An Acetylcholinesterase Inhibition-Based Biosensor for Aflatoxin B1 Detection Using Sodium Alginate as an Immobilization Matrix
Abstract
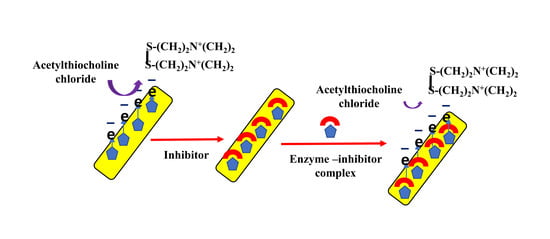
:1. Introduction
2. Results and Discussion
2.1. Optimization of the Amount of Sodium Alginate
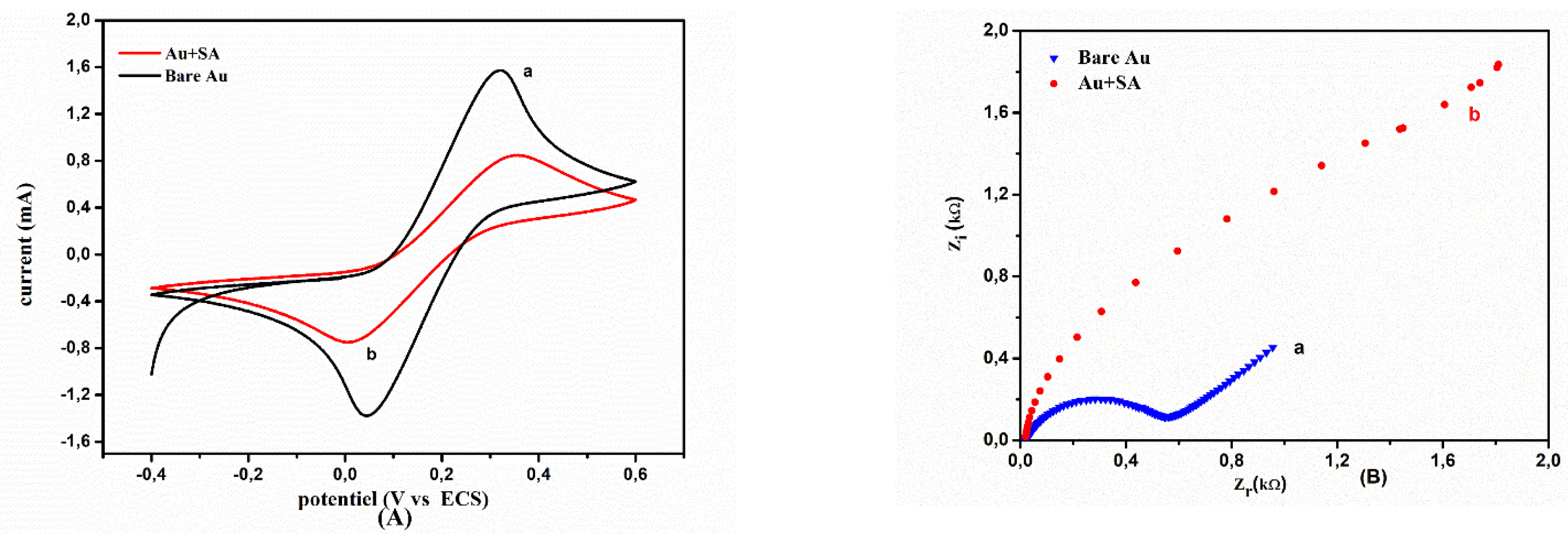
2.2. Electrochemical Behavior of the Modified Electrode with Sodium Alginate Biopolymer
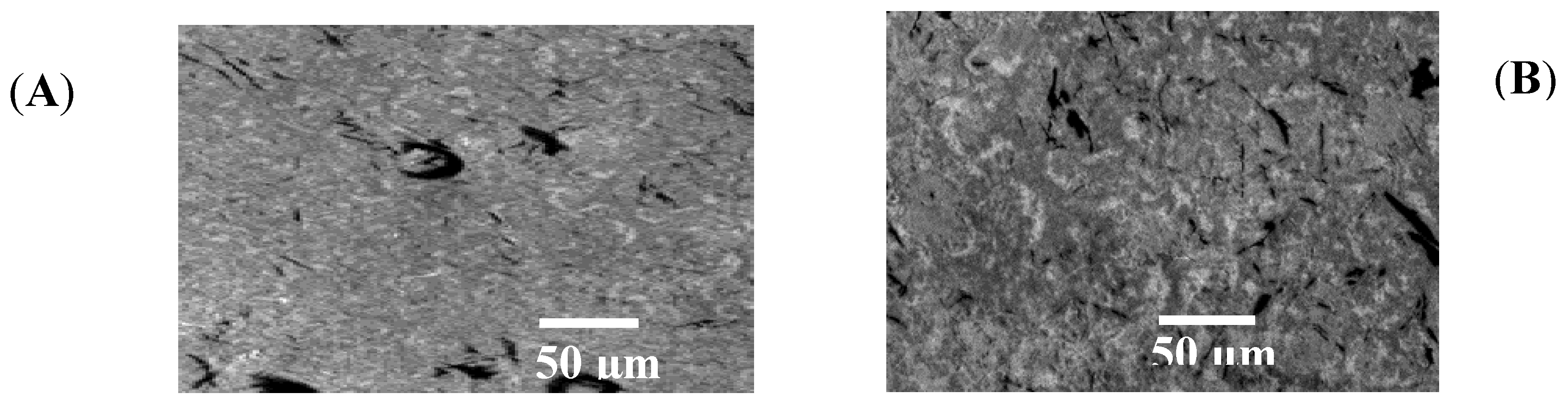
2.3. SEM Analysis of SA Gel Bead
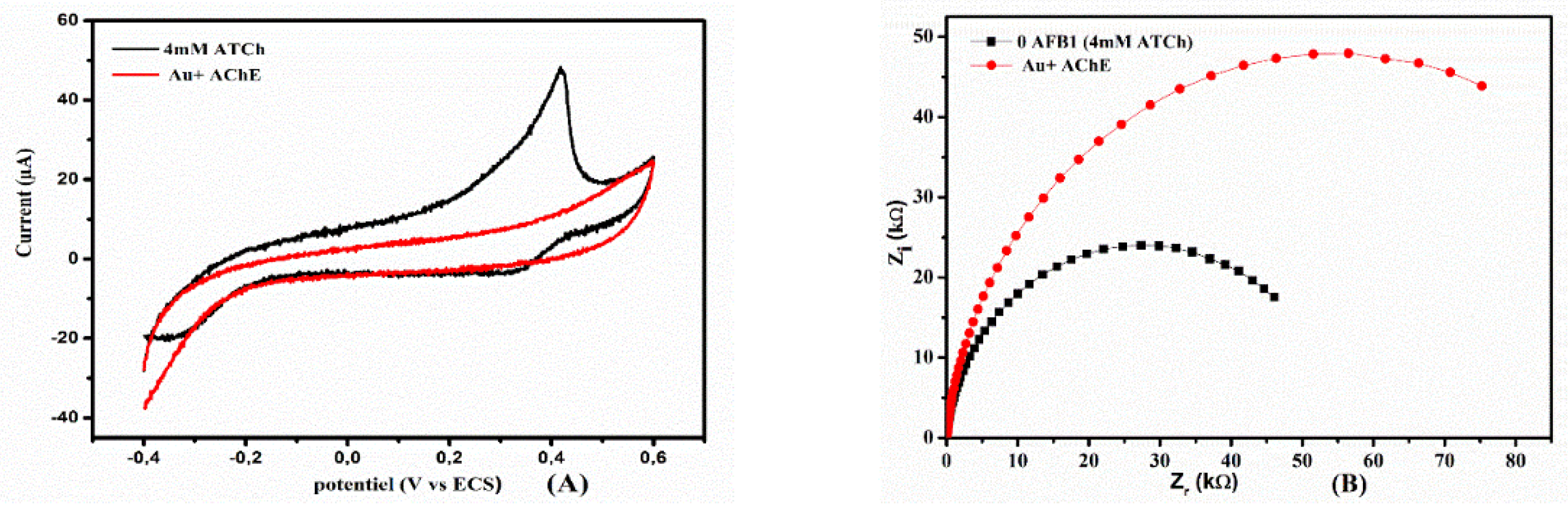
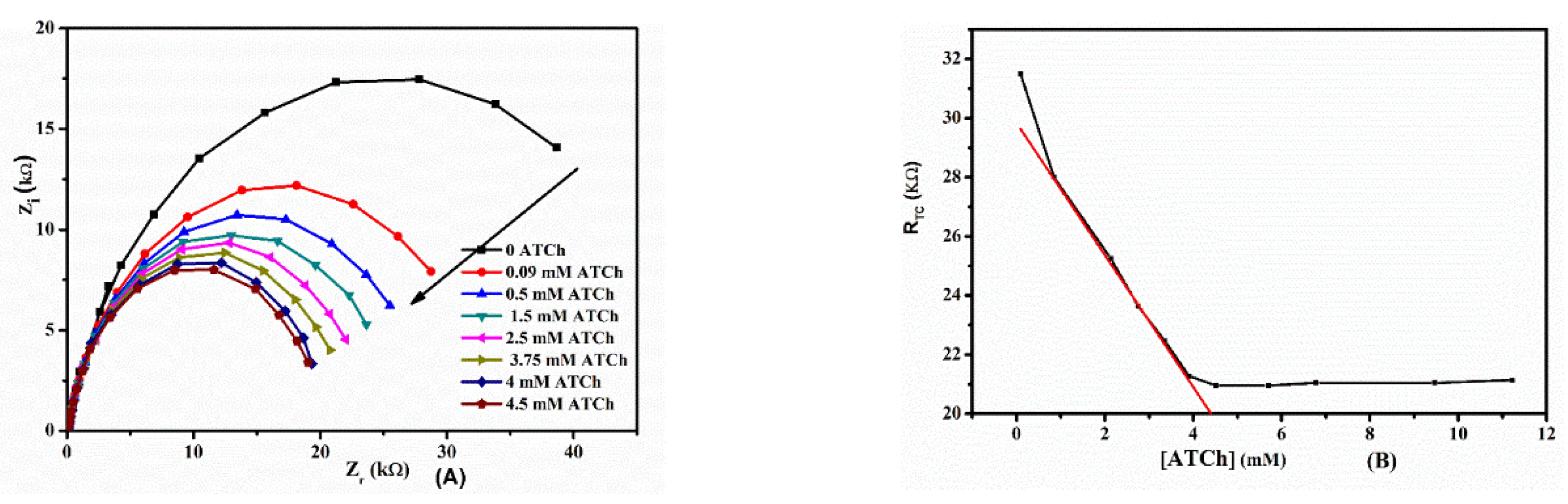
2.4. Electrochemical Behavior of ATCh at AChE/SA/Au Electrode
2.5. Determination of the AChE Activity
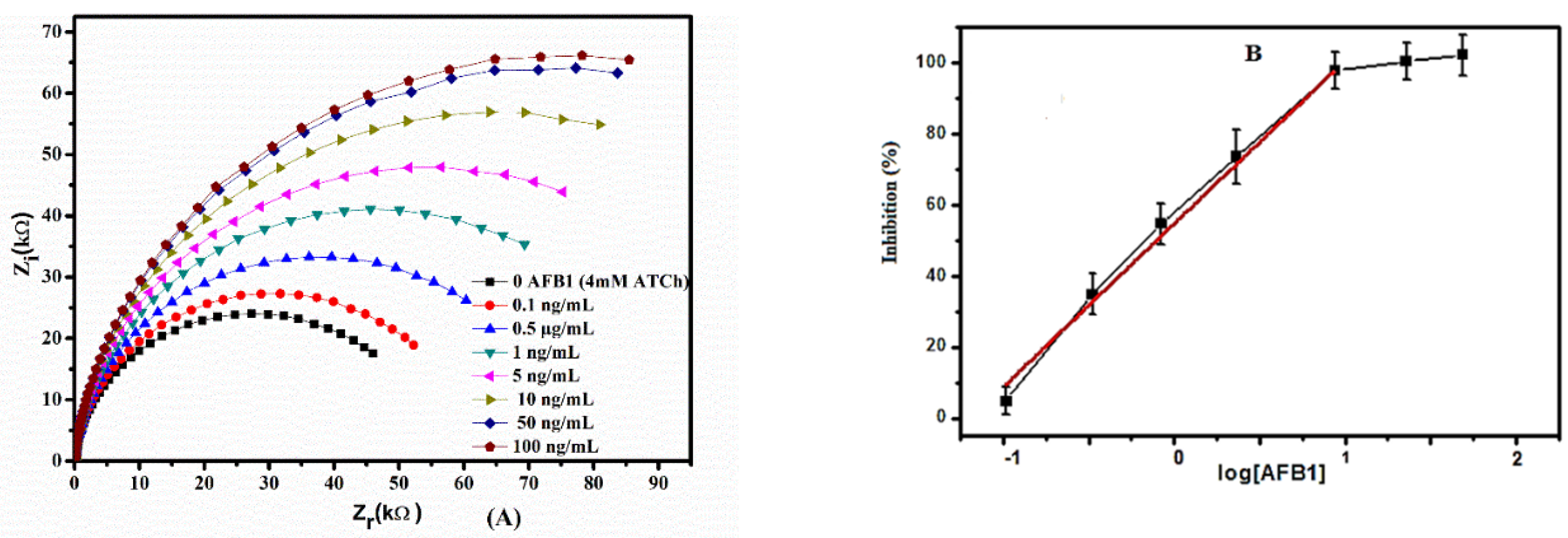
2.6. Calibration of AFB1 Biosensor
2.7. Reproductibility and Stability of the Biosensor
2.8. Specificity
2.9. Rice Sample Analysis
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Instrumention
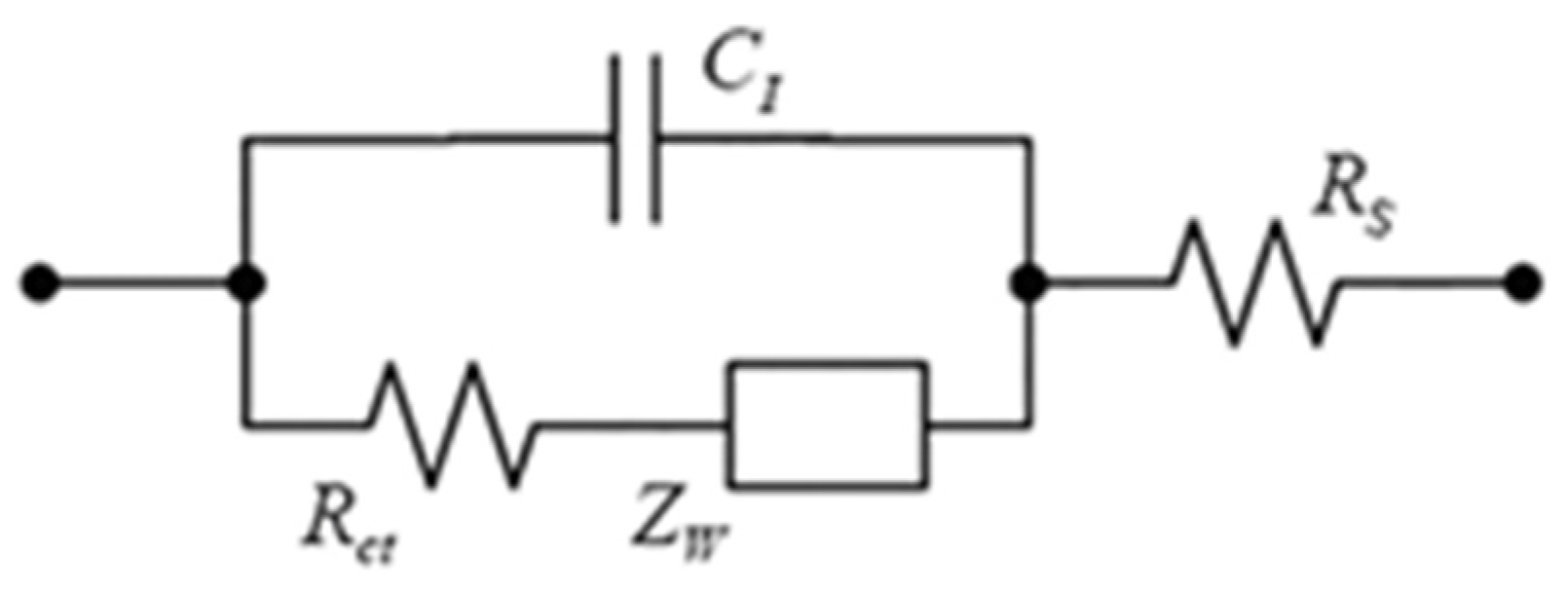
4.3. Electrochemical Characterizations
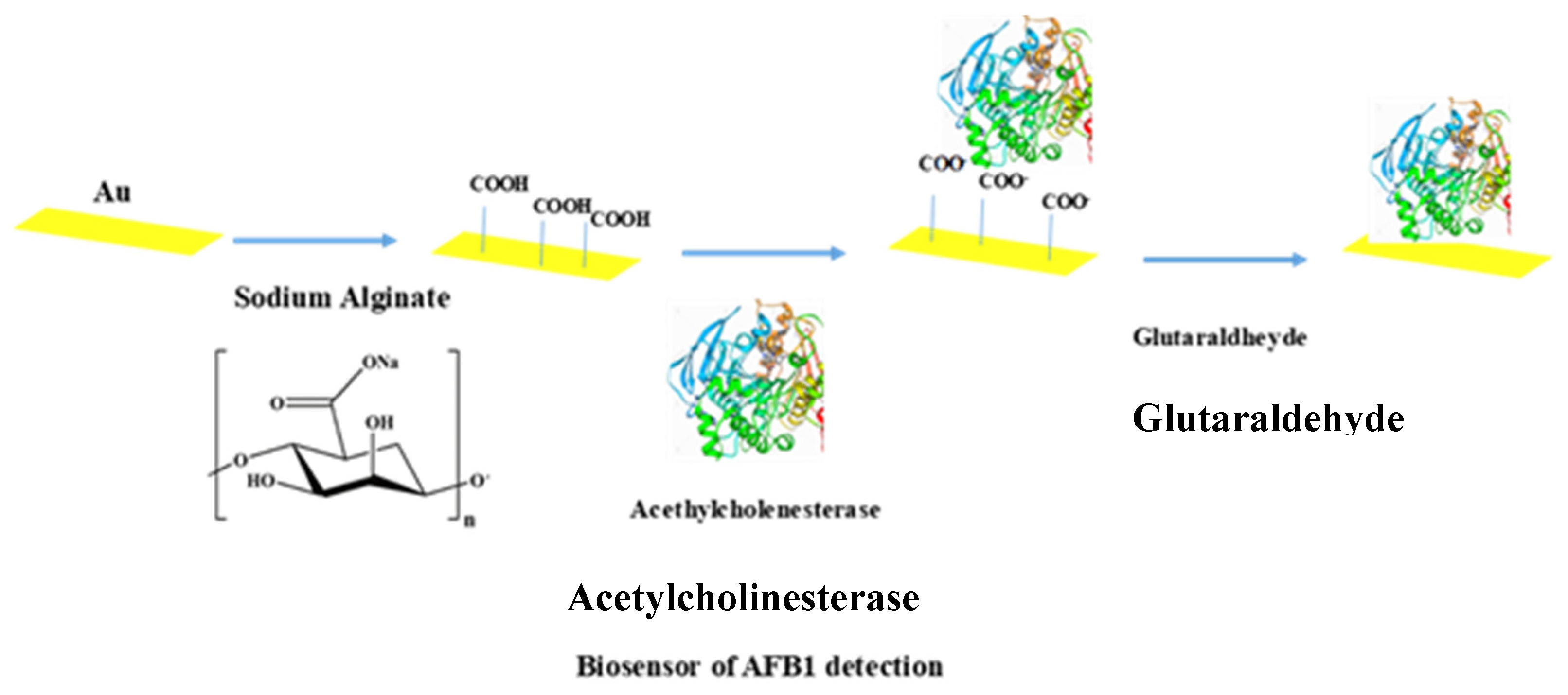
4.4. Development of the Sodium Alginate-Acetylcholinesterase-Based Biosensor
4.5. Immobilization of AChE via GA Cross-Linking
4.6. Fabrication of the Impedimetric Biosensor
4.7. Determination of AFB1 in Rice Samples
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Puiu, M.; Istrate, O.; Rotariu, L.; Bala, C. Kinetic approach of aflatoxin B1-acetylcholinesterase interaction: A tool for developing surface plasmonresonance biosensors. Anal. Biochem. 2012, 421, 587–594. [Google Scholar]
- Benedetti, S.; Iametti, S.; Bonomi, F.; Mannino, S. Head Space Sensor Array for the Detection of Aflatoxin M1 in Raw Ewe’s Milk. Food Protect. 2005, 68, 1089–1092. [Google Scholar]
- Pestka, J.J.; Gaur, P.K.; Chu, F.S. Quantitation of aflatoxin B1 and aflatoxin B1 antibody by an enzyme-linked immunosorbent microassay. Appl. Environ. Microb. 1980, 40, 1027–1031. [Google Scholar]
- Goudm, K.Y.; Sharmaa, A.; Hayata, A.; Catanantea, G.; Gobib, K.V.; Gurbana, A.M.; Marty, J.L. Tetramethyl-6-carboxyrhodamine quenching-based aptasensing platform for aflatoxin B1: Analytical performance comparison of two aptamers. Anal. Biochem. 2016, 508, 19–24. [Google Scholar]
- Abbes, S.; Ben Salah-Abbes, J.; Bouraoui, Y.; Oueslati, S.; Oueslati, R. Natural occurrence of aflatoxins (B1 and M1) in feed, plasma and raw milk of lactating dairy cows in Beja, Tunisia, using ELISA. Food Addit. Contam. Part. B 2012, 5, 11–15. [Google Scholar]
- Rahmani, A.; Jinap, S.; Soleimany, F. Qualitative and Quantitative Analysis of Mycotoxins. Compr. Rev. Food Sci. Food Saf. 2009, 8, 202–251. [Google Scholar]
- Zhanming, L.; Zunzhong, Y.; Yingchun, F.; Yonghua, X.; Li, Y. A portable electrochemical immunosensor for rapid detection of trace aflatoxin B1 in rice. Anal. Methods 2016, 8, 548–553. [Google Scholar]
- Stepurska, K.V.; Soldatkin, O.O.; Kucherenko, I.S.; Arkhypova, V.M.; Soldatkin, A.P.; Dzyadevych, S.V. Inhibitory analysis of toxic compounds of different nature. Anal. Chim. Acta 2015, 854, 161–168. [Google Scholar]
- Pundir, C.S.; Chauhan, N. Acetylcholinesterase inhibition-based biosensors for pesticide determination: A review. Anal. Biochem. 2012, 429, 19–31. [Google Scholar]
- Cometa, M.F.; Lorenzini, P.; Fortuna, S.; Volpe, M.T.; Meneguz, A.; Palmery, M. In vitro inhibitory effect of aflatoxin B1 on acetylcholinesterase activity in mouse brain. Toxicology 2005, 206, 125–135. [Google Scholar]
- Dhull, V.; Gahlaut, A.; Dilbaghi, N.; Hooda, V. Acetylcholinesterase Biosensors for Electrochemical Detection of Organophosphorus Compounds: A Review. Biochem. Res. Int. 2013, 731501, 1–18. [Google Scholar]
- Arduini, F.; Errico, I.; Amine, A.; Micheli, L.; Palleschi, G.; Moscone, D. Enzymatic Spectrophotometric Method for Aflatoxin B Detection Based on Acetylcholinesterase Inhibition. Anal. Chem. 2007, 79, 3409–3415. [Google Scholar]
- Hansmann, T.; Sanson, B.; Stojan, J.; Weik, M.; Marty, J.L.; Fournier, D. Kinetic insight into the mechanism of cholinesterasterase inhibition by aflatoxin B1 to develop biosensors. Biosens. Bioelectron. 2009, 24, 2119–2124. [Google Scholar]
- Ben Rejeb, I.; Arduini, F.; Arvinte, A.; Amine, A.; Gargouri, M.; Micheli, L.; Bala, C.; Moscone, D.; Palleschi, G. Development of a bio-electrochemical assay for AFB1 detection in olive oil. Biosens. Bioelectron. 2009, 24, 1962–1968. [Google Scholar]
- Arduini, F.; Neagu, D.; Pagliarini, V.; Scognamiglio, V.; Leonardis, M.A.; Gatto, E.; Amine, A.; Palleschi, G.; Moscone, D. Rapid and label-free detection of ochratoxin A and aflatoxin B1 using an optical portable instrument. Talanta 2016, 150, 440–448. [Google Scholar]
- Arduini, F.; Guidone, S.; Amine, A.; Palleschi, G.; Moscone, D. Acetylcholinesterase biosensor based on self-assembled monolayer-modified gold-screen printed electrodes for organophosphorus insecticide detection. Sens. Actuators B Chem. 2013, 179, 201–208. [Google Scholar]
- Min, W.; Wang, W.; Chen, J.; Wang, A.; Hu, Z. On-line immobilized acetylcholinesterase microreactor for screening of inhibitors from natural extracts by capillary electrophoresis. Anal. Bioanal. Chem. 2012, 404, 2397–2405. [Google Scholar]
- Ngomsik, A.F.; Bee, A.; Siaugue, J.M.; Talbot, D.; Cabil, V.; Cote, G. Co(II) removal by magnetic alginate beads containing Cyanex 272. J. Hazard. Materials. 2009, 166, 1043–1049. [Google Scholar]
- Zhao, H.Y.; Zheng, W.; Meng, Z.X.; Zhou, H.M.; Xu, X.X.; Li, Z.; Zheng, Y.F. Bioelectrochemistry of hemoglobin immobilized on a sodium alginate-multiwall carbon nanotubes composite film. Biosens Bioelectron. 2009, 24, 2352–2357. [Google Scholar]
- Johnson, F.A.; Craig, D.Q.M.; Mercer, A.D. Characterization of the Block Structure and Molecular Weight of Sodium Alginates. J. Pharm. Pharmacol. 1997, 49, 639–643. [Google Scholar]
- Orive, G.; Hermandez, R.M.; Gascon, A.R.; Calafiore, R.; Chang, T.M.; Hortelano, P.D.V.G.; Hunkeler, D.; Lacik, I.; Shapiro, A.M.; Pedraz, J.L. Cell encapsulation: Promise and progress. Nat. Med. 2003, 9, 1104. [Google Scholar]
- Munjal, N.; Sawhney, S.K. Stability and properties of mushroom tyrosinase entrapped in alginate, polyacrylamide and gelatin gels. Enzym. Microb. Technol. 2002, 30, 613–619. [Google Scholar]
- Ionescu, R.E.; Abu-Rabeah, K.; Cosnier, S.; Marks, R.S. Improved enzyme retention from an electropolymerized polypyrrole-alginate matrix in the development of biosensors. Electrochem. Commun. 2005, 7, 1277–1282. [Google Scholar]
- Cosnier, S.; Novoa, A.; Mousty, C.; Marks, R.S. Biotinylated alginate immobilization matrix in the construction of an amperometric biosensor: Application for the determination of glucose. Anal. Chim. Acta. 2002, 453, 71–79. [Google Scholar]
- Ding, C.F.; Zhang, M.L.; Zhao, F.; Zhang, S.S. Disposable biosensor and biocatalysis of horseradish peroxidase based on sodium alginate film and room temperature ionic liquid. Anal. Biochem. 2008, 378, 32–37. [Google Scholar]
- Sgobbi, L.F.; Razzino, C.A.; Rosset, I.G.; Burtoloso, A.C.B.; Machado, S.A.S. Electrochemistry and UV–vis spectroscopy of synthetic thiocholine: Revisiting the electro-oxidation mechanism. Electrochim. Acta 2013, 112, 500–504. [Google Scholar]
- Naseer, A.; Khan, A.Y. Electrochemical impedance spectroscopic studies of the passive layer on the surface of copper as a function of potential. Turk. J. Chem. 2010, 34, 815–824. [Google Scholar]
- Pohanka, M.; Kuca, K.; Jun, D. Aflatoxin Assay Using an Amperometric Sensor Strip and Acetylcholinesterase as Recognition Element. Sens. Lett. 2008, 6, 450–453. [Google Scholar]
- Tiwari, S.; Shishodia, S.K.; Shankar, J. Docking analysis of hexanoic acid and quercetin with seven domains of polyketide synthase A provided insight into quercetin-mediated aflatoxin biosynthesis inhibition in Aspergillus flavus. Biotech 2019, 9, 149. [Google Scholar]
- Burkin, M.A.; Yakovlev, I.V.; Sviridov, V.V. ELISA Determination of Aflatoxins Using Monoclonal Antibodies/Success in Medicinal Mycology. Natl. Acad. Mycol. 2003, 1, 127. [Google Scholar]
- Tan, Y.; Chu, X.; Shen, G.L.; Yu, R.Q. A signal-amplified electrochemical immunosensor for aflatoxin B1 determination in rice. Anal. Biochem. 2009, 387, 82–86. [Google Scholar]



| Methods | Sample Pretreatment | Assay Time | Detection Limit | Disadvantages | Benefits |
|---|---|---|---|---|---|
| Biosensor developed | easy | 10 min | (0.1 µg/kg) | Cross-reactivity with related mycotoxins | High sensitivity, long storage stability express analysis, low-cost, opportunity of outside laboratory analysis |
| HPLC [30] | very complex | 20 min | 0.007 µg/kg | Long-term sample pretreatment, impossibility of outside laboratory analysis, the use of toxic solvents, high-cost equipment and reagents | Simultaneous testing of several samples, high specificity |
| ELISA [31] | easy | 40 min | 0.25 µg/kg | Long-term analysis, impossibility of outside laboratory analysis, high-cost equipment and reagents | Simultaneous testing of several samples, high specificity |
| TLC [30] | complex | 60 min | 5 µg/kg | Low separation efficiency, low determination limit, use of toxic solvents | low-cost analysis |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrouda, A.; Zinoubi, K.; Soltane, R.; Alzahrani, N.; Osman, G.; Al-Ghamdi, Y.O.; Qari, S.; Al Mahri, A.; Algethami, F.K.; Majdoub, H.; et al. An Acetylcholinesterase Inhibition-Based Biosensor for Aflatoxin B1 Detection Using Sodium Alginate as an Immobilization Matrix. Toxins 2020, 12, 173. https://doi.org/10.3390/toxins12030173
Chrouda A, Zinoubi K, Soltane R, Alzahrani N, Osman G, Al-Ghamdi YO, Qari S, Al Mahri A, Algethami FK, Majdoub H, et al. An Acetylcholinesterase Inhibition-Based Biosensor for Aflatoxin B1 Detection Using Sodium Alginate as an Immobilization Matrix. Toxins. 2020; 12(3):173. https://doi.org/10.3390/toxins12030173
Chicago/Turabian StyleChrouda, Amani, Khouala Zinoubi, Raya Soltane, Noof Alzahrani, Gamal Osman, Youssef O. Al-Ghamdi, Sameer Qari, Albandary Al Mahri, Faisal K. Algethami, Hatem Majdoub, and et al. 2020. "An Acetylcholinesterase Inhibition-Based Biosensor for Aflatoxin B1 Detection Using Sodium Alginate as an Immobilization Matrix" Toxins 12, no. 3: 173. https://doi.org/10.3390/toxins12030173
APA StyleChrouda, A., Zinoubi, K., Soltane, R., Alzahrani, N., Osman, G., Al-Ghamdi, Y. O., Qari, S., Al Mahri, A., Algethami, F. K., Majdoub, H., & Jaffrezic Renault, N. (2020). An Acetylcholinesterase Inhibition-Based Biosensor for Aflatoxin B1 Detection Using Sodium Alginate as an Immobilization Matrix. Toxins, 12(3), 173. https://doi.org/10.3390/toxins12030173