Cardiovascular Calcification in Chronic Kidney Disease—Therapeutic Opportunities
Abstract
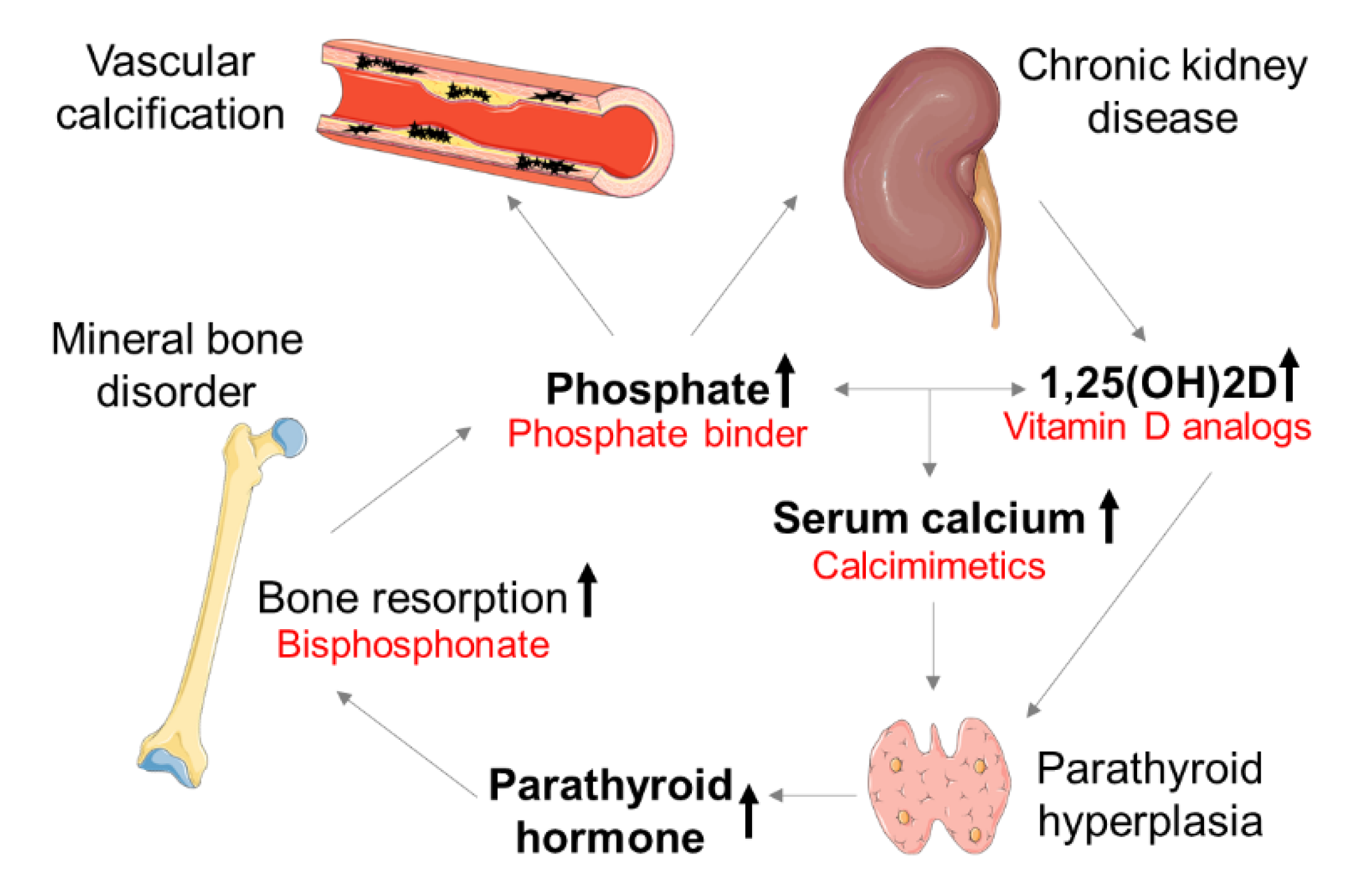
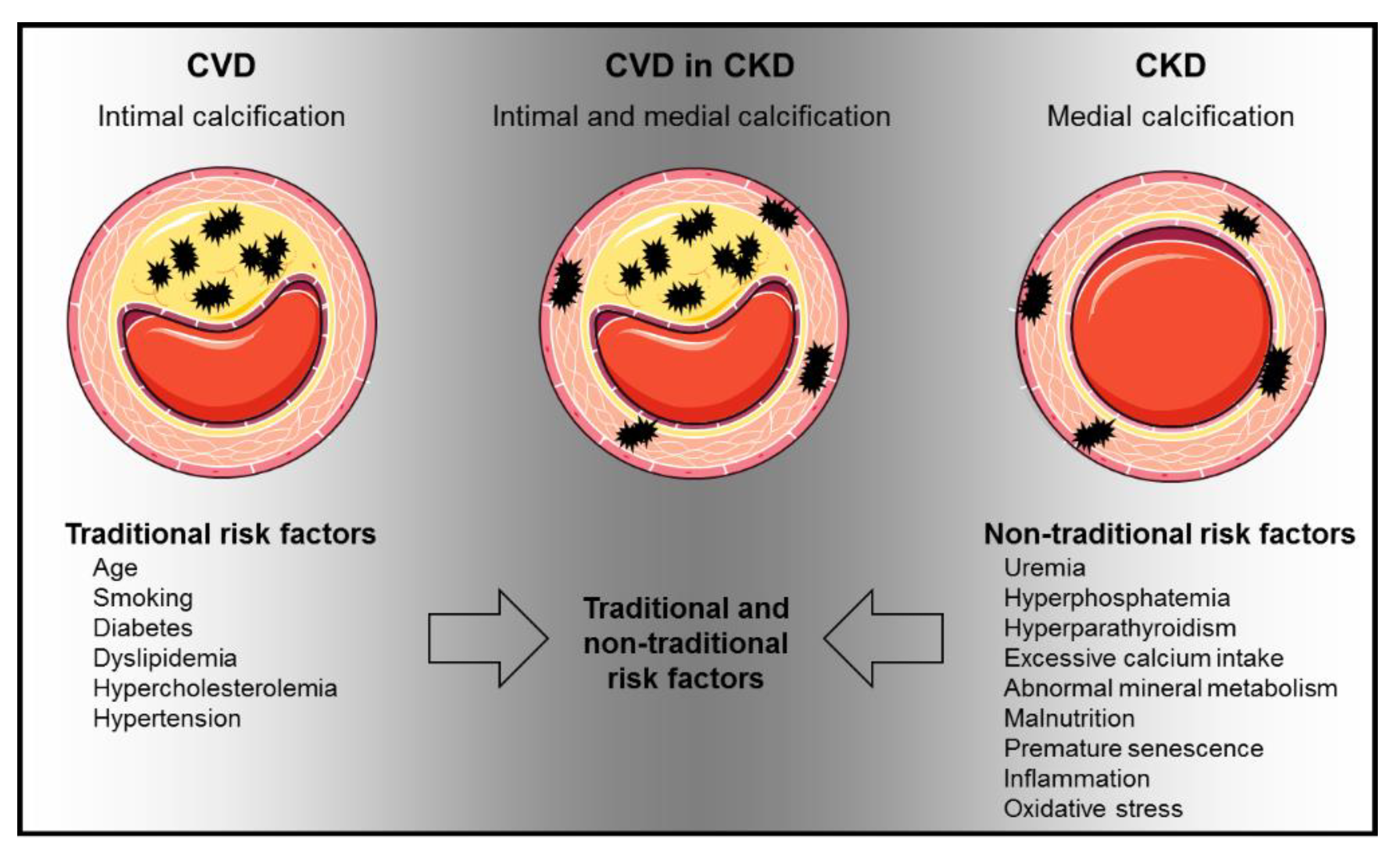
1. Introduction
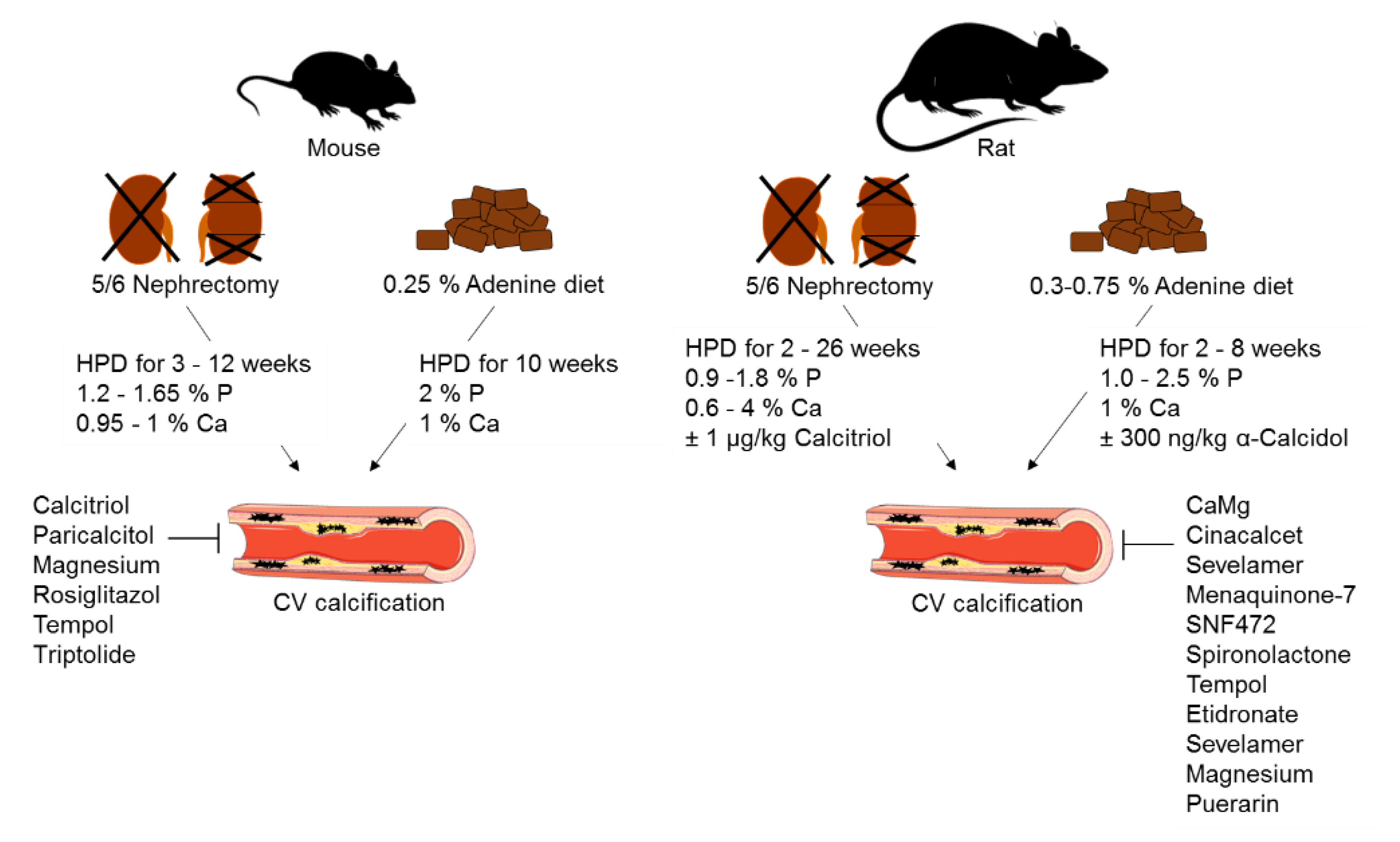
2. Animal Models of CKD
3. Therapeutic Concepts of CV Calcification in CKD
3.1. Phosphate Binder
3.2. Calcimimetics
4. Novel Therapeutic Strategies—from Experimental Models to the Clinic
4.1. Bisphosphonates
| Treatment | Substance | Dosis | Application | Experimental Model | Species, Strain | Ref. |
|---|---|---|---|---|---|---|
| Bisphospho-nate | Etidronate | 5 or 10 mg/kg | s.c., daily, 3 weeks | 5/6 nephrectomy | Wistar rat | [54] |
| Vitamin K | Mena-quinone-7 | 50 µg/kg | Oral gavage, daily 4 weeks | Adenine diet | Sprague-Dawley rat | [66] |
| Omega-3 fatty acid | Eicosapenta-enoic acid | 300 mg/kg | Oral gavage, daily 4 weeks | Adenine diet | Sprague-Dawley rat | [66] |
| Vitamin D receptor agonist | Calcitriol Paricalcitol | 30 ng/kg 100 or 300 ng/kg | i.p., 3 times/week, 3 weeks | 5/6 nephrectomy | DBA/2J mouse | [26] |
| Dietary supplement | Magnesium | 0.1–1.1% | Food intake, 14 days | 5/6 nephrectomy | Wistar rat | [67] |
| Dietary supplement | Magnesium | 3% | Food intake, 7 weeks | 5/6 nephrectomy | Non-agouti mouse | [68] |
| Hexasodium salt | SNF472 | 50 mg/kg | i.v., daily, 19 days | Adenine diet | Wistar rat | [20] |
4.2. Vitamin K
4.3. Vitamin D
4.4. Magnesium
4.5. Hexasodium Salt of Myo-Inositol Hexaphosphate
5. Promising Treatments of CV Calcification in Experimental CKD Models
| Treatment | Substance | Dosis | Application | Experimental Model | Species, Strain | Ref. |
|---|---|---|---|---|---|---|
| Isoflavonoid | Puerarin | 400 mg/kg | Oral gavage, daily; 4 weeks | 5/6 nephrectomy | Sprague-Dawley rat | [104] |
| PPARγ agonist | Rosiglitazol | 10 mg/kg | Oral gavage, daily; 12 weeks | 5/6 nephrectomy | DBA/2J mouse | [107] |
| NF-κB inhibitor | Tempol | 3 mmol/L | Drinking water; 10 weeks | Adenine diet | DBA/2J mouse | [109] |
| NF-κB inhibitor | Tempol | 3 mmol/L | Drinking water; 6 weeks | Adenine diet | Sprague-Dawley rat | [110] |
| NF-κB inhibitor | Triptolide | 70 µg/kg | i.p., daily; 10 weeks | Adenine diet | DBA/2J mouse | [109] |
| MR antagonist | Spirono-lactone | 100 mg/kg | Food intake, daily; 2 weeks | Adenine diet | Sprague-Dawley rat | [111] |
6. Potential Diagnostic Tools for CV Calcification in CKD
6.1. Development of the T50 Assay
6.2. Clinical Association
7. Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Metra, M.; Zaca, V.; Parati, G.; Agostoni, P.; Bonadies, M.; Ciccone, M.; Cas, A.D.; Iacoviello, M.; Lagioia, R.; Lombardi, C.; et al. Cardiovascular and noncardiovascular comorbidities in patients with chronic heart failure. J. Cardiovasc. Med. 2011, 12, 76–84. [Google Scholar] [CrossRef]
- Noels, H.; Boor, P.; Goettsch, C.; Hohl, M.; Jahnen-Dechent, W.; Jankowski, V.; Kindermann, I.; Kramann, R.; Lehrke, M.; Linz, D.; et al. The new SFB/TRR219 Research Centre. Eur. Heart J. 2018, 39, 975–977. [Google Scholar] [CrossRef]
- Ito, S.; Yoshida, M. Protein-bound uremic toxins: New culprits of cardiovascular events in chronic kidney disease patients. Toxins 2014, 6, 665–678. [Google Scholar] [CrossRef]
- Lekawanvijit, S.; Kompa, A.R.; Wang, B.H.; Kelly, D.J.; Krum, H. Cardiorenal syndrome: The emerging role of protein-bound uremic toxins. Circ. Res. 2012, 111, 1470–1483. [Google Scholar] [CrossRef]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef]
- Schlieper, G.; Schurgers, L.; Brandenburg, V.; Reutelingsperger, C.; Floege, J. Vascular calcification in chronic kidney disease: An update. Nephrol. Dial. Transplant. 2016, 31, 31–39. [Google Scholar] [CrossRef]
- Viegas, C.; Araujo, N.; Marreiros, C.; Simes, D. The interplay between mineral metabolism, vascular calcification and inflammation in Chronic Kidney Disease (CKD): Challenging old concepts with new facts. Aging (Albany NY) 2019, 11, 4274–4299. [Google Scholar] [CrossRef]
- Dayanand, P.; Sandhyavenu, H.; Dayanand, S.; Martinez, J.; Rangaswami, J. Role of Bisphosphonates in Vascular calcification and Bone Metabolism: A Clinical Summary. Curr. Cardiol. Rev. 2018, 14, 192–199. [Google Scholar] [CrossRef]
- Kiel, D.P.; Kauppila, L.I.; Cupples, L.A.; Hannan, M.T.; O’Donnell, C.J.; Wilson, P.W. Bone loss and the progression of abdominal aortic calcification over a 25 year period: The Framingham Heart Study. Calcif. Tissue Int. 2001, 68, 271–276. [Google Scholar] [CrossRef]
- Tanko, L.B.; Christiansen, C.; Cox, D.A.; Geiger, M.J.; McNabb, M.A.; Cummings, S.R. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J. Bone Miner. Res. 2005, 20, 1912–1920. [Google Scholar] [CrossRef]
- Beto, J.; Bhatt, N.; Gerbeling, T.; Patel, C.; Drayer, D. Overview of the 2017 KDIGO CKD-MBD Update: Practice Implications for Adult Hemodialysis Patients. J. Ren. Nutr. 2019, 29, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Noels, H.; Jankowski, J.; Floege, J.; Fliser, D.; Bohm, M. Mechanisms of cardiovascular complications in chronic kidney disease: Research focus of the Transregional Research Consortium SFB TRR219 of the University Hospital Aachen (RWTH) and the Saarland University. Clin. Res. Cardiol. 2018, 107, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Blaha, M.J.; Blankstein, R.; Agatston, A.; Rivera, J.J.; Virani, S.S.; Ouyang, P.; Jones, S.R.; Blumenthal, R.S.; Budoff, M.J.; et al. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: Implications for statin therapy from the multi-ethnic study of atherosclerosis. Circulation 2014, 129, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, J.D.; Goettsch, C.; Bertazzo, S.; Maldonado, N.; Ruiz, J.L.; Goh, W.; Yabusaki, K.; Faits, T.; Bouten, C.; Franck, G.; et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat. Mater. 2016, 15, 335–343. [Google Scholar] [CrossRef]
- Shroff, R.C.; Shanahan, C.M. The vascular biology of calcification. Semin. Dial. 2007, 20, 103–109. [Google Scholar] [CrossRef]
- Schlieper, G.; Hess, K.; Floege, J.; Marx, N. The vulnerable patient with chronic kidney disease. Nephrol. Dial. Transplant. 2016, 31, 382–390. [Google Scholar] [CrossRef]
- Ketteler, M.; Schlieper, G.; Floege, J. Calcification and cardiovascular health: New insights into an old phenomenon. Hypertension 2006, 47, 1027–1034. [Google Scholar] [CrossRef]
- Shroff, R.; Long, D.A.; Shanahan, C. Mechanistic insights into vascular calcification in CKD. J. Am. Soc. Nephrol. 2013, 24, 179–189. [Google Scholar] [CrossRef]
- Nitta, K.; Ogawa, T.; Hanafusa, N.; Tsuchiya, K. Recent Advances in the Management of Vascular Calcification in Patients with End-Stage Renal Disease. Contrib. Nephrol. 2019, 198, 62–72. [Google Scholar] [CrossRef]
- Ferrer, M.D.; Ketteler, M.; Tur, F.; Tur, E.; Isern, B.; Salcedo, C.; Joubert, P.H.; Behets, G.J.; Neven, E.; D’Haese, P.C.; et al. Characterization of SNF472 pharmacokinetics and efficacy in uremic and non-uremic rats models of cardiovascular calcification. PLoS ONE 2018, 13, e0197061. [Google Scholar] [CrossRef]
- Raggi, P.; Bellasi, A.; Bushinsky, D.; Bover, J.; Rodriguez, M.; Ketteler, M.; Sinha, S.; Salcedo, C.; Gillotti, K.; Padgett, C.; et al. Slowing Progression of Cardiovascular Calcification with SNF472 in Patients on Hemodialysis: Results of a Randomized, Phase 2b Study. Circulation 2019. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, T.D.; Holt, S.G.; Smith, E.R. Animal models to study links between cardiovascular disease and renal failure and their relevance to human pathology. Front. Immunol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Becker, G.J.; Hewitson, T.D. Animal models of chronic kidney disease: Useful but not perfect. Nephrol. Dial. Transplant. 2013, 28, 2432–2438. [Google Scholar] [CrossRef] [PubMed]
- Shobeiri, N.; Adams, M.A.; Holden, R.M. Vascular calcification in animal models of CKD: A review. Am. J. Nephrol. 2010, 31, 471–481. [Google Scholar] [CrossRef]
- Yokozawa, T.; Oura, H.; Okada, T. Metabolic effects of dietary purine in rats. J. Nutr. Sci. Vitaminol. 1982, 28, 519–526. [Google Scholar] [CrossRef]
- Lau, W.L.; Leaf, E.M.; Hu, M.C.; Takeno, M.M.; Kuro-o, M.; Moe, O.W.; Giachelli, C.M. Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kidney Int. 2012, 82, 1261–1270. [Google Scholar] [CrossRef]
- Rabe, M.; Schaefer, F. Non-Transgenic Mouse Models of Kidney Disease. Nephron 2016, 133, 53–61. [Google Scholar] [CrossRef]
- El-Abbadi, M.M.; Pai, A.S.; Leaf, E.M.; Yang, H.Y.; Bartley, B.A.; Quan, K.K.; Ingalls, C.M.; Liao, H.W.; Giachelli, C.M. Phosphate feeding induces arterial medial calcification in uremic mice: Role of serum phosphorus, fibroblast growth factor-23, and osteopontin. Kidney Int. 2009, 75, 1297–1307. [Google Scholar] [CrossRef]
- Makaryus, A.N.; Sison, C.; Kohansieh, M.; Makaryus, J.N. Implications of gender difference in coronary calcification as assessed by ct coronary angiography. Clin. Med. Insights Cardiol. 2014, 2014, 51–55. [Google Scholar] [CrossRef]
- Ishola, D.A., Jr.; van der Giezen, D.M.; Hahnel, B.; Goldschmeding, R.; Kriz, W.; Koomans, H.A.; Joles, J.A. In mice, proteinuria and renal inflammatory responses to albumin overload are strain-dependent. Nephrol. Dial. Transplant. 2006, 21, 591–597. [Google Scholar] [CrossRef]
- Cozzolino, M.; Staniforth, M.E.; Liapis, H.; Finch, J.; Burke, S.K.; Dusso, A.S.; Slatopolsky, E. Sevelamer hydrochloride attenuates kidney and cardiovascular calcifications in long-term experimental uremia. Kidney Int. 2003, 64, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- De Schutter, T.M.; Behets, G.J.; Geryl, H.; Peter, M.E.; Steppan, S.; Gundlach, K.; Passlick-Deetjen, J.; D’Haese, P.C.; Neven, E. Effect of a magnesium-based phosphate binder on medial calcification in a rat model of uremia. Kidney Int. 2013, 83, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Neven, E.; De Schutter, T.M.; Dams, G.; Gundlach, K.; Steppan, S.; Buchel, J.; Passlick-Deetjen, J.; D’Haese, P.C.; Behets, G.J. A magnesium based phosphate binder reduces vascular calcification without affecting bone in chronic renal failure rats. PLoS ONE 2014, 9, e107067. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.; Gardner, S.; Tonelli, M.; Mavridis, D.; Johnson, D.W.; Craig, J.C.; French, R.; Ruospo, M.; Strippoli, G.F. Phosphate-Binding Agents in Adults With CKD: A Network Meta-analysis of Randomized Trials. Am. J. Kidney Dis. 2016, 68, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.A.; Vandermeer, B.; Raggi, P.; Mendelssohn, D.C.; Chatterley, T.; Dorgan, M.; Lok, C.E.; Fitchett, D.; Tsuyuki, R.T. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: An updated systematic review and meta-analysis. Lancet 2013, 382, 1268–1277. [Google Scholar] [CrossRef]
- Ruospo, M.; Palmer, S.C.; Natale, P.; Craig, J.C.; Vecchio, M.; Elder, G.J.; Strippoli, G.F. Phosphate binders for preventing and treating chronic kidney disease-mineral and bone disorder (CKD-MBD). Cochrane Database Syst. Rev. 2018, 8, CD006023. [Google Scholar] [CrossRef]
- Block, G.A.; Bushinsky, D.A.; Cheng, S.; Cunningham, J.; Dehmel, B.; Drueke, T.B.; Ketteler, M.; Kewalramani, R.; Martin, K.J.; Moe, S.M.; et al. Effect of Etelcalcetide vs Cinacalcet on Serum Parathyroid Hormone in Patients Receiving Hemodialysis With Secondary Hyperparathyroidism: A Randomized Clinical Trial. JAMA 2017, 317, 156–164. [Google Scholar] [CrossRef]
- Wu, M.; Tang, R.N.; Liu, H.; Pan, M.M.; Liu, B.C. Cinacalcet ameliorates aortic calcification in uremic rats via suppression of endothelial-to-mesenchymal transition. Acta Pharmacol. Sin. 2016, 37, 1423–1431. [Google Scholar] [CrossRef]
- Friedl, C.; Zitt, E. Role of etelcalcetide in the management of secondary hyperparathyroidism in hemodialysis patients: A review on current data and place in therapy. Drug Des. Dev. Ther. 2018, 12, 1589–1598. [Google Scholar] [CrossRef]
- Investigators, E.T.; Chertow, G.M.; Block, G.A.; Correa-Rotter, R.; Drueke, T.B.; Floege, J.; Goodman, W.G.; Herzog, C.A.; Kubo, Y.; London, G.M.; et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N. Engl. J. Med. 2012, 367, 2482–2494. [Google Scholar] [CrossRef]
- Raggi, P.; Chertow, G.M.; Torres, P.U.; Csiky, B.; Naso, A.; Nossuli, K.; Moustafa, M.; Goodman, W.G.; Lopez, N.; Downey, G.; et al. The ADVANCE study: A randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol. Dial. Transplant. 2011, 26, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.; Nistor, I.; Craig, J.C.; Pellegrini, F.; Messa, P.; Tonelli, M.; Covic, A.; Strippoli, G.F. Cinacalcet in patients with chronic kidney disease: A cumulative meta-analysis of randomized controlled trials. PLoS Med. 2013, 10, e1001436. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Methven, S.; Gasparini, A.; Barany, P.; Birnie, K.; MacNeill, S.; May, M.T.; Caskey, F.J.; Carrero, J.J. Cinacalcet use and the risk of cardiovascular events, fractures and mortality in chronic kidney disease patients with secondary hyperparathyroidism. Sci. Rep. 2018, 8, 2103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, M.; You, L.; Li, H.; Ni, L.; Gu, Y.; Hao, C.; Chen, J. Effects and safety of calcimimetics in end stage renal disease patients with secondary hyperparathyroidism: A meta-analysis. PLoS ONE 2012, 7, e48070. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, N.D.; Elder, G.J.; Kerr, P.G. Bisphosphonates in chronic kidney disease; balancing potential benefits and adverse effects on bone and soft tissue. Clin. J. Am. Soc. Nephrol. 2009, 4, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Perazella, M.A.; Markowitz, G.S. Bisphosphonate nephrotoxicity. Kidney Int. 2008, 74, 1385–1393. [Google Scholar] [CrossRef]
- Bergner, R.; Diel, I.J.; Henrich, D.; Hoffmann, M.; Uppenkamp, M. Differences in nephrotoxicity of intravenous bisphosphonates for the treatment of malignancy-related bone disease. Onkologie 2006, 29, 534–540. [Google Scholar] [CrossRef]
- Markowitz, G.S.; Appel, G.B.; Fine, P.L.; Fenves, A.Z.; Loon, N.R.; Jagannath, S.; Kuhn, J.A.; Dratch, A.D.; D’Agati, V.D. Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J. Am. Soc. Nephrol. 2001, 12, 1164–1172. [Google Scholar]
- Verhulst, A.; Sun, S.; McKenna, C.E.; D’Haese, P.C. Endocytotic uptake of zoledronic acid by tubular cells may explain its renal effects in cancer patients receiving high doses of the compound. PLoS ONE 2015, 10, e0121861. [Google Scholar] [CrossRef][Green Version]
- Otto, S.; Pautke, C.; Hafner, S.; Hesse, R.; Reichardt, L.F.; Mast, G.; Ehrenfeld, M.; Cornelius, C.P. Pathologic fractures in bisphosphonate-related osteonecrosis of the jaw-review of the literature and review of our own cases. Craniomaxillofac. Trauma Reconstr. 2013, 6, 147–154. [Google Scholar] [CrossRef]
- Miller, P.D.; Roux, C.; Boonen, S.; Barton, I.P.; Dunlap, L.E.; Burgio, D.E. Safety and efficacy of risedronate in patients with age-related reduced renal function as estimated by the Cockcroft and Gault method: A pooled analysis of nine clinical trials. J. Bone Miner. Res. 2005, 20, 2105–2115. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J. Bisphosphonates in the renal patient. Nephrol. Dial. Transplant. 2007, 22, 1505–1507. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, K.I.; Rajamannan, N.M.; Towler, D.A. The regulation of valvular and vascular sclerosis by osteogenic morphogens. Circ. Res. 2011, 109, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Suzuki, Y.; Matsushita, M.; Fujii, H.; Miyaura, C.; Aizawa, S.; Kogo, H. Prevention of aortic calcification by etidronate in the renal failure rat model. Eur. J. Pharmacol. 2007, 558, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Caffarelli, C.; Montagnani, A.; Nuti, R.; Gonnelli, S. Bisphosphonates, atherosclerosis and vascular calcification: Update and systematic review of clinical studies. Clin. Interv. Aging 2017, 12, 1819–1828. [Google Scholar] [CrossRef]
- Toussaint, N.D.; Lau, K.K.; Strauss, B.J.; Polkinghorne, K.R.; Kerr, P.G. Effect of alendronate on vascular calcification in CKD stages 3 and 4: A pilot randomized controlled trial. Am. J. Kidney Dis. 2010, 56, 57–68. [Google Scholar] [CrossRef]
- Hartle, J.E.; Tang, X.; Kirchner, H.L.; Bucaloiu, I.D.; Sartorius, J.A.; Pogrebnaya, Z.V.; Akers, G.A.; Carnero, G.E.; Perkins, R.M. Bisphosphonate therapy, death, and cardiovascular events among female patients with CKD: A retrospective cohort study. Am. J. Kidney Dis. 2012, 59, 636–644. [Google Scholar] [CrossRef]
- Kranenburg, G.; Bartstra, J.W.; Weijmans, M.; de Jong, P.A.; Mali, W.P.; Verhaar, H.J.; Visseren, F.L.J.; Spiering, W. Bisphosphonates for cardiovascular risk reduction: A systematic review and meta-analysis. Atherosclerosis 2016, 252, 106–115. [Google Scholar] [CrossRef]
- McCloskey, E.V.; Johansson, H.; Oden, A.; Austin, M.; Siris, E.; Wang, A.; Lewiecki, E.M.; Lorenc, R.; Libanati, C.; Kanis, J.A. Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J. Bone Miner. Res. 2012, 27, 1480–1486. [Google Scholar] [CrossRef]
- Feng, X.; Teitelbaum, S.L. Osteoclasts: New Insights. Bone Res. 2013, 1, 11–26. [Google Scholar] [CrossRef]
- Panizo, S.; Cardus, A.; Encinas, M.; Parisi, E.; Valcheva, P.; Lopez-Ongil, S.; Coll, B.; Fernandez, E.; Valdivielso, J.M. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ. Res. 2009, 104, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Helas, S.; Goettsch, C.; Schoppet, M.; Zeitz, U.; Hempel, U.; Morawietz, H.; Kostenuik, P.J.; Erben, R.G.; Hofbauer, L.C. Inhibition of receptor activator of NF-kappaB ligand by denosumab attenuates vascular calcium deposition in mice. Am. J. Pathol. 2009, 175, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Iseri, K.; Watanabe, M.; Yoshikawa, H.; Mitsui, H.; Endo, T.; Yamamoto, Y.; Iyoda, M.; Ryu, K.; Inaba, T.; Shibata, T.; et al. Effects of Denosumab and Alendronate on Bone Health and Vascular Function in Hemodialysis Patients: A Randomized, Controlled Trial. J. Bone Miner. Res. 2019, 34, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Hjortnaes, J.; Bouten, C.V.; Van Herwerden, L.A.; Grundeman, P.F.; Kluin, J. Translating autologous heart valve tissue engineering from bench to bed. Tissue Eng. Part B Rev. 2009, 15, 307–317. [Google Scholar] [CrossRef]
- Rajamannan, N.M.; Evans, F.J.; Aikawa, E.; Grande-Allen, K.J.; Demer, L.L.; Heistad, D.D.; Simmons, C.A.; Masters, K.S.; Mathieu, P.; O’Brien, K.D.; et al. Calcific aortic valve disease: Not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation 2011, 124, 1783–1791. [Google Scholar] [CrossRef]
- An, W.S.; Lee, S.M.; Son, Y.K.; Kim, S.E. Combination of omega-3 fatty acid and menaquinone-7 prevents progression of aortic calcification in adenine and low protein diet induced rat model. Nephrol. Dial. Transplant. 2017, 32, iii253–iii254. [Google Scholar] [CrossRef]
- Diaz-Tocados, J.M.; Peralta-Ramirez, A.; Rodriguez-Ortiz, M.E.; Raya, A.I.; Lopez, I.; Pineda, C.; Herencia, C.; Montes de Oca, A.; Vergara, N.; Steppan, S.; et al. Dietary magnesium supplementation prevents and reverses vascular and soft tissue calcifications in uremic rats. Kidney Int. 2017, 92, 1084–1099. [Google Scholar] [CrossRef]
- Kaesler, N.; Goettsch, C.; Weis, D.; Schurgers, L.; Hellmann, B.; Floege, J.; Kramann, R. Magnesium but not nicotinamide prevents vascular calcification in experimental uraemia. Nephrol. Dial. Transplant. 2019. [Google Scholar] [CrossRef]
- O’Young, J.; Liao, Y.; Xiao, Y.; Jalkanen, J.; Lajoie, G.; Karttunen, M.; Goldberg, H.A.; Hunter, G.K. Matrix Gla protein inhibits ectopic calcification by a direct interaction with hydroxyapatite crystals. J. Am. Chem. Soc. 2011, 133, 18406–18412. [Google Scholar] [CrossRef]
- Tesfamariam, B. Involvement of Vitamin K-Dependent Proteins in Vascular Calcification. J. Cardiovasc. Pharmacol. Ther. 2019, 24, 323–333. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Teunissen, K.J.; Knapen, M.H.; Kwaijtaal, M.; van Diest, R.; Appels, A.; Reutelingsperger, C.P.; Cleutjens, J.P.; Vermeer, C. Novel conformation-specific antibodies against matrix gamma-carboxyglutamic acid (Gla) protein: Undercarboxylated matrix Gla protein as marker for vascular calcification. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1629–1633. [Google Scholar] [CrossRef]
- Halder, M.; Petsophonsakul, P.; Akbulut, A.C.; Pavlic, A.; Bohan, F.; Anderson, E.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K: Double Bonds beyond Coagulation Insights into Differences between Vitamin K1 and K2 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 896. [Google Scholar] [CrossRef] [PubMed]
- Cranenburg, E.C.; Schurgers, L.J.; Uiterwijk, H.H.; Beulens, J.W.; Dalmeijer, G.W.; Westerhuis, R.; Magdeleyns, E.J.; Herfs, M.; Vermeer, C.; Laverman, G.D.; et al. Vitamin K intake and status are low in hemodialysis patients. Kidney Int. 2012, 82, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; An, W.S. Supplementary nutrients for prevention of vascular calcification in patients with chronic kidney disease. Korean J. Intern. Med. 2019, 34, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Schlieper, G.; Westenfeld, R.; Kruger, T.; Cranenburg, E.C.; Magdeleyns, E.J.; Brandenburg, V.M.; Djuric, Z.; Damjanovic, T.; Ketteler, M.; Vermeer, C.; et al. Circulating nonphosphorylated carboxylated matrix gla protein predicts survival in ESRD. J. Am. Soc. Nephrol. 2011, 22, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Keyzer, C.A.; de Borst, M.H.; van den Berg, E.; Jahnen-Dechent, W.; Arampatzis, S.; Farese, S.; Bergmann, I.P.; Floege, J.; Navis, G.; Bakker, S.J.; et al. Calcification Propensity and Survival among Renal Transplant Recipients. J. Am. Soc. Nephrol. 2016, 27, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Masuda, K.; Yamazaki, M.; Kiyohara, C.; Itoh, S.; Wasaki, M.; Inoue, H. Metal ion and vitamin adsorption profiles of phosphate binder ion-exchange resins. Clin. Nephrol. 2010, 73, 30–35. [Google Scholar] [CrossRef]
- Neradova, A.; Schumacher, S.P.; Hubeek, I.; Lux, P.; Schurgers, L.J.; Vervloet, M.G. Phosphate binders affect vitamin K concentration by undesired binding, an in vitro study. BMC Nephrol. 2017, 18, 149. [Google Scholar] [CrossRef]
- Jansz, T.T.; Neradova, A.; van Ballegooijen, A.J.; Verhaar, M.C.; Vervloet, M.G.; Schurgers, L.J.; van Jaarsveld, B.C. The role of kidney transplantation and phosphate binder use in vitamin K status. PLoS ONE 2018, 13, e0203157. [Google Scholar] [CrossRef]
- Aoun, M.; Makki, M.; Azar, H.; Matta, H.; Chelala, D.N. High Dephosphorylated-Uncarboxylated MGP in Hemodialysis patients: Risk factors and response to vitamin K2, A pre-post intervention clinical trial. BMC Nephrol. 2017, 18, 191. [Google Scholar] [CrossRef] [PubMed]
- Westenfeld, R.; Krueger, T.; Schlieper, G.; Cranenburg, E.C.; Magdeleyns, E.J.; Heidenreich, S.; Holzmann, S.; Vermeer, C.; Jahnen-Dechent, W.; Ketteler, M.; et al. Effect of vitamin K2 supplementation on functional vitamin K deficiency in hemodialysis patients: A randomized trial. Am. J. Kidney Dis. 2012, 59, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Caluwe, R.; Pyfferoen, L.; De Boeck, K.; De Vriese, A.S. The effects of vitamin K supplementation and vitamin K antagonists on progression of vascular calcification: Ongoing randomized controlled trials. Clin. Kidney J. 2016, 9, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Kurnatowska, I.; Grzelak, P.; Masajtis-Zagajewska, A.; Kaczmarska, M.; Stefanczyk, L.; Vermeer, C.; Maresz, K.; Nowicki, M. Effect of vitamin K2 on progression of atherosclerosis and vascular calcification in nondialyzed patients with chronic kidney disease stages 3–5. Pol. Arch. Med. Wewn. 2015, 125, 631–640. [Google Scholar] [CrossRef]
- Zheng, Z.; Shi, H.; Jia, J.; Li, D.; Lin, S. Vitamin D supplementation and mortality risk in chronic kidney disease: A meta-analysis of 20 observational studies. BMC Nephrol. 2013, 14, 199. [Google Scholar] [CrossRef]
- Chitalia, N.; Recio-Mayoral, A.; Kaski, J.C.; Banerjee, D. Vitamin D deficiency and endothelial dysfunction in non-dialysis chronic kidney disease patients. Atherosclerosis 2012, 220, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Tang, M.; Perry, T.; Zalunardo, N.; Beaulieu, M.; Dubland, J.A.; Zerr, K.; Djurdjev, O. Randomized Controlled Trial for the Effect of Vitamin D Supplementation on Vascular Stiffness in CKD. Clin. J. Am. Soc. Nephrol. 2017, 12, 1447–1460. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Yadav, A.K.; Lal, A.; Kumar, V.; Singhal, M.; Billot, L.; Gupta, K.L.; Banerjee, D.; Jha, V. A Randomized Trial of Vitamin D Supplementation on Vascular Function in CKD. J. Am. Soc. Nephrol. 2017, 28, 3100–3108. [Google Scholar] [CrossRef]
- Samaan, F.; Carvalho, A.B.; Pillar, R.; Rocha, L.A.; Cassiolato, J.L.; Cuppari, L.; Canziani, M.E.F. The Effect of Long-Term Cholecalciferol Supplementation on Vascular Calcification in Chronic Kidney Disease Patients With Hypovitaminosis D. J. Ren. Nutr. 2019, 29, 407–415. [Google Scholar] [CrossRef]
- Levin, A.; Le Barbier, M.; Er, L.; Andress, D.; Sigrist, M.K.; Djurdjev, O. Incident isolated 1,25(OH)(2)D(3) deficiency is more common than 25(OH)D deficiency in CKD. J. Nephrol. 2012, 25, 204–210. [Google Scholar] [CrossRef]
- Louvet, L.; Buchel, J.; Steppan, S.; Passlick-Deetjen, J.; Massy, Z.A. Magnesium prevents phosphate-induced calcification in human aortic vascular smooth muscle cells. Nephrol. Dial. Transplant. 2013, 28, 869–878. [Google Scholar] [CrossRef]
- Massy, Z.A.; Drueke, T.B. Magnesium and outcomes in patients with chronic kidney disease: Focus on vascular calcification, atherosclerosis and survival. Clin. Kidney J. 2012, 5, i52–i61. [Google Scholar] [CrossRef]
- Briet, M.; Schiffrin, E.L. Vascular actions of aldosterone. J. Vasc. Res. 2013, 50, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Mizuiri, S.; Nishizawa, Y.; Yamashita, K.; Naito, T.; Ono, K.; Tanji, C.; Usui, K.; Doi, S.; Masaki, T.; Shigemoto, K. Hypomagnesemia is not an independent risk factor for mortality in Japanese maintenance hemodialysis patients. Int. Urol. Nephrol. 2019, 51, 1043–1052. [Google Scholar] [CrossRef]
- Wu, L.; Cai, K.; Luo, Q.; Wang, L.; Hong, Y. Baseline Serum Magnesium Level and Its Variability in Maintenance Hemodialysis Patients: Associations with Mortality. Kidney Blood Press. Res. 2019, 44, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Fujii, N.; Shoji, T.; Hayashi, T.; Rakugi, H.; Iseki, K.; Tsubakihara, Y.; Isaka, Y.; Committee of Renal Data Registry of the Japanese Society for Dialysis Therapy. Magnesium modifies the cardiovascular mortality risk associated with hyperphosphatemia in patients undergoing hemodialysis: A cohort study. PLoS ONE 2014, 9, e116273. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Luo, Q.; Dai, Z.; Zhu, B.; Fei, J.; Xue, C.; Wu, D. Hypomagnesemia Is Associated with Increased Mortality among Peritoneal Dialysis Patients. PLoS ONE 2016, 11, e0152488. [Google Scholar] [CrossRef]
- Bundy, J.D.; Cai, X.; Scialla, J.J.; Dobre, M.A.; Chen, J.; Hsu, C.Y.; Leonard, M.B.; Go, A.S.; Rao, P.S.; Lash, J.P.; et al. Serum Calcification Propensity and Coronary Artery Calcification Among Patients With CKD: The CRIC (Chronic Renal Insufficiency Cohort) Study. Am. J. Kidney Dis. 2019, 73, 806–814. [Google Scholar] [CrossRef]
- Mortazavi, M.; Moeinzadeh, F.; Saadatnia, M.; Shahidi, S.; McGee, J.C.; Minagar, A. Effect of magnesium supplementation on carotid intima-media thickness and flow-mediated dilatation among hemodialysis patients: A double-blind, randomized, placebo-controlled trial. Eur. Neurol. 2013, 69, 309–316. [Google Scholar] [CrossRef]
- Turgut, F.; Kanbay, M.; Metin, M.R.; Uz, E.; Akcay, A.; Covic, A. Magnesium supplementation helps to improve carotid intima media thickness in patients on hemodialysis. Int. Urol. Nephrol. 2008, 40, 1075–1082. [Google Scholar] [CrossRef]
- Bressendorff, I.; Hansen, D.; Schou, M.; Kragelund, C.; Brandi, L. The effect of magnesium supplementation on vascular calcification in chronic kidney disease-a randomised clinical trial (MAGiCAL-CKD): Essential study design and rationale. BMJ Open 2017, 7, e016795. [Google Scholar] [CrossRef]
- Salcedo, C.; Joubert, P.H.; Ferrer, M.D.; Canals, A.Z.; Maduell, F.; Torregrosa, V.; Campistol, J.M.; Ojeda, R.; Perello, J. A phase 1b randomized, placebo-controlled clinical trial with SNF472 in haemodialysis patients. Br. J. Clin. Pharmacol. 2019, 85, 796–806. [Google Scholar] [CrossRef]
- Perello, J.; Joubert, P.H.; Ferrer, M.D.; Canals, A.Z.; Sinha, S.; Salcedo, C. First-time-in-human randomized clinical trial in healthy volunteers and haemodialysis patients with SNF472, a novel inhibitor of vascular calcification. Br. J. Clin. Pharmacol. 2018, 84, 2867–2876. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.X.; Zhang, H.; Peng, C. Puerarin: A review of pharmacological effects. Phytother. Res. 2014, 28, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, X.; Zhong, X.; Li, Z.; Cai, S.; Yang, P.; Ou, C.; Chen, M. Puerarin inhibits vascular calcification of uremic rats. Eur. J. Pharmacol. 2019, 855, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Millar, J.S. Novel benefits of peroxisome proliferator-activated receptors on cardiovascular risk. Curr. Opin. Lipidol. 2013, 24, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Ikejima, H.; Imanishi, T.; Tsujioka, H.; Kuroi, A.; Kobayashi, K.; Shiomi, M.; Muragaki, Y.; Mochizuki, S.; Goto, M.; Yoshida, K.; et al. Effects of telmisartan, a unique angiotensin receptor blocker with selective peroxisome proliferator-activated receptor-gamma-modulating activity, on nitric oxide bioavailability and atherosclerotic change. J. Hypertens. 2008, 26, 964–972. [Google Scholar] [CrossRef]
- Bostom, A.; Pasch, A.; Madsen, T.; Roberts, M.B.; Franceschini, N.; Steubl, D.; Garimella, P.S.; Ix, J.H.; Tuttle, K.R.; Ivanova, A.; et al. Serum Calcification Propensity and Fetuin-A: Biomarkers of Cardiovascular Disease in Kidney Transplant Recipients. Am. J. Nephrol. 2018, 48, 21–31. [Google Scholar] [CrossRef]
- Zhao, G.; Xu, M.J.; Zhao, M.M.; Dai, X.Y.; Kong, W.; Wilson, G.M.; Guan, Y.; Wang, C.Y.; Wang, X. Activation of nuclear factor-kappa B accelerates vascular calcification by inhibiting ankylosis protein homolog expression. Kidney Int. 2012, 82, 34–44. [Google Scholar] [CrossRef]
- Yoshida, T.; Yamashita, M.; Horimai, C.; Hayashi, M. Smooth Muscle-Selective Nuclear Factor-kappaB Inhibition Reduces Phosphate-Induced Arterial Medial Calcification in Mice With Chronic Kidney Disease. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Yamada, S.; Taniguchi, M.; Tokumoto, M.; Toyonaga, J.; Fujisaki, K.; Suehiro, T.; Noguchi, H.; Iida, M.; Tsuruya, K.; Kitazono, T. The antioxidant tempol ameliorates arterial medial calcification in uremic rats: Important role of oxidative stress in the pathogenesis of vascular calcification in chronic kidney disease. J. Bone Miner. Res. 2012, 27, 474–485. [Google Scholar] [CrossRef]
- Tatsumoto, N.; Yamada, S.; Tokumoto, M.; Eriguchi, M.; Noguchi, H.; Torisu, K.; Tsuruya, K.; Kitazono, T. Spironolactone ameliorates arterial medial calcification in uremic rats: The role of mineralocorticoid receptor signaling in vascular calcification. Am. J. Physiol. Ren. Physiol. 2015, 309, F967–F979. [Google Scholar] [CrossRef]
- Scialla, J.J.; Kao, W.H.; Crainiceanu, C.; Sozio, S.M.; Oberai, P.C.; Shafi, T.; Coresh, J.; Powe, N.R.; Plantinga, L.C.; Jaar, B.G.; et al. Biomarkers of vascular calcification and mortality in patients with ESRD. Clin. J. Am. Soc. Nephrol. 2014, 9, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Lu, R.; Zhang, M.; Pang, H.; Zhu, M.; Zhang, W.; Ni, Z.; Qian, J.; Yan, Y. Serum Soluble Klotho Level Is Associated with Abdominal Aortic Calcification in Patients on Maintenance Hemodialysis. Blood Purif. 2015, 40, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Pasch, A.; Farese, S.; Graber, S.; Wald, J.; Richtering, W.; Floege, J.; Jahnen-Dechent, W. Nanoparticle-based test measures overall propensity for calcification in serum. J. Am. Soc. Nephrol. 2012, 23, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Heiss, A.; DuChesne, A.; Denecke, B.; Grotzinger, J.; Yamamoto, K.; Renne, T.; Jahnen-Dechent, W. Structural basis of calcification inhibition by alpha 2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles. J. Biol. Chem. 2003, 278, 13333–13341. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Ford, M.L.; Tomlinson, L.A.; Bodenham, E.; McMahon, L.P.; Farese, S.; Rajkumar, C.; Holt, S.G.; Pasch, A. Serum calcification propensity predicts all-cause mortality in predialysis CKD. J. Am. Soc. Nephrol. 2014, 25, 339–348. [Google Scholar] [CrossRef]
- Lorenz, G.; Steubl, D.; Kemmner, S.; Pasch, A.; Koch-Sembdner, W.; Pham, D.; Haller, B.; Bachmann, Q.; Mayer, C.C.; Wassertheurer, S.; et al. Worsening calcification propensity precedes all-cause and cardiovascular mortality in haemodialyzed patients. Sci. Rep. 2017, 7, 13368. [Google Scholar] [CrossRef]
- Dahle, D.O.; Asberg, A.; Hartmann, A.; Holdaas, H.; Bachtler, M.; Jenssen, T.G.; Dionisi, M.; Pasch, A. Serum Calcification Propensity Is a Strong and Independent Determinant of Cardiac and All-Cause Mortality in Kidney Transplant Recipients. Am. J. Transplant. 2016, 16, 204–212. [Google Scholar] [CrossRef]
- Brandenburg, V.M.; Reinartz, S.; Kaesler, N.; Kruger, T.; Dirrichs, T.; Kramann, R.; Peeters, F.; Floege, J.; Keszei, A.; Marx, N.; et al. Slower Progress of Aortic Valve Calcification With Vitamin K Supplementation: Results From a Prospective Interventional Proof-of-Concept Study. Circulation 2017, 135, 2081–2083. [Google Scholar] [CrossRef]
| Treatment | Substance | Dosis | Medication | Experimental Model | Species, Strain | Ref. |
|---|---|---|---|---|---|---|
| Phosphate binder | Sevelamer | 750 mg/kg | Daily oral gavage, 4 weeks | Adenine diet | Wistar rat | [32] |
| Phosphate binder | Sevelamer | 3% | Diet, 6 months | 5/6 nephrectomy | Sprague-Dawley rat | [31] |
| Phosphate binder | CaMg | 185 mg/kg | Daily oral gavage, 6 weeks | Adenine diet | Wistar rat | [33] |
| Calcimimetic | Cinacalcet | 10 mg/kg | Daily oral gavage, 12 weeks | Adenine diet | Wistar rat | [38] |
| Patients | Follow-up | Main Results | Ref. |
|---|---|---|---|
| CKD stages 4 to 5D (n = 107) | 2.2 years | dp-ucMGP: positive association with progressive CKD stages and increased all-cause mortality | [75] |
| HD patients (n = 188) | 3 years | - 6.5-fold elevated dp-ucMGP - dp-cMGP associated with increased all-cause and CV mortality | [75] |
| KTR (n = 518) | 9.8 years | dp-ucMGP: association with increased all-cause mortality | [76] |
| Patients | Treatment | Study Design | Main Results | Ref. |
|---|---|---|---|---|
| CKD stage 3–5 (n = 42) | 90 μg/d MK-7 + 10 μg/d cholecalciferol, or 10 μg/d cholecalciferol (control), 38.5 weeks | Prospective, randomized, double-blind | Decrease of dp-ucMGP, smaller increase of CAC and CCA-IMT compared to control | [83] |
| HD patients (n = 50) | 360 μg/d MK-7, 4 weeks | Prospective, pre-post intervention clinical trial | 86% decrease of dp-ucMGP | [80] |
| HD patients (n = 17) | 135 μg/d MK-7, 6 weeks | Interventional pilot study | Decrease of dp-ucMGP but not dp-cMGP | [75] |
| HD patients (n = 53), Healthy controls (n = 50) | 45, 135, 360 μg/d MK-7, 6 weeks | Interventional, randomized, non-placebo-controlled trial | Dose-dependent decrease of dp-ucMGP | [81] |
| Patients | Mean/Median T50 (Baseline) | Follow up, Years | Findings | Ref. |
|---|---|---|---|---|
| CKD stages 2 to 4 (n = 1274), In follow up n = 780 | Median: 321 min | 3.2 | Association of low T50 with increased CAC prevalence and progression | [97] |
| CKD stages 3 and 4 (n = 184) | Mean: 329 ± 95 min | 5.3 | Association of low T50 with increased all-cause mortality and APWV | [116] |
| HD patients (n = 2785), control group (n = 1366) | Mean: 212 min (10th–90th percentile: 109–328 min) | 1.7 | Association of low T50 with increased all-cause mortality and CVD | [117] |
| HD patients (n = 188) | Mean: 246 ± 64 min | 3.7 | Association of low T50 and T50 decline with all-cause and CV mortality | [117] |
| KTR (n = 699) | Mean: 286 ± 62 min | 3.1 | Association of low T50 with increased all-cause and CV mortality and graft failure | [76] |
| KTR (n = 433) | Mean: 340 ± 70 min | 3.7 | Association of low T50 with increased CVD event risk | [107] |
| KTR during 10 weeks after transplantation (n = 1435), Follow-up: APWV after 1 year (n = 589) | Median: 188 min (25th–75th percentile: 139–248 min) | 5.1 | Association of low T50 with increased all-cause and CV mortality APWV not associated with T50 baseline | [118] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Himmelsbach, A.; Ciliox, C.; Goettsch, C. Cardiovascular Calcification in Chronic Kidney Disease—Therapeutic Opportunities. Toxins 2020, 12, 181. https://doi.org/10.3390/toxins12030181
Himmelsbach A, Ciliox C, Goettsch C. Cardiovascular Calcification in Chronic Kidney Disease—Therapeutic Opportunities. Toxins. 2020; 12(3):181. https://doi.org/10.3390/toxins12030181
Chicago/Turabian StyleHimmelsbach, Anika, Carina Ciliox, and Claudia Goettsch. 2020. "Cardiovascular Calcification in Chronic Kidney Disease—Therapeutic Opportunities" Toxins 12, no. 3: 181. https://doi.org/10.3390/toxins12030181
APA StyleHimmelsbach, A., Ciliox, C., & Goettsch, C. (2020). Cardiovascular Calcification in Chronic Kidney Disease—Therapeutic Opportunities. Toxins, 12(3), 181. https://doi.org/10.3390/toxins12030181





