Phytotoxic Metabolites Isolated from Neufusicoccum batangarum, the Causal Agent of the Scabby Canker of Cactus Pear (Opuntia ficus-indica L.)
Abstract
:1. Introduction
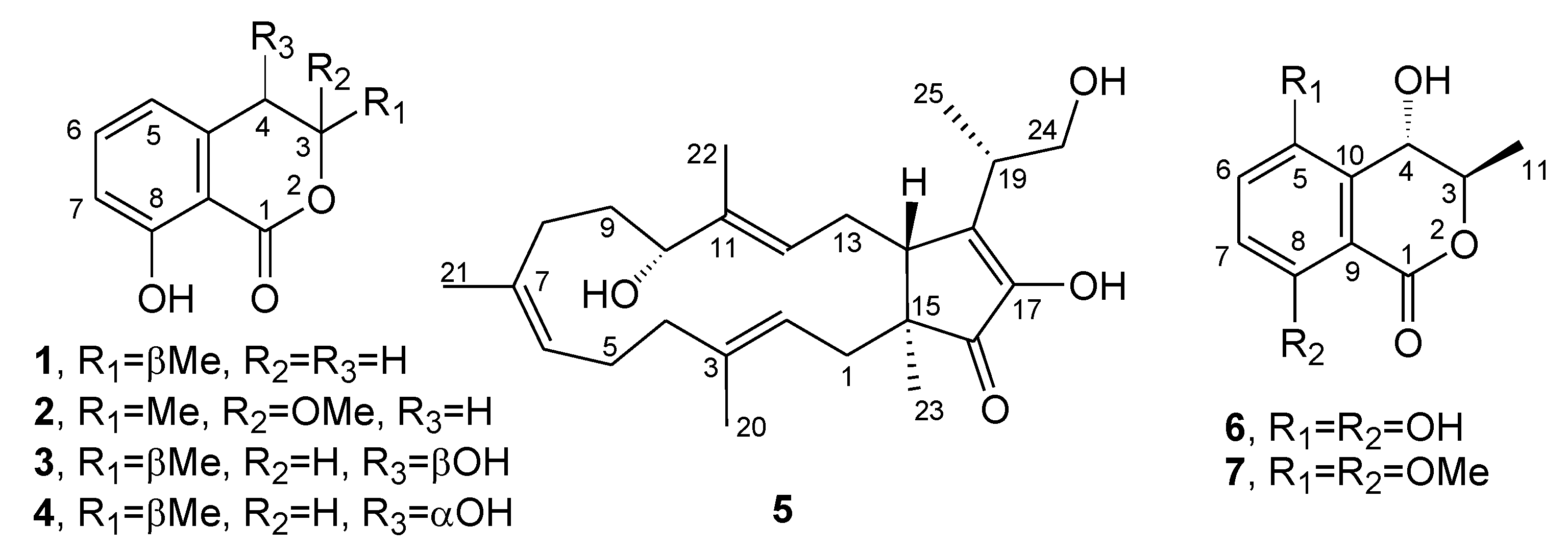
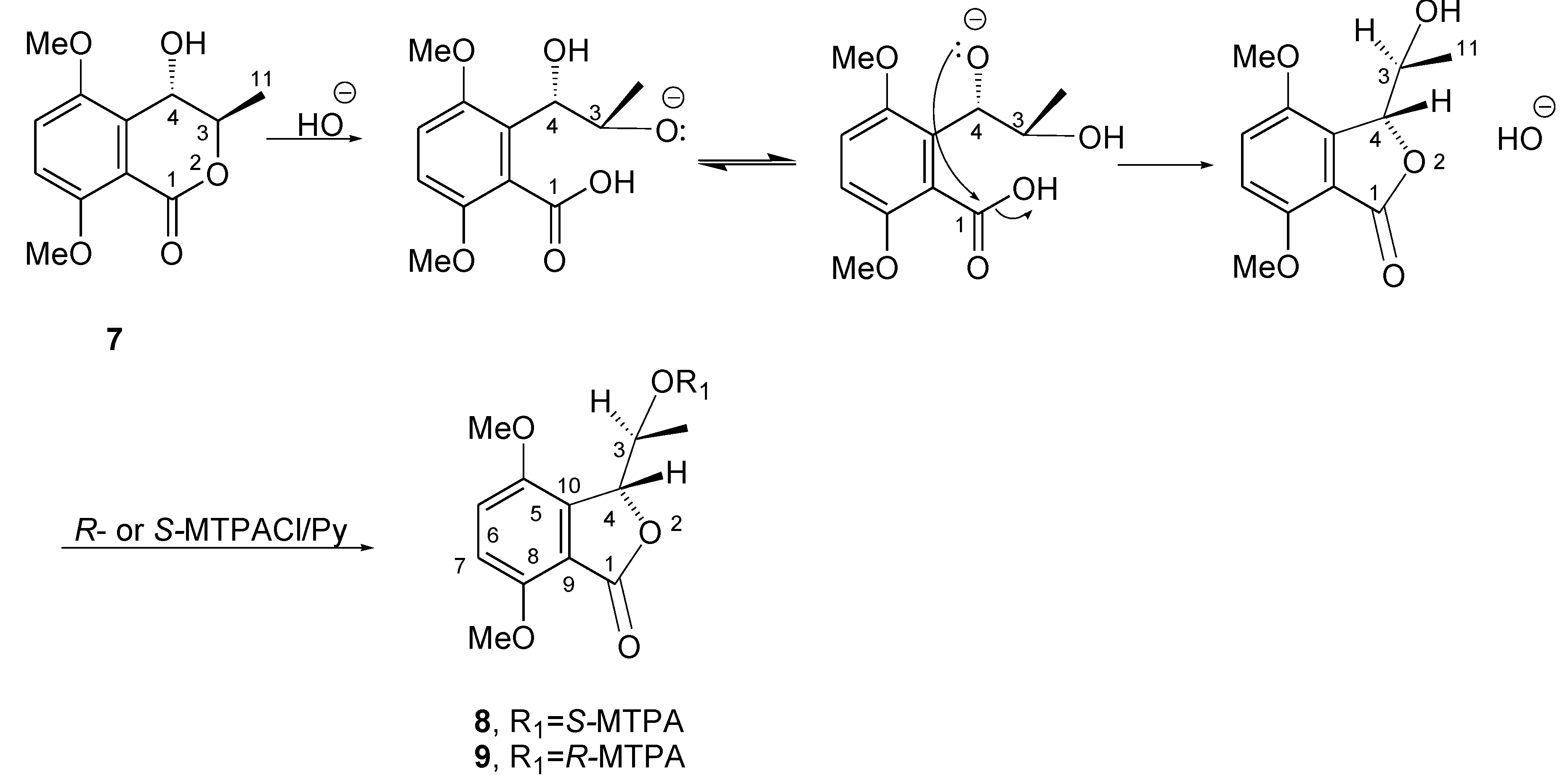
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Fungal Strain
4.3. Production, Extraction, and Isolation of Secondary Metabolites
4.4. Biological Assays
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kieling, R.; Metzing, D. Origin and Taxonomy of Opuntia ficus-indica. In Crop Ecology, Cultivation and User of Cactus Pear; Inglese, P., Mondragon, C., Nefzaoui, A., Saenz, C., Eds.; FAO and ICARDA: Rome, Italy, 2017; pp. 14–19. [Google Scholar]
- Ochoa, M.J.; Barbera, G. History and economic and agro-ecological importance. In Crop Ecology, Cultivation and Uses of Cactus Pear; Inglese, P., Mondragon, C., Nefzaoui, A., Saenz, C., Eds.; FAO and ICARDA: Rome, Italy, 2017; pp. 1–11. [Google Scholar]
- Schena, L.; Surico, G.; Burruano, S.; Giambra, S.; Pane, A.; Evoli, M.; Cacciola, S.O. First report of Neofusicoccum batangarum as causal agent of scabby cankers of Cactus pear (Opuntia ficus-indica) in minor islands of Sicily. Plant Dis. 2018, 102, 445. [Google Scholar] [CrossRef]
- Phillips, A.J.L.; Alves, A.; Abdollahzadeh, J.; Slippers, B.; Wingfield, M.J.; Groenewald, J.Z.; Crous, P.W. The Botryosphaeriaceae: Genera and species known from culture. Studies Mycol. 2013, 76, 51–167. [Google Scholar] [CrossRef] [Green Version]
- Conforto, C.; Lima, N.B.; Garcete-Gómez, J.M.; Câmara, M.P.S.; Michereff, S.J. First report of cladode brown spot in cactus prickly pear caused by Neofusicoccum batangarum in Brazil. Plant Dis. 2016, 100, 1238. [Google Scholar] [CrossRef]
- Netto, M.S.B.; Lima, W.G.; Correia, K.G.; Da Silva, C.F.B.; Thon, M.; Martins, R.B.; Miller, R.N.G.; Michereff, F.J.; Camâra, M.P.F. Analysis of phylogeny, distribution and pathogenicity of Botryosphaeriaceae species associated with gummosis of Anacardium in Brasil, with a new species of Lasiodipplodia. Fungal Biol. 2017, 121, 437–451. [Google Scholar] [CrossRef] [Green Version]
- Dissanayake, A.J.; Phillips, A.J.L.; Li, X.H.; Hyde, K.D. Botryosphaeriaceae: Current status of genera and species. Mycosphere 2016, 7, 1001–1073. [Google Scholar] [CrossRef]
- Garcete-Gómez, J.M.; Conforto, C.; Domínguez-Monge, S.; Flores-Sánchez, J.L.F.; Mora-Aguilera, G.; Michereff, S.J. Sample size for assessment of cladode brown spot in prickly pear cactus. Eur. J. Plant Pathol. 2017, 149, 759–763. [Google Scholar] [CrossRef]
- Conforto, C.; Lima, N.B.; Silva, F.J.A.; Câmara, M.P.S.; Maharachchikumbura, S.; Michereff, S.J. Characterization of fungal species associated with cladode brown spot on Nopalea cochenillifera in Brazil. Eur. J. Plant Pathol. 2019, 155, 1179–1194. [Google Scholar] [CrossRef]
- Feijo, F.M.; Silva, M.J.S.; Nascimento, A.D.; Infante, N.B.; Ramos-Sobrinho, R.; Assunção, I.P.; Lima, G.S.A. Botryosphaeriaceae species associated with the prickly pear cactus, Nopalea cochinellifera. Trop. Plant Pathol. 2019, 44, 452–459. [Google Scholar] [CrossRef]
- Dissanayake, A.J.; Camporesi, E.; Hyde, K.D.; Phillips, A.J.L.; Fu, C.Y.; Yan, J.Y.; Li, X.H. Dothiorella species associated with woody hosts in Italy. Mycosphere 2016, 7, 51–63. [Google Scholar] [CrossRef]
- Andolfi, A.; Maddau, L.; Cimmino, A.; Linaldeddu, B.T.; Basso, S.; Deidda, A.; Evidente, A. Lasiojasmonates A–C, three jasmonic acid esters produced by Lasiodiplodia sp., a grapevine pathogen. Phytochemistry 2014, 103, 145–153. [Google Scholar] [CrossRef]
- Cimmino, A.; Cinelli, T.; Masi, M.; Reveglia, P.; da Silva, M.A.; Mugnai, L.; Evidente, A. Phytotoxic lipophilic metabolites produced by grapevine strains of Lasiodiplodia species in Brazil. J. Agric. Food Chem. 2017, 65, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Reveglia, P.; Savocchia, S.; Billones-Baaijens, R.; Masi, M.; Cimmino, A.; Evidente, A. Phytotoxic metabolites by nine species of Botryosphaeriaceae involved in grapevine dieback in Australia and identification of those produced by Diplodia mutila, Diplodia seriata, Neofusicoccum australe and Neofusicoccum luteum. Nat. Prod. Res. 2019, 33, 2223–2229. [Google Scholar] [CrossRef]
- Masi, M.; Cimmino, A.; Reveglia, P.; Mugnai, L.; Surico, G.; Evidente, A. Advances on fungal phytotoxins and their role in grapevine trunk diseases. J. Agric. Food Chem. 2018, 66, 5948–5958. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, C.; Zheng, Z. A new 3,4-dihydroisocoumarin isolated from Botryosphaeria sp. F00741. Chem. Nat. Compd. 2012, 48, 205–207. [Google Scholar] [CrossRef] [Green Version]
- Cole, R.J.; Cox, R.H. Handbook of Toxic Fungal Metabolites; Academic Press: New York, NY, USA,, 1981; Volume 3, pp. 623–624.
- Cabras, A.; Mannoni, M.A.; Serra, S.; Andolfi, A.; Fiore, M.; Evidente, A. Occurrence, isolation and biological activity of phytotoxic metabolites produced in vitro by Sphaeropsis sapinea, pathogenic fungus of Pinus radiata. Eur. J. Plant Pathol. 2006, 115, 187–193. [Google Scholar] [CrossRef]
- Evidente, A.; Punzo, B.; Andolfi, A.; Cimmino, A.; Melck, D.; Luque, J. Lipophilic phytotoxins produced by Neofusicoccum parvum, a grapevine canker agent. Phytopathol. Mediterr. 2010, 49, 74–79. [Google Scholar]
- Abou-Mansour, E.; Débieux, J.L.; Ramírez-Suero, M.; Bénard-Gellon, M.; Magnin-Robert, M.; Spagnolo, A.; Serrano, M. Phytotoxic metabolites from Neofusicoccum parvum, a pathogen of Botryosphaeria dieback of grapevine. Phytochemistry 2015, 115, 207–215. [Google Scholar] [CrossRef]
- Djoukeng, J.D.; Polli, S.; Larignon, P.; Abou-Mansour, E. Identification of phytotoxins from Botryosphaeria obtusa, a pathogen of black dead arm disease of grapevine. Eur. J. Plant. Pathol. 2009, 124, 303–308. [Google Scholar] [CrossRef] [Green Version]
- Devys, M.; Barbier, M.; Bousquet, J.F.; Kollmann, A. Isolation of the new (−)-(3R, 4S)-4-hydroxymellein from the fungus Septoria nodorum Berk. Z. Naturforsch. C J. Biosci. 1992, 47, 779–781. [Google Scholar] [CrossRef]
- Oka, M.; Iimura, S.; Tenmyo, O.; Sawada, Y.; Sugawara, M.; Ohkusa, N.; Oki, T. Terpestacin, a new syncytium formation inhibitor from Arthrinium sp. J. Antibiot. 1993, 46, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Trost, B.M.; Dong, G.; Vance, J.A. A Diosphenol-based strategy for the total synthesis of (−)-terpestacin. J. Am. Chem. Soc. 2007, 129, 4540–4541. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Sarrocco, S.; Masi, M.; Diquattro, S.; Evidente, M.; Vannacci, G.; Evidente, A. Fusaproliferin, terpestacin and their derivatives display variable allelopathic activity against some Ascomycetous fungi. Chem. Biodivers. 2016, 13, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.X.; Chen, Y.; Huang, C.; She, Z.; Lin, Y. A new isochroman derivative from the marine fungus Phomopsis sp. (No. ZH-111). Chem. Nat. Compd. 2011, 47, 13. [Google Scholar] [CrossRef]
- Krohn, K.; Bahramsari, R.; Flörke, U.; Ludewig, K.; Kliche-Spory, C.; Michel, A.; Antus, S. Dihydroisocoumarins from fungi: Isolation, structure elucidation, circular dichroism and biological activity. Phytochemistry 1997, 45, 313–320. [Google Scholar] [CrossRef]
- Rukachaisirikul, V.; Arunpanichlert, J.; Sukpondma, Y.; Phongpaichit, S.; Sakayaroj, J. Metabolites from the endophytic fungi Botryosphaeria rhodina PSU-M35 and PSU-M114. Tetrahedron 2009, 65, 10590–10595. [Google Scholar] [CrossRef]
- Salazar, A.; Rios, I. Sustainable Agriculture: Technology, Planning and Management; Nova Science Publishers: New York, NY, USA, 2010. [Google Scholar]
- Ramírez-Suero, M.; Bénard-Gellon, M.; Chong, J.; Laloue, H.; Stempien, E.; Abou-Mansour, E.; Bertsch, C. Extracellular compounds produced by fungi associated with Botryosphaeria dieback induce differential defence gene expression patterns and necrosis in Vitis vinifera cv. Chardonnay cells. Protoplasma 2014, 251, 1417–1426. [Google Scholar] [CrossRef] [Green Version]
- Cimmino, A.; Maddau, L.; Masi, M.; Linaldeddu, B.T.; Evidente, A. Secondary metabolites produced by Sardiniella urbana, a new emerging pathogen on European hackberry. Nat. Prod. Res. 2019, 33, 1862–1869. [Google Scholar] [CrossRef]
- Bestmann, H.J.; Kern, F.; Schäfer, D.; Witschel, M.C. 3, 4-dihydroisocoumarins, a new class of ant trail pheromones. Angew. Chem. 1992, 31, 795–796. [Google Scholar] [CrossRef]
- Kern, F.; Klein, R.W.; Janssen, E.; Bestmann, H.J.; Attygalle, A.B.; Schäfer, D.; Maschwitz, U. Mellein, a trail pheromone component of the ant Lasius fuliginosus. J. Chem. Ecol. 1997, 23, 779–792. [Google Scholar] [CrossRef]
- Manigaunha, A.; Ganesh, N.; Kharya, M.D. Morning glory: A new thirst in-search of de-novo therapeutic approach. Int. J. Phytomed. 2010, 2, 18–21. [Google Scholar]
- Herzner, G.; Schlecht, A.; Dollhofer, V.; Parzefall, C.; Harrar, K.; Kreuzer, A.; Ruther, J. Larvae of the parasitoid wasp Ampulex compressa sanitize their host, the American cockroach, with a blend of antimicrobials. Proc. Natl. Acad. Sci. USA 2013, 110, 1369–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cimmino, A.; Maddau, L.; Masi, M.; Linaldeddu, B.T.; Pescitelli, G.; Evidente, A. Fraxitoxin, a new isochromanone isolated from Diplodia fraxini. Chem. Biodivers. 2017, 14, e1700325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masi, M.; Maddau, L.; Linaldeddu, B.T.; Scanu, B.; Evidente, A.; Cimmino, A. Bioactive metabolites from pathogenic and endophytic fungi of forest trees. Curr. Med. Chem. 2018, 25, 208–252. [Google Scholar] [CrossRef] [PubMed]
- Höller, U.; König, G.M.; Wright, A.D. Three new metabolites from marine-derived fungi of the genera Coniothyrium and Microsphaeropsis. J. Nat. Prod. 1999, 62, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.R.; Carté, B.K.; Sidebottom, P.J.; Sek Yew, A.L.; Ng, S.B.; Huang, Y.; Butler, M.S. Circumdatin G, a new alkaloid from the fungus Aspergillus ochraceus. J. Nat. Prod. 2001, 64, 125–126. [Google Scholar] [CrossRef]
- Zhao, J.H.; Zhang, Y.L.; Wang, L.W.; Wang, J.Y.; Zhang, C.L. Bioactive secondary metabolites from Nigrospora sp. LLGLM003, an endophytic fungus of the medicinal plant Moringa oleifera Lam. World J. Microbiol. Biotechnol. 2012, 28, 2107–2112. [Google Scholar] [CrossRef]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 2015, 32, 1629–1653. [Google Scholar] [CrossRef]
- Evidente, M.; Cimmino, A.; Zonno, M.C.; Masi, M.; Berestetskyi, A.; Santoro, E.; Evidente, A. Phytotoxins produced by Phoma chenopodiicola, a fungal pathogen of Chenopodium album. Phytochemistry 2015, 117, 482–488. [Google Scholar] [CrossRef]
- Ju, Z.; Lin, X.; Lu, X.; Tu, Z.; Wang, J.; Kaliyaperumal, K.; Liu, Y. Botryoisocoumarin A, a new COX-2 inhibitor from the mangrove Kandelia candel endophytic fungus Botryosphaeria sp. KcF6. J. Antibiot. 2015, 68, 653–656. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, X.M.; Meng, L.H.; Wang, B.G. Polyketides from the marine mangrove-derived fungus Aspergillus ochraceus MA-15 and their activity against aquatic pathogenic bacteria. Phytochem. Lett. 2015, 12, 232–236. [Google Scholar] [CrossRef]
- Fredimoses, M.; Zhou, X.; Ai, W.; Tian, X.; Yang, B.; Lin, X.; Liu, Y. Westerdijkin A, a new hydroxyphenylacetic acid derivative from deep sea fungus Aspergillus westerdijkiae SCSIO 05233. Nat. Prod. Res. 2015, 29, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, X.M.; Li, C.S.; Wang, B.G. Sesterterpenes and 2H-pyran-2-ones (= α-pyrones) from the mangrove-derived endophytic fungus Fusarium proliferatum MA84. Helv. Chim. Acta 2013, 96, 437–444. [Google Scholar] [CrossRef]
- Masi, M.; Meyer, S.; Górecki, M.; Pescitelli, G.; Clement, S.; Cimmino, A.; Evidente, A. Phytotoxic activity of metabolites isolated from Rutstroemia sp. n., the causal agent of bleach blonde syndrome on cheatgrass (Bromus tectorum). Molecules 2018, 23, 1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Application of Mosher’s method for absolute configuration assignment to bioactive plants and fungi metabolites. J. Pharm. Biomed. 2017, 144, 59–89. [Google Scholar] [CrossRef]
- Shetty, K.G.; Minnis, A.M.; Rossman, A.Y.; Jayachandran, K. The Brazilian peppertree seedborne pathogen, Neofusicoccum batangarum. Biol. Control 2011, 56, 91–97. [Google Scholar] [CrossRef]
- Dawkins, K.; Esiobu, N. Emerging insights on Brazilian pepper tree (Schinus terebinthifolius) invasion: The potential role of soil microorganisms. Front. Plant Sci. 2016, 7, 712. [Google Scholar] [CrossRef] [Green Version]
- Masi, M.; Nocera, P.; Reveglia, P.; Cimmino, A.; Evidente, A. Fungal metabolites antagonists towards plant pests and human pathogens: Structure-activity relationship studies. Molecules 2018, 23, 834. [Google Scholar] [CrossRef] [Green Version]




| Position | 8 | 9 |
|---|---|---|
| δH (J in Hz) | δH (J in Hz) | |
| 3 | 5.960 (1H) dq (2.2, 6.6) | 5.958 (1H) dq (2.2, 6.6) |
| 4 | 5.694 (1H) d (2.2) | 5.829 (1H) d (2.2) |
| 6 | 7.361 (1H) d (8.9) | 7.368 (1H) d (8.9) |
| 7 | 7.129 (1H) d (8.9) | 7.141 (1H) d (8.9) |
| Me-C3O Me 2 OMe 2 OMe | 1.092 (3H) d (6.6) 3.950(3H) s 3.888 (3H) s 3.574 (3H) s | 0.982 (3H) d (6.6) 3.955 (3H) s 3.896 (3H) s 3.626 (3H) s |
| Ph | 7.585–7.485 (5H) m | 7.584–7.481 (5H) m |
| Compound | Concentration (M) | Mean Lesion Area (mm2) 1 | Compound | Concentration (M) | Mean Lesion Area (mm2) 1 |
|---|---|---|---|---|---|
| 1 | 5.6 × 10−3 | 2.75 ± 0.68 | 4 | 5.2 × 10−3 | 3.14 ± 0.00 |
| 2.8 × 10−3 | 2.36 ± 0.79 | 2.6 × 10−3 | 2.75 ± 0.68 | ||
| 1.4 × 10−3 | 0.00 ± 0.00 | 1.3 × 10−3 | 0.00 ± 0.00 | ||
| 5.6 × 10−4 | 0.00 ± 0.00 | 5.2 × 10−4 | 0.00 ± 0.00 | ||
| 2.8 × 10−4 | 0.00 ± 0.00 | 2.6 × 10−4 | 0.00 ± 0.00 | ||
| 2 | 4.8 × 10−3 | 7.98 ± 1.28 | 5 | 2.5 × 10−3 | 5.10 ± 1.29 |
| 2.4 × 10−3 | 7.46 ± 1.5 | 1.2 × 10−3 | 5.50 ± 1.04 | ||
| 1.2 × 10−3 | 6.67 ± 1.5 | 6.2 × 10−4 | 4.81 ± 1.17 | ||
| 4.8 × 10−4 | 6.80 ± 1.71 | 2.4 × 10−4 | 4.81 ± 1.3 | ||
| 2.4 × 10−4 | 6.28 ± 1.03 | 1.2 × 10−4 | 4.61 ± 1.6 | ||
| 3 | 5.2 × 10−3 | 3.14 ± 0.00 | 6 | 4.8 × 10−3 | 7.98 ± 1.28 |
| 2.6 × 10−3 | 2.36 ± 1.36 | 2.4 × 10−3 | 7.07 ± 1.26 | ||
| 1.3 × 10−3 | 0.00 ± 0.00 | 1.2 × 10−3 | 6.67 ± 0.81 | ||
| 5.2 × 10−4 | 0.00 ± 0.00 | 4.8 × 10−4 | 4.97 ± 1.55 | ||
| 2.6 × 10−4 | 0.00 ± 0.00 | 2.4 × 10−4 | 5.36 ± 1.65 |
| Compound | Concentration (M) | Mean Lesion Area (mm2) 1 | Compound | Concentration (M) | Mean Lesion Area (mm2) 1 |
|---|---|---|---|---|---|
| 1 | 5.6 × 10−3 | 3.14 ± 0.00 | 4 | 5.2 × 10−3 | 6.28 ± 1.56 |
| 2.8 × 10−3 | 2.36 ± 0.79 | 2.6 × 10−3 | 2.36 ± 0.79 | ||
| 1.4 × 10−3 | 2.75 ± 0.68 | 1.3 × 10−3 | 3.14 ± 0.00 | ||
| 5.6 × 10−4 | 0.00 ± 0.00 | 5.2 × 10−4 | 0.00 ± 0.00 | ||
| 2.8 × 10−4 | 0.00 ± 0.00 | 2.6 × 10−4 | 0.00 ± 0.00 | ||
| 2 | 4.8 × 10−3 | 11.00 ± 1.57 | 5 | 2.5 × 10−3 | 11.58 ± 1.57 |
| 2.4 × 10−3 | 9.81 ± 1.75 | 1.2 × 10−3 | 7.07 ± 1.67 | ||
| 1.2 × 10−3 | 11.78 ± 1.36 | 6.2 × 10−4 | 6.28 ± 1.32 | ||
| 4.8 × 10−4 | 5.50 ± 0.79 | 2.4 × 10−4 | 3.53 ± 0.68 | ||
| 2.4 × 10−4 | 7.07 ± 0.00 | 1.2 × 10−4 | 2.94 ± 1.40 | ||
| 3 | 5.2 × 10−3 | 3.14 ± 0.00 | 6 | 4.8 × 10−3 | 11.78 ± 1.60 |
| 2.6 × 10−3 | 2.61 ± 0.93 | 2.4 × 10−3 | 9.62 ± 1.55 | ||
| 1.3 × 10−3 | 3.14 ± 0.00 | 1.2 × 10−3 | 9.62 ± 1.55 | ||
| 5.2 × 10−4 | 0.00 ± 0.00 | 4.8 × 10−4 | 4.51 ± 1.61 | ||
| 2.6 × 10−4 | 0.00 ± 0.00 | 2.4 × 10−4 | 3.50 ± 0.68 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masi, M.; Aloi, F.; Nocera, P.; Cacciola, S.O.; Surico, G.; Evidente, A. Phytotoxic Metabolites Isolated from Neufusicoccum batangarum, the Causal Agent of the Scabby Canker of Cactus Pear (Opuntia ficus-indica L.). Toxins 2020, 12, 126. https://doi.org/10.3390/toxins12020126
Masi M, Aloi F, Nocera P, Cacciola SO, Surico G, Evidente A. Phytotoxic Metabolites Isolated from Neufusicoccum batangarum, the Causal Agent of the Scabby Canker of Cactus Pear (Opuntia ficus-indica L.). Toxins. 2020; 12(2):126. https://doi.org/10.3390/toxins12020126
Chicago/Turabian StyleMasi, Marco, Francesco Aloi, Paola Nocera, Santa Olga Cacciola, Giuseppe Surico, and Antonio Evidente. 2020. "Phytotoxic Metabolites Isolated from Neufusicoccum batangarum, the Causal Agent of the Scabby Canker of Cactus Pear (Opuntia ficus-indica L.)" Toxins 12, no. 2: 126. https://doi.org/10.3390/toxins12020126
APA StyleMasi, M., Aloi, F., Nocera, P., Cacciola, S. O., Surico, G., & Evidente, A. (2020). Phytotoxic Metabolites Isolated from Neufusicoccum batangarum, the Causal Agent of the Scabby Canker of Cactus Pear (Opuntia ficus-indica L.). Toxins, 12(2), 126. https://doi.org/10.3390/toxins12020126









