Distribution of Domoic Acid in the Digestive Gland of the King Scallop Pecten maximus
Abstract
1. Introduction
2. Results
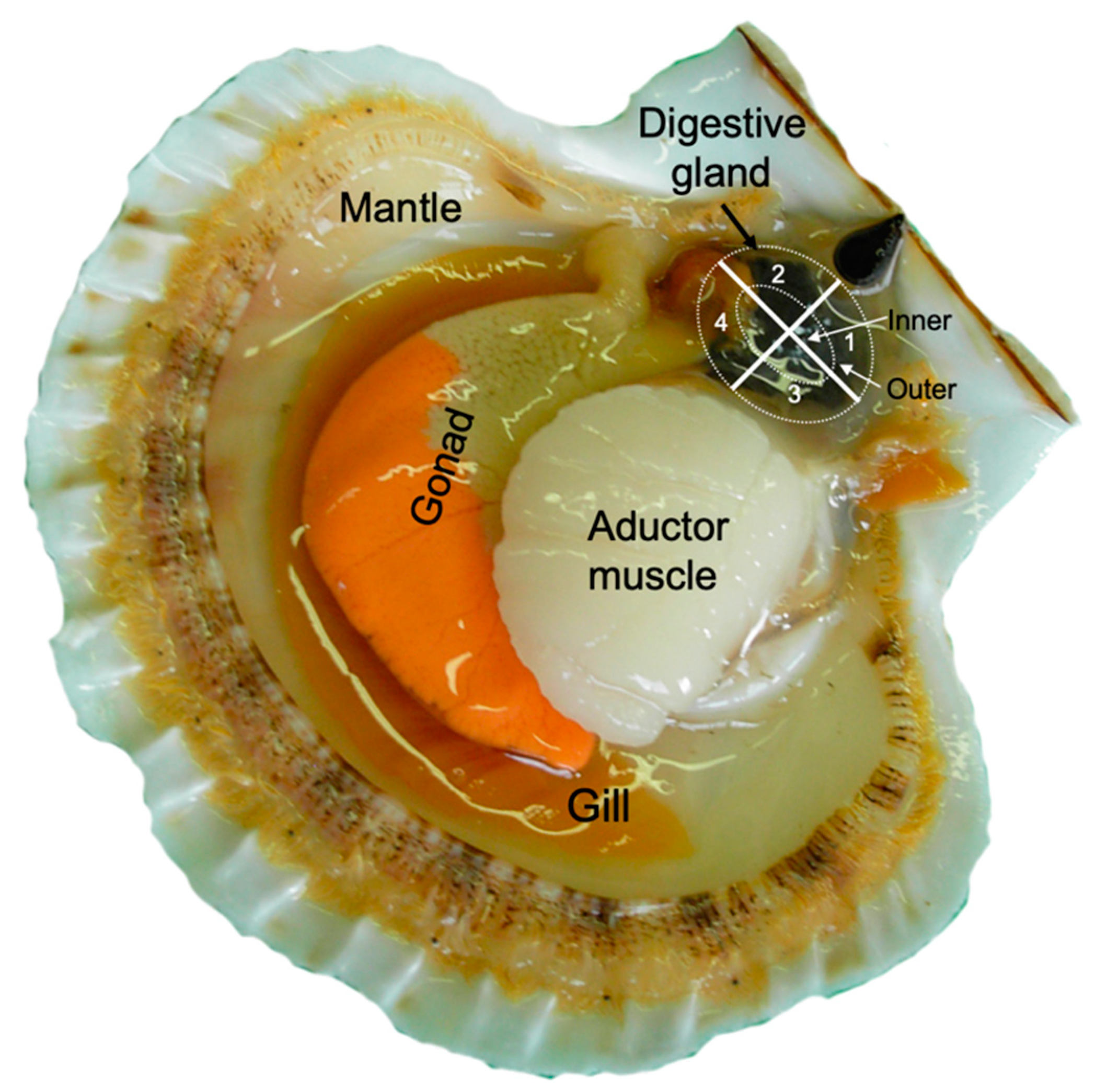
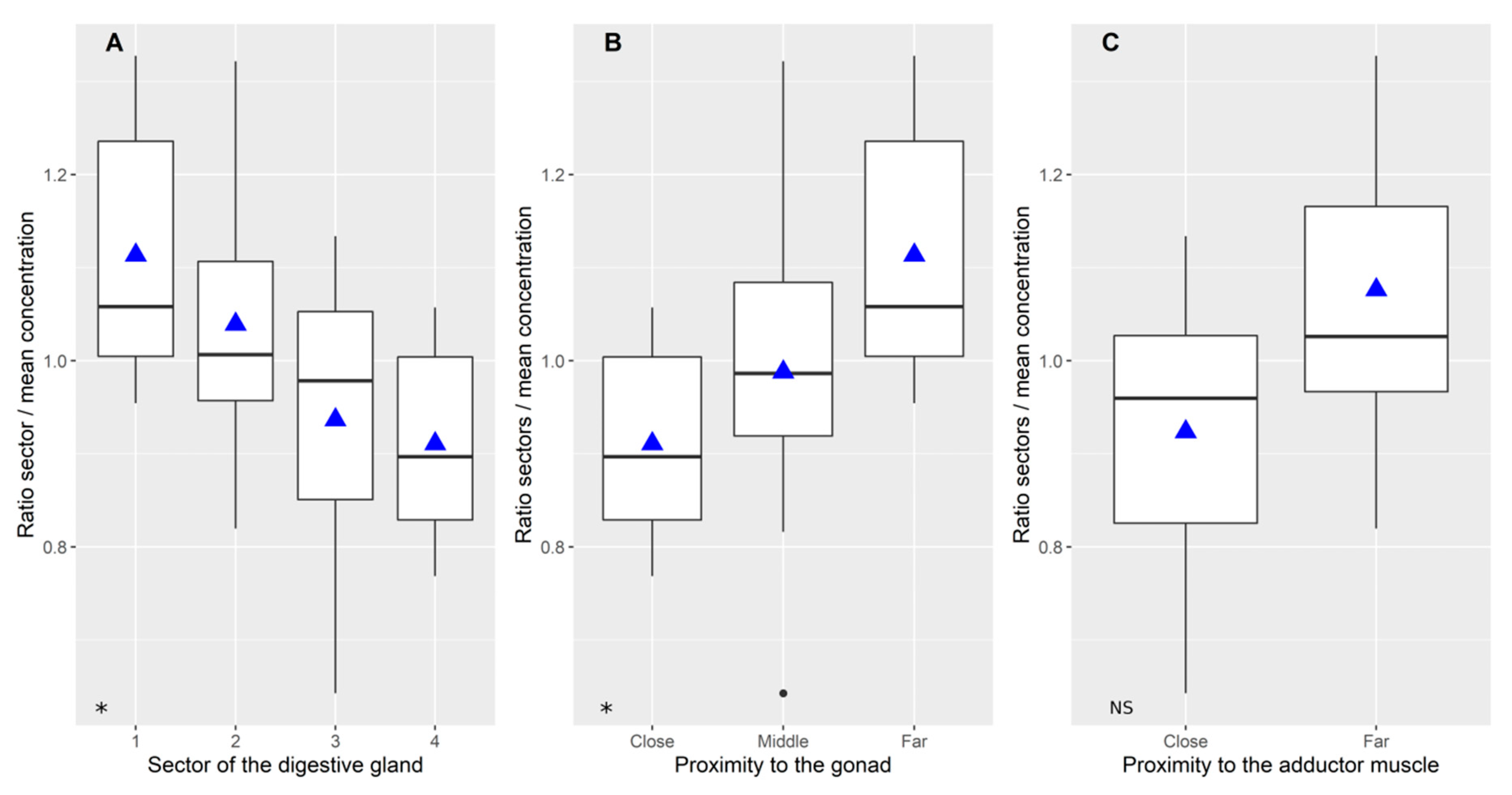
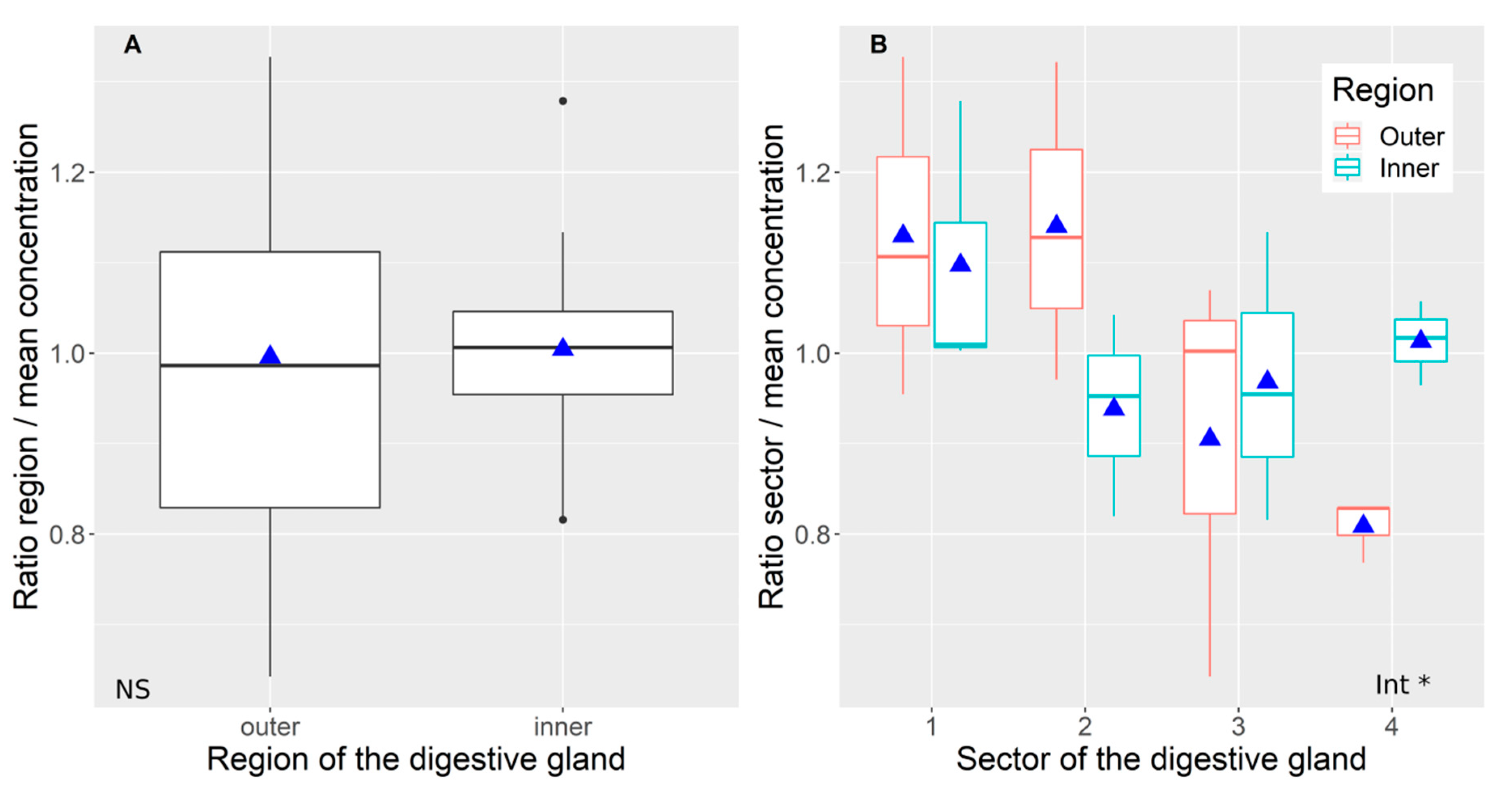
2.1. Variability of Toxin Distribution in Different Parts of the Digestive Gland
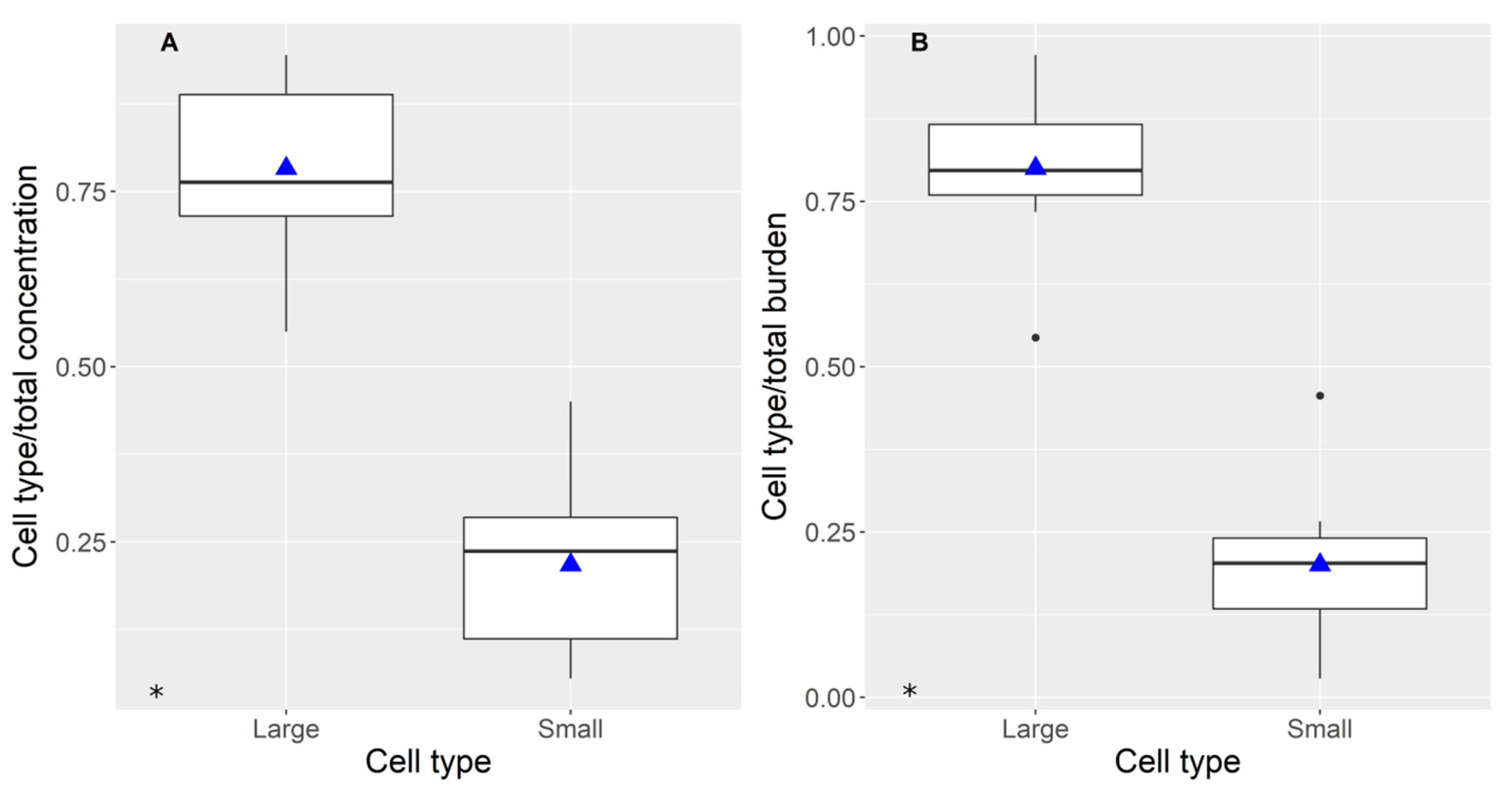
2.2. Distribution of Domoic Acid between Cellular Types
3. Discussion
4. Materials and Methods
4.1. Biological Material
4.2. Sector Distribution
4.3. Cell Type Separations
4.4. Chromatographic Analysis
4.5. Computations and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pulido, O.M. Domoic Acid Toxicologic Pathology: A Review. Mar. Drugs 2008, 6, 180–219. [Google Scholar] [CrossRef] [PubMed]
- Perl, T.M.; Bedard, L.; Kosatsky, T.; Hockin, J.C.; Todd, E.C.; McNutt, L.A.; Remis, R.S. Amnesic shellfish poisoning: A new clinical syndrome due to domoic acid. Can. Dis. Wkly. Rep. 1990, 16, 7–8. [Google Scholar]
- Perl, T.M.; Bédard, L.; Kosatsky, T.; Hockin, J.C.; Todd, E.C.; Remis, R.S. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N. Engl. J. Med. 1990, 322, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, T.; Daigo, K. Constituents of Chondria armata. Chem. Pharm. Bull. 1958, 6, 578–580. [Google Scholar] [CrossRef]
- Wright, J.L.C.; Boyd, R.K.; Freitas, A.S.W.; Falk, M.; Foxall, R.A.; Jamieson, W.D.; Laycock, M.V.; McCulloch, A.W.; McInnes, A.G.; Odense, P. Identification of domoic acid, a neuroexcitatory amino acid, in toxic mussels from eastern Prince Edward Island. Can. J. Chem. 1989, 67, 481–490. [Google Scholar] [CrossRef]
- Bates, S.; Bird, C.; Defreitas, A.; Foxall, R.; Gilgan, M.; Hanic, L.; Johnson, G.; McCulloch, A.; Odense, P.; Pocklington, R. , et al. Pennate diatom Nitzschia pungens as the primary source of domoic acid, a toxin in shellfish from eastern Prince Edward Island, Canada. Can. J. Fish. Aquat. Sci. 1989, 46, 1203–1215. [Google Scholar] [CrossRef]
- Míguez, A.; Ferná>ndez, M.L.; Fraga, S. First detection of domoic acid in Galicia (NW Spain). In Harmful and Toxic Algal Blooms; Yasumoto, T., Oshima, Y., Fukuyo, Y., Eds.; IOC of UNESCO: Paris, France, 1996; pp. 143–145. [Google Scholar]
- Vale, P.; Sampayo, M.A.M. Domoic acid in portuguese shellfish and fish. Toxicon 2001, 39, 893–904. [Google Scholar] [CrossRef]
- Amzil, Z.; Fresnel, J.; Le Gal, D.; Billard, C. Domoic Acid Accumulation in French Shellfish in Relation to Toxic Species of Pseudo-nitzschia multiseries and P. pseudodelicatissima. Toxicon 2001, 39, 1245–1251. [Google Scholar] [CrossRef]
- Ujevic, I.; Nincevic-Gladan, Z.; Roje, R.; Skejic, S.; Arapov, J.; Marasovic, I. Domoic Acid—A New Toxin in the Croatian Adriatic Shellfish Toxin Profile. Molecules 2010, 15, 6835–6849. [Google Scholar] [CrossRef]
- Cusack, C.K.; Bates, S.S.; Quilliam, M.A.; Patching, J.W.; Raine, R. Confirmation of domoic acid production by Pseudo-nitzschia australis (Bacillariophyceae) isolated from Irish waters. J. Phycol. 2002, 38, 1106–1112. [Google Scholar] [CrossRef]
- Gilgan, M.W.; Burns, B.G.; Landry, G.J. Distribution and Magnitude of Domoic Acid Contamination of Shellfish in Atlantic Canada during 1988; Elsevier Science Publishing, Co.: Amsterdam, The Netherlands, 1990; pp. 469–474. [Google Scholar]
- Martin, J.L.; Haya, K.; Burridge, L.E.; Wildish, D.J. Nitzschia pseudodelicatissima—A source of domoic acid in the Bay of Fundy, eastern Canada. Mar. Ecol.-Prog. Ser. 1990, 6, 177–182. [Google Scholar] [CrossRef]
- Dickey, R.W.; Fryxell, G.A.; Granade, H.R.; Roelke, D. Detection of the marine toxins okadaic acid and domoic acid in shellfish and phytoplankton in the Gulf of Mexico. Toxicon 1992, 30, 355–359. [Google Scholar] [CrossRef]
- Drum, A.S.; Siebens, T.L.; Crecelius, E.A.; Elston, R.A. Domoic acid in the Pacific razor clam Siliqua patula (Dixon, 1789). J. Shellfish Res. 1993, 12, 443–450. [Google Scholar]
- Langlois, G.W.; Kizer, K.W.; Hansgen, K.H.; Howell, R.; Loscutoff, S.M. A note on domoic acid in California coastal molluscs and crabs. J. Shellfish Res. 1993, 12, 467–468. [Google Scholar]
- Marchetti, A.; Trainer, V.L.; Harrison, P.J. Environmental conditions and phytoplankton dynamics associated with pseudo-nitzschia abundance and domoic acid in the juan de fuca eddy. Mar. Ecol.-Prog. Ser. 2004, 281, 1–12. [Google Scholar] [CrossRef]
- Álvarez, G.; Uribe, E.; Quijano-Scheggia, S.; Lopez-Rivera, A.; Marino, C.; Blanco, J. Domoic acid production by Pseudo-nitzschia australis and Pseudo-nitzschia calliantha isolated from North Chile. Harmful Algae 2009, 8, 938–945. [Google Scholar] [CrossRef]
- MacKenzie, A.; White, D.A.; Sim, P.G.; Holland, A.J. Domoic acid and the New Zealand Greenshell mussel (Perna canaliculus). In Toxic Phytoplankton Blooms in the Sea; Smayda, T.J., Shimizu, Y., Eds.; Elsevier Sci. Publ. BV: Amsterdam, The Netherlands, 1993; pp. 607–612. [Google Scholar]
- Kotaki, Y.; Koike, K.; Sato, S.; Ogata, T.; Fukuyo, Y.; Kodama, M. Confirmation of domoic acid production of Pseudo-nitzschia multiseries isolated from Ofunato Bay, Japan. Toxicon 1999, 37, 677–682. [Google Scholar] [CrossRef]
- Besiktepe, S.; Ryabushko, L.; Ediger, D.; Yilmaz, D.; Zenginer, A.; Ryabushko, V.; Lee, R. Domoic acid production by Pseudo-nitzschia calliantha Lundholm, Moestrup et Hasle (bacillariophyta) isolated from the Black Sea. Harmful Algae 2008, 7, 438–442. [Google Scholar] [CrossRef]
- Sahraoui, I.; Bates, S.S.; Bouchouicha, D.; Mabrouk, H.H.; Hlaili, A.S.; Steleyn G, T.G.U.B. Toxicity of Pseudo-nitzschia populations from Bizerte Lagoon, Tunisia, southwest Mediterranean, and first report of domoic acid production by P. brasiliana. Diatom Res. 2011, 26, 293–303. [Google Scholar] [CrossRef]
- Leblad, B.R.; Lundholm, N.; Goux, D.; Veron, B.; Sagou, R.; Taleb, H.; Nhhala, H.; Er-Raioui, H. Pseudo-nitzschia Peragallo (Bacillariophyceae) diversity and domoic acid accumulation in tuberculate cockles and sweet clams in M’diq Bay, Morocco. Acta Bot. Croat. 2013, 72, 35–47. [Google Scholar] [CrossRef]
- Liu, H.; Kelly, M.; Campbell, D.; Dong, S.; Zhu, J.; Wang, S. Ingestion of domoic acid and its impact on king scallop (Pecten maximus, Linnaeus 1758). J. Ocean Univ. China (English Edition) 2007, 6, 175–181. [Google Scholar] [CrossRef]
- Dursun, F.; Yurdun, T.; Unlu, S. The First Observation of Domoic Acid in Plankton Net Samples from the Sea of Marmara, Turkey. Bull. Environ. Contam. Toxicol. 2016, 96, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.N.; Teng, S.T.; Lim, H.C.; Kotaki, Y.; Bates, S.S.; Leaw, C.P.; Lim, P.T. Diatom Nitzschia navis-varingica (Bacillariophyceae) and its domoic acid production from the mangrove environments of Malaysia. Harmful Algae 2016, 60, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Livramento, F.; Rangel, I.M. Amnesic shellfish poisoning (ASP) toxins in plankton and molluscs from Luanda Bay, Angola. Toxicon 2010, 55, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, G.C.; Cembella, A.D.; Krock, B.; Macey, B.M.; Mansfield, L.; Probyn, T.A. Identification of the marine diatom Pseudo-nitzschia multiseries (Bacillariophyceae) as a source of the toxin domoic acid in Algoa Bay, South Africa. Afr. J. Mar. Sci. 2014, 36, 523–528. [Google Scholar] [CrossRef]
- Louw, D.C.; Doucette, G.J.; Voges, E. Annual patterns, distribution and long-term trends of Pseudo-nitzschia species in the northern Benguela upwelling system. J. Plankton Res. 2017, 39, 35–47. [Google Scholar] [CrossRef]
- Lefebvre, K.A.; Powell, C.L. Detection of Domoic Acid in Northern Anchovies and California Sea Lions Associated with an Unusual Mortality Event. Nat. Toxins 1999, 7, 85–92. [Google Scholar] [CrossRef]
- Scholin, C.A.; Gulland, F.; Doucette, G.J.; Benson, S.; Busman, M.; Chavez, F.P.; Cordaro, J.; DeLong, R.; DeVogelaere, A.; Harvey, J.; et al. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature 2000, 403, 80–84. [Google Scholar] [CrossRef]
- McHuron, E.A.; Greig, D.J.; Colegrove, K.M.; Fleetwood, M.; Spraker, T.R.; Gulland, F.M.D.; Harvey, J.T.; Lefebvre, K.A.; Frame, E.R. Domoic acid exposure and associated clinical signs and histopathology in Pacific harbor seals (Phoca vitulina richardii). Harmful Algae 2013, 23, 28–33. [Google Scholar] [CrossRef]
- Fire, S.E.; Adkesson, M.J.; Wang, Z.; Jankowski, G.; Cardenas-Alayza, S.; Broadwater, M. Peruvian fur seals (Arctocephalus australis ssp.) and South American sea lions (Otaria byronia) in Peru are exposed to the harmful algal toxins domoic acid and okadaic acid. Mar. Mammal Sci. 2017, 33, 630–644. [Google Scholar] [CrossRef]
- Kreuder, C.; Miller, M.A.; Lowenstine, L.J.; Conrad, P.A.; Carpenter, T.E.; Jessup, D.A.; Mazet, J.A. Evaluation of cardiac lesions and risk factors associated with myocarditis and dilated cardiomyopathy in southern sea otters (Enhydra lutris nereis). Am. J. Vet. Res. 2005, 66, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Fire, S.E.; Wang, Z.H.; Berman, M.; Langlois, G.W.; Morton, S.L.; Sekula-Wood, E.; Benitez-Nelson, C.R. Trophic Transfer of the Harmful Algal Toxin Domoic Acid as a Cause of Death in a Minke Whale (Balaenoptera acutorostrata) Stranding in Southern California. Aquat. Mamm. 2010, 36, 342–350. [Google Scholar] [CrossRef]
- De la Riva, G.T.; Johnson, C.K.; Gulland, F.M.D.; Langlois, G.W.; Heyning, J.E.; Rowles, T.K.; Mazet, J.A.K. Association of an Unusual Marine Mammal Mortality Event with Pseudo-nitzschia Spp. Blooms Along the Southern California Coastline. J. Wildl. Dis. 2009, 45, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Fritz, L.; Quilliam, M.A.; Wright, J.L.C.; Beale, A.M.; Work, T.M. An outbreak of domoic acid poisoning attributed to the pennate diatom Pseudonitzschia australis. J. Phycol. 1992, 28, 439–442. [Google Scholar] [CrossRef]
- Work, T.M. Epidemiology of domoic acid poisoning in Brown pelicans (Pelecanus occidentalis) and Brandt’s Cormoran (Phalacrocorax penicillatus) in California. J. Zoo Wildl. Med. 1993, 24, 54–62. [Google Scholar]
- Sierra Beltrán, A.; Palafox-Uribe, M.; Grajales-Montiel, J.; Cruz-Villacorta, A.; Ochoa, J.L. Sea bird mortality at Cabo San Lucas, Mexico: Evidence that toxic diatom blooms are spreading. Toxicon 1997, 35, 447–453. [Google Scholar] [CrossRef]
- Trainer, V.L.; Bates, S.S.; Lundholm, N.; Thessen, A.E.; Cochlan, W.P.; Adams, N.G.; Trick, C.G. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 2012, 14, 271–300. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on marine biotoxins in shellfish—Domoic acid. EFSA J. 2009, 1181, 1–61. [Google Scholar]
- Novaczek, I.; Madhyastha, M.S.; Ablett, R.F.; Donald, A.; Johnson, G.; Nijjar, M.S.; Sims, D.E. Depuration of domoic acid from live blue mussels (Mytilus edulis). Can. J. Fish. Aquat. Sci. 1992, 49, 312–318. [Google Scholar] [CrossRef]
- Wohlgeschaffen, G.D.; Mann, K.H.; Subba Rao, D.V.; Pocklington, R. Dynamics of the phycotoxin domoic acid: Accumulation and excretion in two commercially important bivalves. J. Appl. Phycol. 1992, 4, 297–310. [Google Scholar] [CrossRef]
- Whyte, J.N.C.; Ginther, N.G.; Townsend, T.D. Accumulation and depuration of domoic acid by the mussel, Mytilus californianus. In Harmful Marine Algal Blooms; Lassus, P., Arzul, G., Erard, E., Gentien, P., Marcaillou, C., Eds.; Technique et documentation-Lavoisier, Intercept Ltd.: París, France, 1995; pp. 531–537. [Google Scholar]
- Blanco, J.; Bermúdez de la Puente, M.; Arévalo, F.; Salgado, C.; Moroño, A. Depuration of mussels (Mytilus galloprovincialis) contaminated with domoic acid. Aquat. Living Resour. 2002, 15, 53–60. [Google Scholar] [CrossRef]
- Mafra, L.L.; Bricelj, V.M.; Fennel, K. Domoic acid uptake and elimination kinetics in oysters and mussels in relation to body size and anatomical distribution of toxin. Aquat. Toxicol. 2010, 100, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, G.; Uribe, E.; Regueiro, J.; Martin, H.; Gajardo, T.; Jara, L.; Blanco, J. Depuration and anatomical distribution of domoic acid in the surf clam Mesodesma donacium. Toxicon 2015, 102, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Douglas, D.J.; Kenchington, E.R.; Bird, C.J.; Pocklington, R.; Bradford, B.; Silvert, W. Accumulation of domoic acid by the sea scallop (Placopecten magellanicus) fed cultured cells of toxic Pseudo-nitzschia multiseries. Can. J. Fish. Aquat. Sci. 1997, 54, 907–913. [Google Scholar] [CrossRef]
- Horner, R.A.; Kusske, M.B.; Moynihan, B.P.; Skinner, R.N.; Wekell, J.C. Retention of domoic acid by Pacific razor clams, Siliqua patula (Dixon, 1789): Preliminary study. J. Shellfish Res. 1993, 12, 451–456. [Google Scholar]
- Trainer, V.L.; Bill, B.D. Characterization of a domoic acid binding site from Pacific razor clam. Aquat. Toxicol. 2004, 69, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Marco, J.; Moreno, O.; Santamaría, M. VI Reunión Ibérica sobre Fitoplancton Tóxico y Biotoxinas. In Ensayos de Desintoxicación se ASP en Vieiras (Pecten spp.); Márquez, I., Ed.; Junta de Andalucía: Sevilla, Spain, 1999; pp. 175–181. [Google Scholar]
- Campbell, D.A.; Kelly, M.S.; Busman, M.; Bolch, C.J.; Wiggins, E.; Moeller, P.D.R.; Morton, S.L.; Hess, P.; Shumway, S.E. Amnesic shellfish poisoning in the king scallop, Pecten maximus, from the west coast of Scotland. J. Shellfish Res. 2001, 20, 75–84. [Google Scholar]
- Blanco, J.; Acosta, C.P.; Bermúdez de la Puente, M.; Salgado, C. Depuration and anatomical distribution of the amnesic shellfish poisoning (ASP) toxin domoic acid in the king scallop Pecten maximus. Aquat. Toxicol. 2002, 60, 111–121. [Google Scholar] [CrossRef]
- Blanco, J.; Acosta, C.P.; Mariño, C.; Muñíz, S.; Martín, H.; Moroño, A.; Correa, J.; Arévalo, F.; Salgado, C. Depuration of domoic acid from different body compartments of the King Scallop Pecten maximus grown in raft culture and natural bed. Aquat. Living Resour. 2006, 19, 257–265. [Google Scholar] [CrossRef]
- Mauríz, A.; Blanco, J. Distribution and linkage of domoic acid (amnesic shellfish poisoning toxins) in subcellular fractions of the digestive gland of the scallop Pecten maximus. Toxicon 2010, 55, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Moroño, A.; Pazos, Y.; Doval, M.D.; Maneiro, J. Floraciones algales nocivas y condiciones oceanográficas en las rías gallegas durante los años 2001 y 2002. In Viii Reunión Ibérica Sobre Fitoplancton Tóxico Y Biotoxinas; Norte, M., Fernández, J.J., Eds.; Instituto de Bio-orgánica.: La Laguna, Spain, 2004; pp. 195–210. [Google Scholar]
- Pazos, Y.; Moroño, A.; Miranda, M.; Maneiro, J. Evolución de las condiciones oceanográficas y fitoplancton tóxico/nocivo en los años 1999–2000 en las Rías Gallegas. In Actas de la VII Reunión Ibérica Sobre Fitoplancton Tóxico Y Biotoxinas; Consellería de Agricultura, Pesca y Alimención, Ed.; Generalitat de Valencia: Valencia, Spain, 2003; pp. 195–210. [Google Scholar]
- Salgado, C.; Maneiro, J.; Correa, J.; Pérez, J.L.; Arévalo, F. ASP biotoxins in scallops: The practical application in Galicia of Commision Decision 2002/226/EC. In Molluscan Shellfish Safety; Villalba, A., Reguera, B., Romalde, J.L., Beiras, R., Eds.; Xunta de Galicia and IOC of UNESCO: Santiago de Compostela, Spain, 2003; pp. 169–177. [Google Scholar]
- Arévalo, F.F.; Bermúdez, M.; Salgado, C. ASP toxicity in scallops: Individual variability and tissue distribution. In Harmful Microalgae; Reguera, B., Blanco, J., Fernández, M.L., Wyatt, T., Eds.; Xunta de Galicia and the IOC of UNESCO: Santiago de Compostela, Spain, 1998; pp. 499–502. [Google Scholar]
- EU. Commission Decision of 15 March 2002 establishing special health checks for the harvesting and processing of certain bivalve molluscs with a level of amnesic shellfish poison (ASP) exceeding the limit laid down by Council Directive 91/492/EEC. Off. J. Eur. Communities 2002, 16, 0065–0066. [Google Scholar]
- Blanco, J.; Mariño, C.; Acosta, C.P.; Martin, H. The use of biopsies to quantify domoic acid concentration in the king scallop Pecten maximus. In Proceedings of the 12th International Conference on Harmful Algae, Copenhagen, Denmark, 4–8 September 2006. [Google Scholar] [CrossRef]
- Beninger, P.G.; Le Pennec, M. Scallop structure and function. In Scallops: Biology, Ecology, Aquaculture, and Fisheries, Third edition; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 85–159. [Google Scholar]
- Henry, M.; Boucaud-Camou, E.; Lefort, Y. Functional micro-anatomy of the digestive gland of the scallop Pecten maximus (L.). Aquat. Living Resour. 1991, 4, 191–202. [Google Scholar] [CrossRef]
- Wekell, J.C.; Trainer, V.L.; Ayres, D.; Simons, D. A study of spatial variability of domoic acid in razor clams: Recommendations for resource management on the washington coast. Harmful Algae 2002, 1, 35–43. [Google Scholar] [CrossRef]
- Ha, D.V.; Takata, Y.; Sato, S.; Fukuyo, Y.; Kodama, M. Domoic acid in a bivalve Spondylus cruentus in Nha Trang bay, Khanh Hoa province, Vietnam. Coast. Mar. Sci. 2006, 30, 130–132. [Google Scholar] [CrossRef]
- Blanco, J. Informe Final del Proyecto INIA ACU01-014. Efecto del Crecimiento, de las Condiciones Ambientales y del Ciclo Reproductivo en la Acumulación y la Distribución Anatómica de Toxinas de Tipo Paralítico (PST) y de Tipo Amnésico (AST) en la Vieira Pecten Maximus, 2004; p. 27. [CrossRef]
- Blanco, J.; Cano, J.; Marino, M.D.C.; Campos, M.J. Effect of Phytoplankton Containing Paralytic Shellfish and Amnesic Shellfish Toxins on the Culture of the King Scallop Pecten maximus in Málaga (SE Spain). Aquat. Living Resour. 2006, 19, 267–273. [Google Scholar] [CrossRef]
- Pennec, G.L.; Pennec, M.L.; Beninger, G. Seasonal digestive gland dynamics of the scallop Pecten maximus in the Bay of Brest (France). J. Mar. Biol. Assoc. UK 2001, 81, 663–671. [Google Scholar] [CrossRef]
- Rossignoli, A.; Blanco, J. Cellular distribution of okadaic acid in the digestive gland of Mytilus galloprovincialis (Lamarck, 1819). Toxicon 2008, 52, 957–959. [Google Scholar] [CrossRef]
- Giard, W.; Favrel, P.; Boucaud-Camou, E. In vitro investigation of α-amylase release from the digestive cells of the bivalve mollusc Pecten maximus: Effect of second messengers and biogenic amines. J. Comp. Physiol. B 1995, 164. [Google Scholar] [CrossRef]
- Regueiro, J.; Álvarez, G.; Mauríz, A.; Blanco, J. High throughput analysis of amnesic shellfish poisoning toxins in bivalve molluscs by dispersive solid-phase extraction and high-performance liquid chromatography using a monolithic column. Food Chem. 2011, 127, 1884–1891. [Google Scholar] [CrossRef]
- R. Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016; p. 260. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, J.; Mauríz, A.; Álvarez, G. Distribution of Domoic Acid in the Digestive Gland of the King Scallop Pecten maximus. Toxins 2020, 12, 371. https://doi.org/10.3390/toxins12060371
Blanco J, Mauríz A, Álvarez G. Distribution of Domoic Acid in the Digestive Gland of the King Scallop Pecten maximus. Toxins. 2020; 12(6):371. https://doi.org/10.3390/toxins12060371
Chicago/Turabian StyleBlanco, Juan, Aida Mauríz, and Gonzalo Álvarez. 2020. "Distribution of Domoic Acid in the Digestive Gland of the King Scallop Pecten maximus" Toxins 12, no. 6: 371. https://doi.org/10.3390/toxins12060371
APA StyleBlanco, J., Mauríz, A., & Álvarez, G. (2020). Distribution of Domoic Acid in the Digestive Gland of the King Scallop Pecten maximus. Toxins, 12(6), 371. https://doi.org/10.3390/toxins12060371






