Tolerability and Efficacy of Customized IncobotulinumtoxinA Injections for Essential Tremor: A Randomized, Double-Blind, Placebo-Controlled Study
Abstract
1. Introduction
2. Results
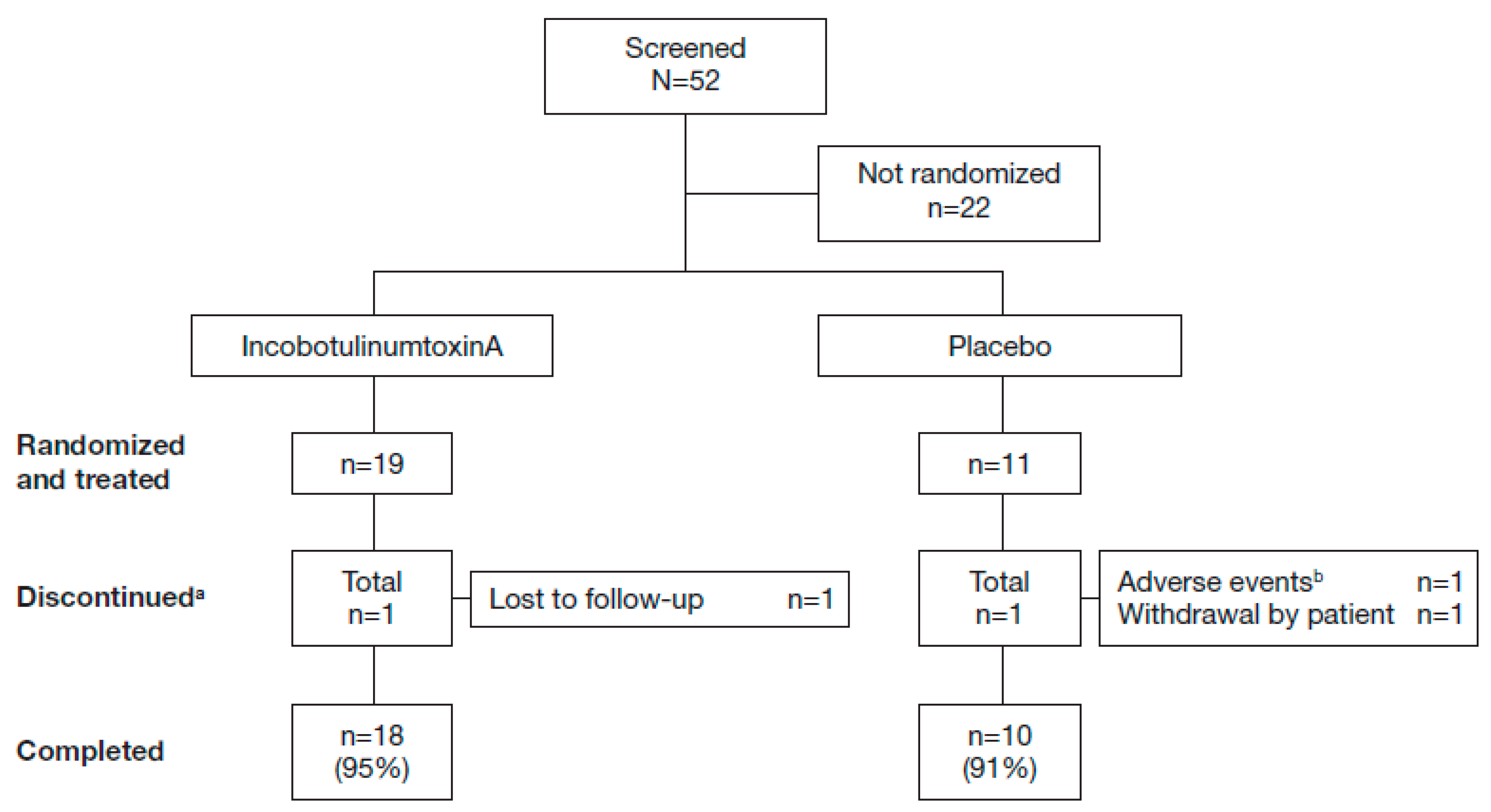
2.1. Study Population
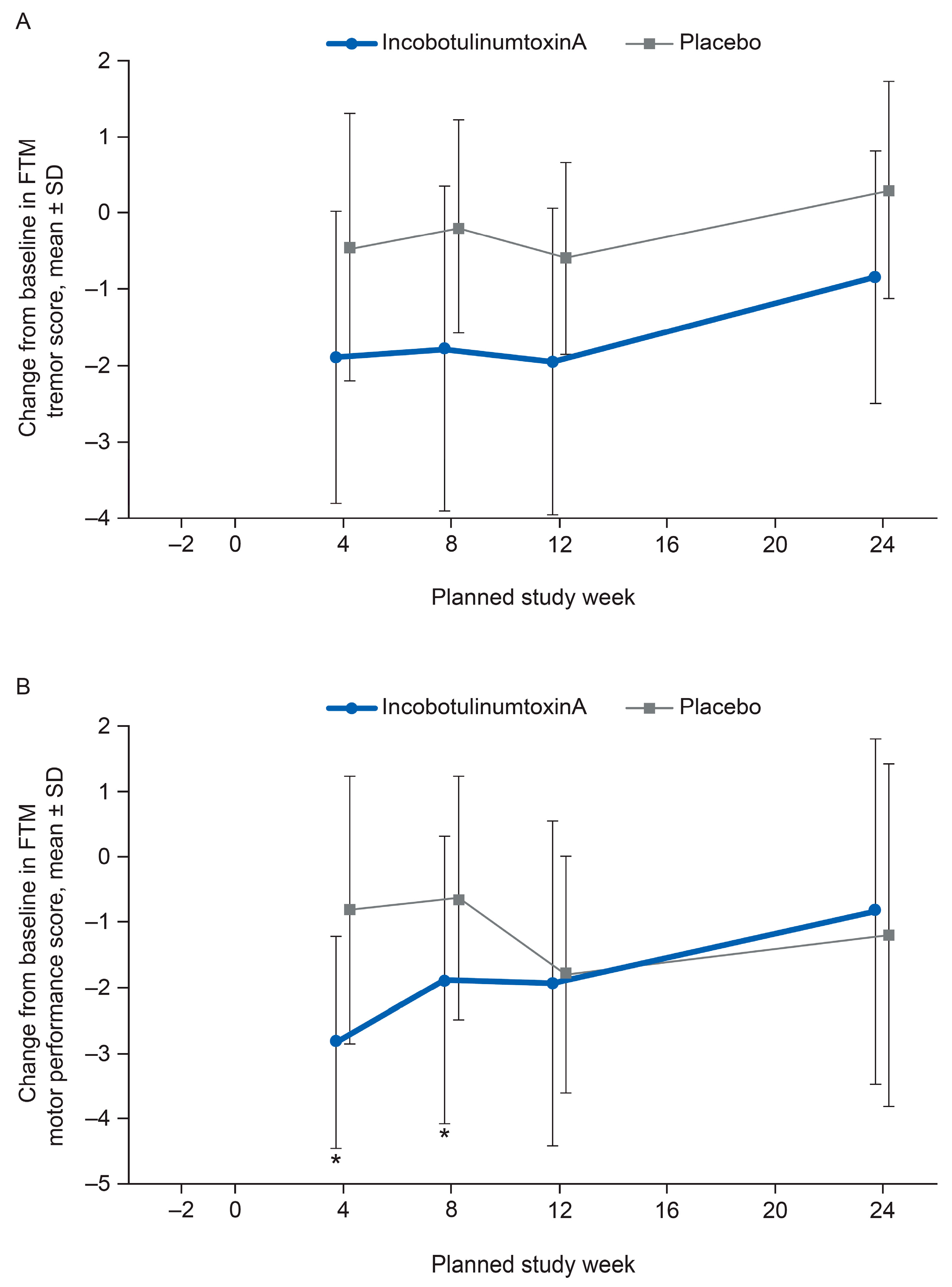
2.2. FTM Tremor Rating and Motor Performance
2.3. Global Impression of Change
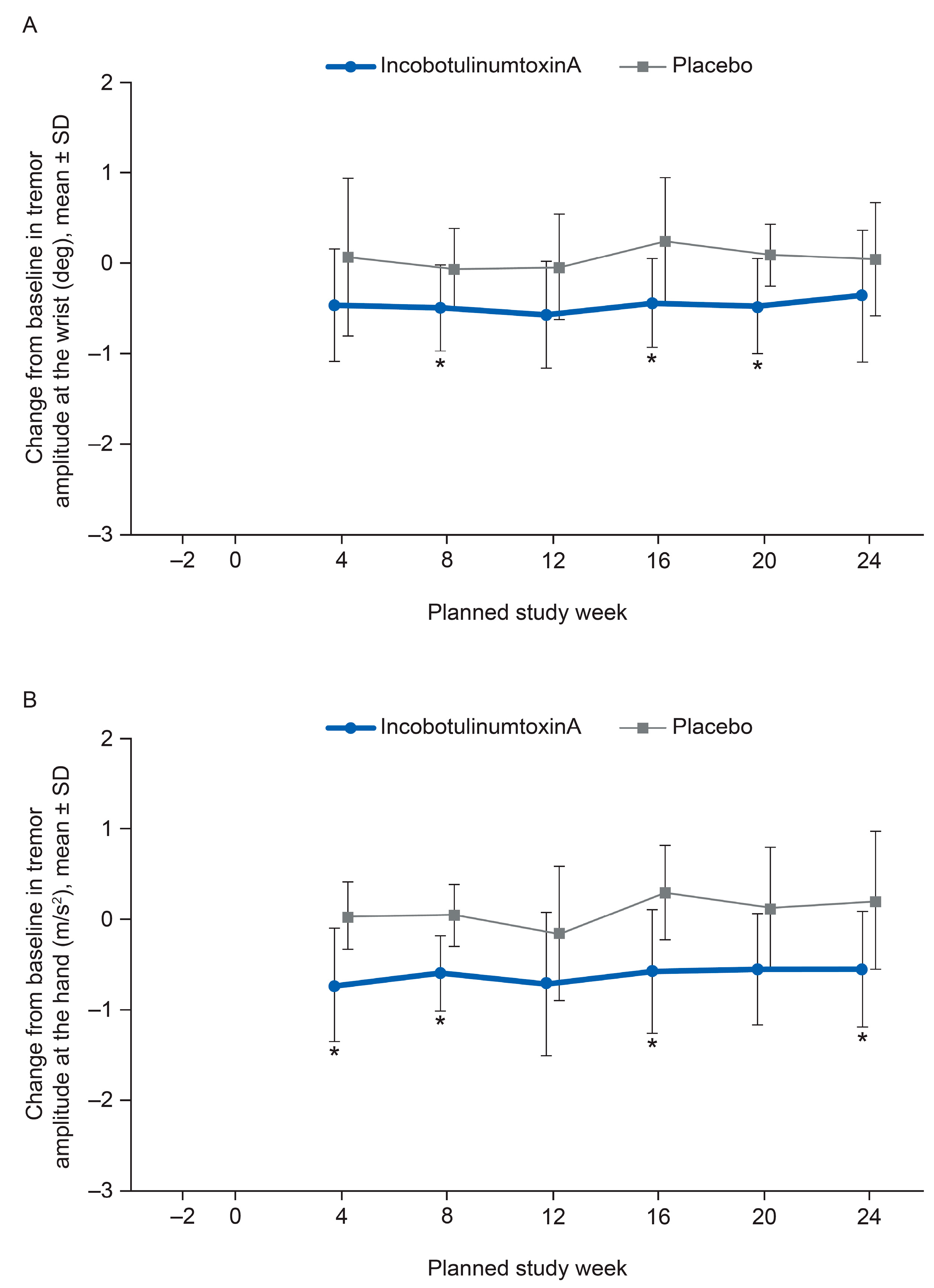
2.4. Kinematic Tremor Analysis
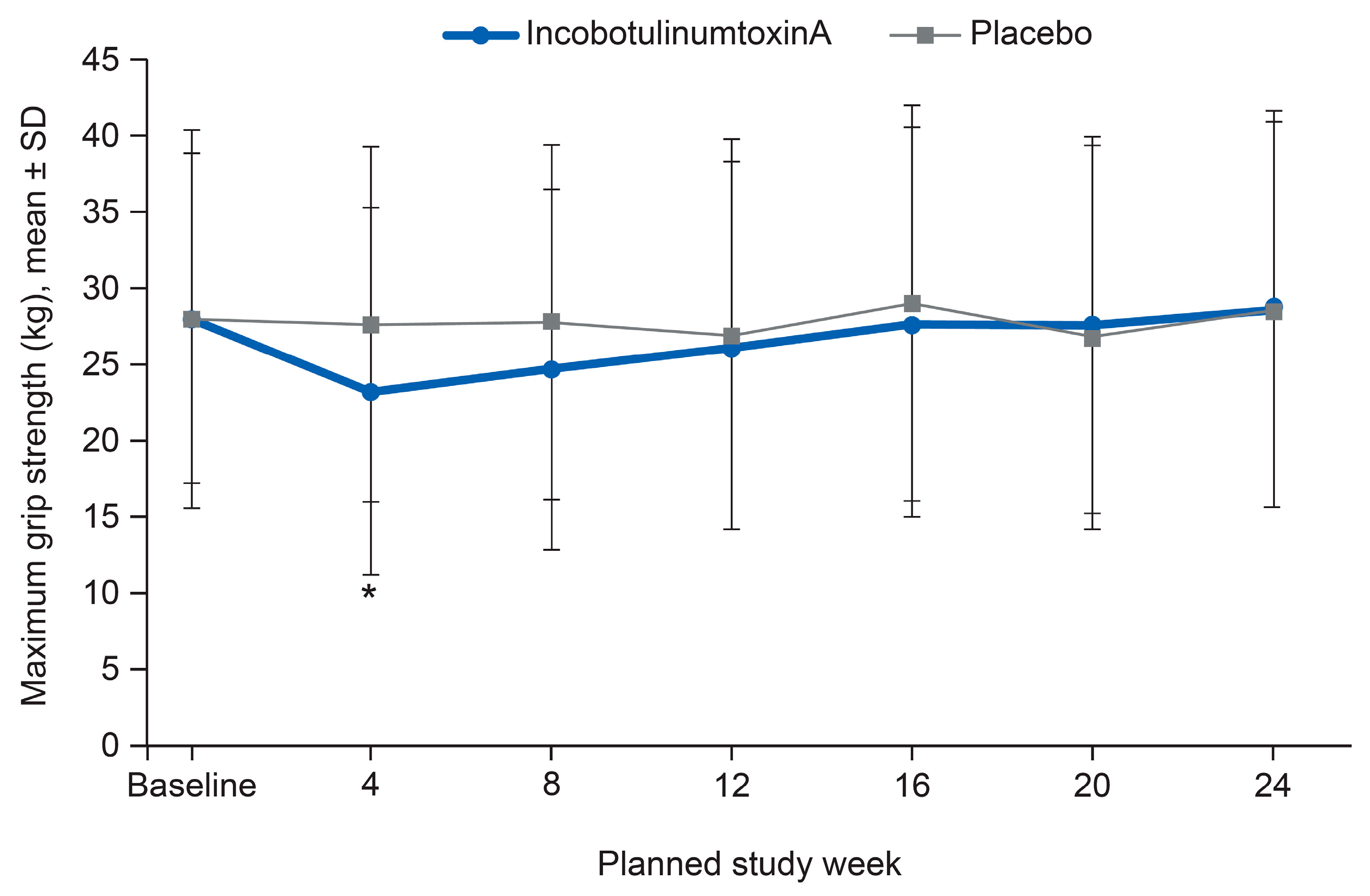
2.5. Safety Outcomes
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Design and Patients
5.2. Treatment
5.3. Efficacy Assessments
5.4. Kinematic Tremor Analysis
5.4.1. FTM Tremor Rating Scale
5.4.2. Global Impression of Change
5.5. Safety Assessments
5.5.1. Adverse Events
5.5.2. Other Safety Assessments
5.6. Statistical Analysis
5.7. Standard Protocol Approvals, Registrations, and Patient Consents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Sharing Statement
Abbreviations
| ADL | Activities of daily living |
| ANCOVA | Analysis of covariance |
| BoNT-A | Botulinum neurotoxin type A |
| CI | Confidence interval |
| ET | Essential tremor |
| FAS | Full analysis set |
| FTM | Fahn–Tolosa–Marin |
| GICS | Global Impression of Change Scale |
| IRB | Institutional review board |
| LS | Least squares |
| MMRM | Mixed model repeated measures |
| MRC | Medical Research Council |
| MRC MMT | Medical Research Council manual muscle testing |
| RMS | Root mean square |
| SD | Standard deviation |
| TEAE | Treatment-emergent adverse event |
| U | Unit |
References
- Louis, E.D.; Ferreira, J.J. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov. Disord. 2010, 25, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Hedera, P.; Cibulcik, F.; Davis, T.L. Pharmacotherapy of essential tremor. J. Cent. Nerv. Syst. Dis. 2013, 5, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.D.; Gerbin, M.; Galecki, M. Essential tremor 10, 20, 30, 40: Clinical snapshots of the disease by decade of duration. Eur. J. Neurol. 2013, 20, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, K.P.; Bain, P.; Bajaj, N.; Elble, R.J.; Hallett, M.; Louis, E.D.; Raethjen, J.; Stamelou, M.; Testa, C.M.; Deuschl, G.; et al. Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov. Disord. 2018, 33, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.H.; Rajput, A. Medical treatment of essential tremor. J. Cent. Nerv. Syst. Dis. 2014, 6, 29–39. [Google Scholar] [CrossRef]
- Koller, W.C.; Vetere-Overfield, B. Acute and chronic effects of propranolol and primidone in essential tremor. Neurology 1989, 39, 1587–1588. [Google Scholar] [CrossRef]
- Brin, M.F.; Lyons, K.E.; Doucette, J.; Adler, C.H.; Caviness, J.N.; Comella, C.L.; Dubinsky, R.M.; Friedman, J.H.; Manyam, B.V.; Matsumoto, J.Y.; et al. A randomized, double masked, controlled trial of botulinum toxin type A in essential hand tremor. Neurology 2001, 56, 1523–1528. [Google Scholar] [CrossRef]
- Jankovic, J.; Schwartz, K.; Clemence, W.; Aswad, A.; Mordaunt, J. A randomized, double-blind, placebo-controlled study to evaluate botulinum toxin type A in essential hand tremor. Mov. Disord. 1996, 11, 250–256. [Google Scholar] [CrossRef]
- Mittal, S.O.; Machado, D.; Richardson, D.; Dubey, D.; Jabbari, B. Botulinum toxin in essential hand tremor—A randomized double-blind placebo-controlled study with customized injection approach. Parkinsonism Relat. Disord. 2018, 56, 65–69. [Google Scholar] [CrossRef]
- Kamel, J.T.; Cordivari, C.; Catania, S. Treatment of Upper Limb Tremor With Botulinum Toxin: An Individualized Approach. Mov. Disord. Clin. Pract. 2019, 6, 652–655. [Google Scholar] [CrossRef]
- Kreisler, A.; Bouchain, B.; Defebvre, L.; Krystkowiak, P. Treatment with Botulinum Neurotoxin Improves Activities of Daily Living and Quality of Life in Patients with Upper Limb Tremor. Tremor Other Hyperkinet. Mov. 2019, 9. [Google Scholar] [CrossRef]
- Samotus, O.; Lee, J.; Jog, M. Personalized Bilateral Upper Limb Essential Tremor Therapy with Botulinum Toxin Using Kinematics. Toxins 2019, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, F.; Bee, C.; Debicki, D.; Roberts, A.C.; Bapat, P.; Jog, M. Effectiveness of BoNT A in Parkinson’s disease upper limb tremor management. Can. J. Neurol. Sci. 2013, 40, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Casellato, C.; Zorzi, G.; Pedrocchi, A.; Ferrigno, G.; Nardocci, N. Reaching and writing movements: Sensitive and reliable tools to measure genetic dystonia in children. J. Child Neurol. 2011, 26, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Supuk, T.; Bajd, T.; Kurillo, G. Assessment of reach-to-grasp trajectories toward stationary objects. Clin. Biomech. 2011, 26, 811–818. [Google Scholar] [CrossRef]
- Rahimi, F.; Samotus, O.; Lee, J.; Jog, M. Effective management of upper limb parkinsonian tremor by incobotulinumtoxinA injections using sensor-based biomechanical patterns. Tremor Other Hyperkinet. Mov. 2015, 5, 348. [Google Scholar] [CrossRef]
- Samotus, O.; Rahimi, F.; Lee, J.; Jog, M. Functional ability improved in essential tremor by incobotulinumtoxinA injections using kinematically determined biomechanical patterns—A new future. PLoS ONE 2016, 11, e0153739. [Google Scholar] [CrossRef]
- Samotus, O.; Lee, J.; Jog, M. Long-term tremor therapy for Parkinson and essential tremor with sensor-guided botulinum toxin type A injections. PLoS ONE 2017, 12, e0178670. [Google Scholar] [CrossRef]
- Fahn, S.; Tolosa, E.; Marin, C. Clinical Rating Scale for Tremor. In Parkinson’s Disease and Movement Disorders; Jankovic, J., Tolosa, E., Eds.; Urban & Schwarzenberg: Baltimore-Münich, WA, USA, 1988; pp. 225–234. [Google Scholar]
- Zesiewicz, T.A.; Elble, R.J.; Louis, E.D.; Gronseth, G.S.; Ondo, W.G.; Dewey, R.B., Jr.; Okun, M.S.; Sullivan, K.L.; Weiner, W.J. Evidence-based guideline update: Treatment of essential tremor: Report of the Quality Standards subcommittee of the American Academy of Neurology. Neurology 2011, 77, 1752–1755. [Google Scholar] [CrossRef]
- Louis, E.D. From neurons to neuron neighborhoods: The rewiring of the cerebellar cortex in essential tremor. Cerebellum 2014, 13, 501–512. [Google Scholar] [CrossRef]
- Simpson, D.M.; Blitzer, A.; Brashear, A.; Comella, C.; Dubinsky, R.; Hallett, M.; Jankovic, J.; Karp, B.; Ludlow, C.L.; Miyasaki, J.M.; et al. Assessment: Botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2008, 70, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Deuschl, G.; Bain, P.; Brin, M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov. Disord. 1998, 13 (Suppl. 3), 2–23. [Google Scholar] [CrossRef]
- Jost, W. Pictorial Atlas of Botulinum Toxin Injection: Dosage, Localization, Application; Quintessence Publishing Company: New Malden, UK, 2008. [Google Scholar]
- Likert, R. A technique for the measurement of attitudes. Arch. Psychol. 1932, 140, 55. [Google Scholar]
| Characteristic | IncobotulinumtoxinA (n = 19) | Placebo (n = 11) | Total (n = 30) |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 10 (52.6) | 5 (45.5) | 15 (50.0) |
| Male | 9 (47.4) | 6 (54.5) | 15 (50.0) |
| Age, years | |||
| Mean (SD) | 68.1 (10.6) | 68.2 (10.2) | 68.1 (10.3) |
| Min, max | 41, 88 | 49, 85 | 41, 88 |
| Ethnic origin, n (%) | |||
| White | 17 (89.5) | 11 (100.0) | 28 (93.3) |
| Black or African American | 1 (5.3) | 0 (0.0) | 1 (3.3) |
| Other | 1 (5.3) | 0 (0.0) | 1 (3.3) |
| Time since onset of ET, years; mean (SD) | 24.7 (19.6) | 35.3 (26.4) | 28.1 (22.4) |
| Concomitant medication for ET, n (%) a | |||
| Beta-blockers b | 10 (52.6) | 4 (36.4) | 14 (46.7) |
| Anti-epileptics c | 5 (26.3) | 4 (36.4) | 9 (30.0) |
| Clinical Pattern | IncobotulinumtoxinA (N = 19) | Placebo (N = 11) | |
|---|---|---|---|
| Muscle | n | Dose, U; Mean (SD) | n |
| Total | 19 | 116.3 (53.0) | 11 |
| Wrist (mandatory treatment) a,b | |||
| Total | 19 | 47.4 (18.1) | 11 |
| Extensor carpi radialis | 14 | 9.6 (6.0) | 10 |
| Extensor carpi ulnaris | 11 | 8.6 (3.9) | 8 |
| Flexor carpi radialis | 16 | 10.6 (5.1) | 11 |
| Flexor carpi ulnaris | 16 | 7.2 (2.6) | 7 |
| Pronator quadratus | 13 | 8.1 (4.8) | 8 |
| Pronator teres | 14 | 8.9 (4.5) | 8 |
| Supinator | 15 | 10.3 (6.4) | 7 |
| Shoulder c | |||
| Total | 14 | 45.0 (7.6) | 9 |
| Deltoid | 5 | 9.0 (2.2) | 1 |
| Latissimus dorsi | 12 | 11.3 (3.8) | 8 |
| Pectoralis major | 14 | 21.1 (4.5) | 9 |
| Supraspinatus | 14 | 11.1 (4.0) | 8 |
| Teres major | 0 | 0.0 (0.0) | 1 |
| Elbow d | |||
| Total | 16 | 42.5 (13.9) | 10 |
| Biceps brachii | 2 | 30.0 (0.0) | 1 |
| Brachialis | 14 | 20.0 (6.5) | 9 |
| Triceps brachii | 16 | 21.3 (7.0) | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jog, M.; Lee, J.; Scheschonka, A.; Chen, R.; Ismail, F.; Boulias, C.; Hobson, D.; King, D.; Althaus, M.; Simon, O.; et al. Tolerability and Efficacy of Customized IncobotulinumtoxinA Injections for Essential Tremor: A Randomized, Double-Blind, Placebo-Controlled Study. Toxins 2020, 12, 807. https://doi.org/10.3390/toxins12120807
Jog M, Lee J, Scheschonka A, Chen R, Ismail F, Boulias C, Hobson D, King D, Althaus M, Simon O, et al. Tolerability and Efficacy of Customized IncobotulinumtoxinA Injections for Essential Tremor: A Randomized, Double-Blind, Placebo-Controlled Study. Toxins. 2020; 12(12):807. https://doi.org/10.3390/toxins12120807
Chicago/Turabian StyleJog, Mandar, Jack Lee, Astrid Scheschonka, Robert Chen, Farooq Ismail, Chris Boulias, Douglas Hobson, David King, Michael Althaus, Olivier Simon, and et al. 2020. "Tolerability and Efficacy of Customized IncobotulinumtoxinA Injections for Essential Tremor: A Randomized, Double-Blind, Placebo-Controlled Study" Toxins 12, no. 12: 807. https://doi.org/10.3390/toxins12120807
APA StyleJog, M., Lee, J., Scheschonka, A., Chen, R., Ismail, F., Boulias, C., Hobson, D., King, D., Althaus, M., Simon, O., Dersch, H., Frucht, S., & Simpson, D. M., on behalf of the Essential Tremor Study Team. (2020). Tolerability and Efficacy of Customized IncobotulinumtoxinA Injections for Essential Tremor: A Randomized, Double-Blind, Placebo-Controlled Study. Toxins, 12(12), 807. https://doi.org/10.3390/toxins12120807






