonal distribution of phytoplankton assemblages and nutrient enriched bioassays as indicators of nutrient limitation of phytoplankton growth in Gwangyang Bay, Korea. Estuar. Coast. Shelf Sci. 2015, 163, 265–278. [Google Scholar] [CrossRef]
- Lee, M.; Park, B.S.; Baek, S.H. Tidal influences on biotic and abiotic factors in the Seomjin River Estuary and Gwangyang Bay, Korea. Estuar. Coast. 2018, 41, 1977–1993. [Google Scholar] [CrossRef]
- Smayda, T.J. Turbulence, watermass stratification and harmful algal bloom: An alternative view and frontal zones as “pelagic and banks”. Harmful Algae 2002, 1, 95–112. [Google Scholar] [CrossRef]
- Anderson, D.M.; Pitcher, G.C.; Estrada, M. The comparative systems approach to HAB research. Oceanography 2005, 18, 148–157. [Google Scholar] [CrossRef][Green Version]
- Baek, S.H.; Shimode, S.; Han, M.S.; Kikuchi, T. Growth of dinofalgellate, Ceratium furca and Ceratium fusus in Sagami Bay, Japan: The role of nutrients. Harmful Algae 2008, 7, 729–739. [Google Scholar] [CrossRef]
- Baek, S.H.; Shimode, S.; Shin, K.S.; Han, M.S.; Kikuchi, T. Growth of dinoflagellates, Ceratium furca and Ceratium fusus in Sagami Bay, Japan: The role of vertical migration and cell division. Harmful Algae 2009, 8, 843–856. [Google Scholar] [CrossRef]
- Lim, A.S.; Jeong, H.J.; Jang, T.Y.; Kang, N.S.; Jang, S.H.; Lee, M.J. Differential effects of typhoons on ichthyotoxic Cochlodinium polykrikoides red tides in the South Sea of Korea during 2012-2014. Harmful Algae 2015, 45, 26–32. [Google Scholar] [CrossRef]
- Trainer, V.L.; Hickey, B.M.; Horner, R.A. Biological and physical dynamics of domoic acid production off the Washington Coast. Limnol. Oceanogr. 2002, 47, 1438–1446. [Google Scholar] [CrossRef]
- Chen, Y.L.; Chen, H.; Jan, S.; Tuo, S. Phytoplankton productivity enhancement and assemblage change in the upstream Kuroshio after typhoons. Mar. Ecol. Prog. Ser. 2009, 385, 111–126. [Google Scholar] [CrossRef]
- Roegner, G.C.; Hickey, B.M.; Newton, J.A.; Shanks, A.L.; Armstrong, D.A. Wind-introduced plume and bloom intrusions into Willapa Bay, Washington. Limnol. Oceanogr. 2002, 47, 1033–1042. [Google Scholar] [CrossRef]
- Ryan, J.P.; Polito, P.S.; Strutton, P.G.; Chavez, F.P. Unusual large-scale phytoplankton blooms in the Equatorial Pacific. Progr. Oceanogr. 2002, 55, 263–285. [Google Scholar] [CrossRef]
- Chung, C.; Gong, G.; Hung, C. Effect of typhoon Morakot on micro phytoplankton population dynamics in the subtropical Northwest Pacific. Mar. Ecol. Prog. Ser. 2012, 448, 39–49. [Google Scholar] [CrossRef]
- Lee, D. Cochlodinium polykrikoides blooms and eco-physical conditions in the South Sea of Korea. Harmful Algae 2008, 7, 315–323. [Google Scholar] [CrossRef]
- Lee, Y.S.; Park, Y.T.; Kim, Y.S.; Kim, K.Y.; Park, J.S.; Go, W.J.; Jo, Y.J.; Park, S.Y. Countermeasure and outbreak mechanism of Cochlodinium polykrikoides red tide 1. Environmental characteristics on outbreak and disappearance of Cochlodinium polykrikoides bloom. Korean Soc. Oceanogr. 2001, 6, 259–264. [Google Scholar]
- Lim, W.A.; Jung, C.S.; Lee, C.K.; Cho, Y.C.; Lee, S.G.; Kim, H.G.; Chung, I.K. The outbreak, maintenance and decline of the red tide dominated by Cochlodinium polykrikoides in the coastal waters off southern Korea from August to October, 2000. Korean Soc. Oceanogr. 2002, 7, 68–77. [Google Scholar]
- Lie, H.J.; Cho, C.H. On the origin of the Tsushima Warm Current. J. Geophy. Res. 1994, 99, 25081–25091. [Google Scholar] [CrossRef]
- Senjyu, T.; Enomoto, H.; Matsuno, T.; Matsu, S. Interannual salinity variations in the Tsushima Strait and its relation to the Changjiang discharge. J. Oceanogr. 2006, 62, 681–692. [Google Scholar] [CrossRef]
- Sournia, A. Phytoplankton Manual; UNESCO: Paris, France, 1978; p. 337. [Google Scholar]

| Year | F | ||||||
|---|---|---|---|---|---|---|---|
| 2012 | 2013 | 2014 | 2015 | 2016 | |||
| Temperature (°C) | Range Mean ± SD | 24.5–27.1 (25.9 ± 0.81) b | 17.7–27.4 (21.8 ± 2.56) d | 22.8–25.1 (23.9 ± 0.69) c | 21.5–27.7 (24.5 ± 1.58) c | 26.1–29.5 (28.0 ± 0.81) a | 102 *** |
| Salinity | Range Mean ± SD | 31.8–32.9 (32.4 ± 0.31) a | 32.0–33.8 (33.1 ± 0.47) a | 24.1–32.7 (30.1 ± 1.97) c | 31.9–33.0 (32.5 ± 0.38) a | 29.3–32.7 (30.9 ± 0.86) b | 21.6 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.H.; Kim, Y.; Lee, M.; Ahn, C.-Y.; Cho, K.H.; Park, B.S. Potential Cause of Decrease in Bloom Events of the Harmful Dinoflagellate Cochlodinium polykrikoides in Southern Korean Coastal Waters in 2016. Toxins 2020, 12, 390. https://doi.org/10.3390/toxins12060390
Baek SH, Kim Y, Lee M, Ahn C-Y, Cho KH, Park BS. Potential Cause of Decrease in Bloom Events of the Harmful Dinoflagellate Cochlodinium polykrikoides in Southern Korean Coastal Waters in 2016. Toxins. 2020; 12(6):390. https://doi.org/10.3390/toxins12060390
Chicago/Turabian StyleBaek, Seung Ho, Yunji Kim, Minji Lee, Chi-Yong Ahn, Kyung Hwa Cho, and Bum Soo Park. 2020. "Potential Cause of Decrease in Bloom Events of the Harmful Dinoflagellate Cochlodinium polykrikoides in Southern Korean Coastal Waters in 2016" Toxins 12, no. 6: 390. https://doi.org/10.3390/toxins12060390
APA StyleBaek, S. H., Kim, Y., Lee, M., Ahn, C.-Y., Cho, K. H., & Park, B. S. (2020). Potential Cause of Decrease in Bloom Events of the Harmful Dinoflagellate Cochlodinium polykrikoides in Southern Korean Coastal Waters in 2016. Toxins, 12(6), 390. https://doi.org/10.3390/toxins12060390






