Grape Seed Waste Counteracts Aflatoxin B1 Toxicity in Piglet Mesenteric Lymph Nodes
Abstract
1. Introduction
2. Results
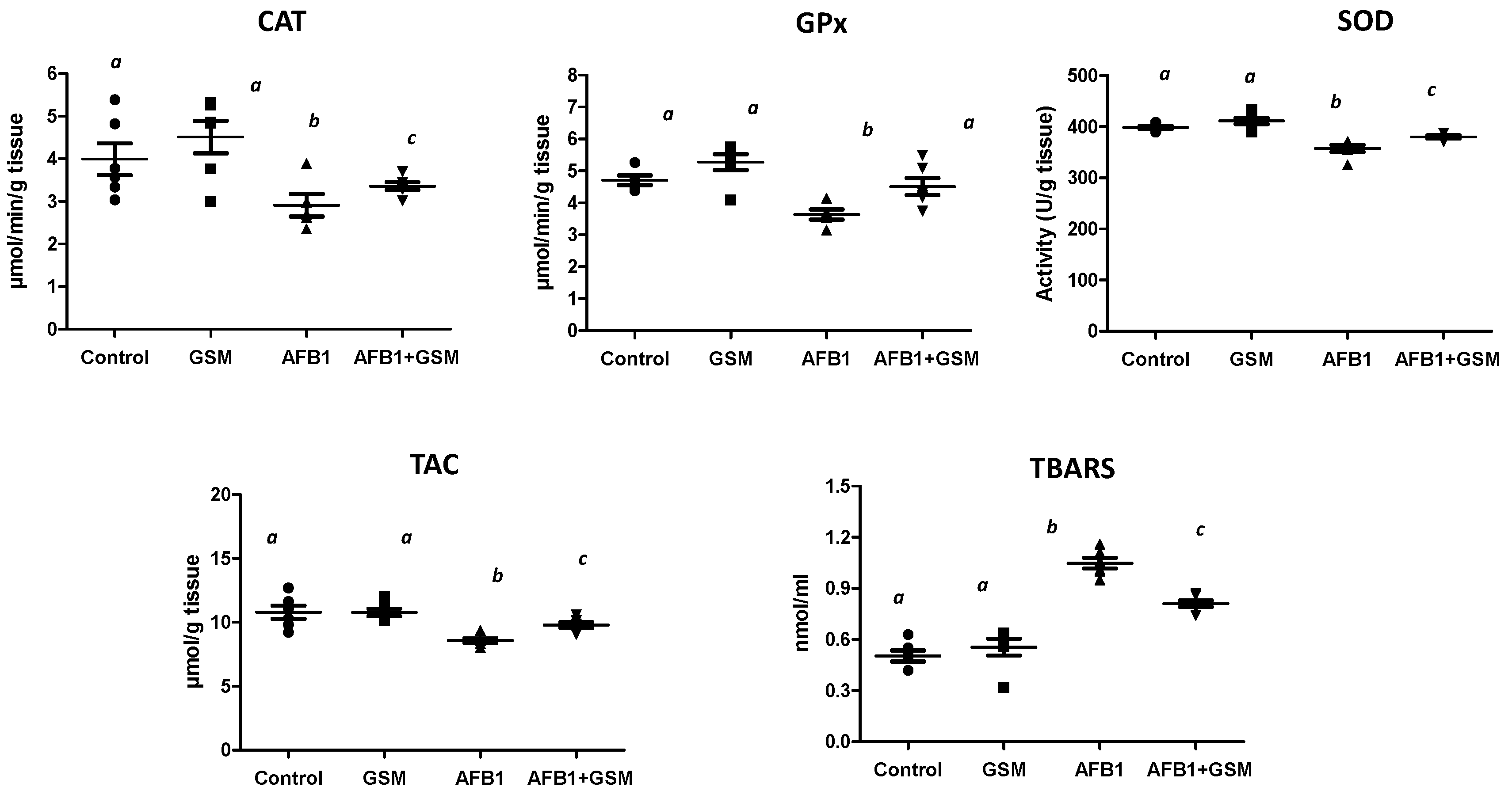
2.1. Effect of Grape Seed Meal and Aflatoxin B1 on Oxidative Damage in Mesenteric Lymph Nodes
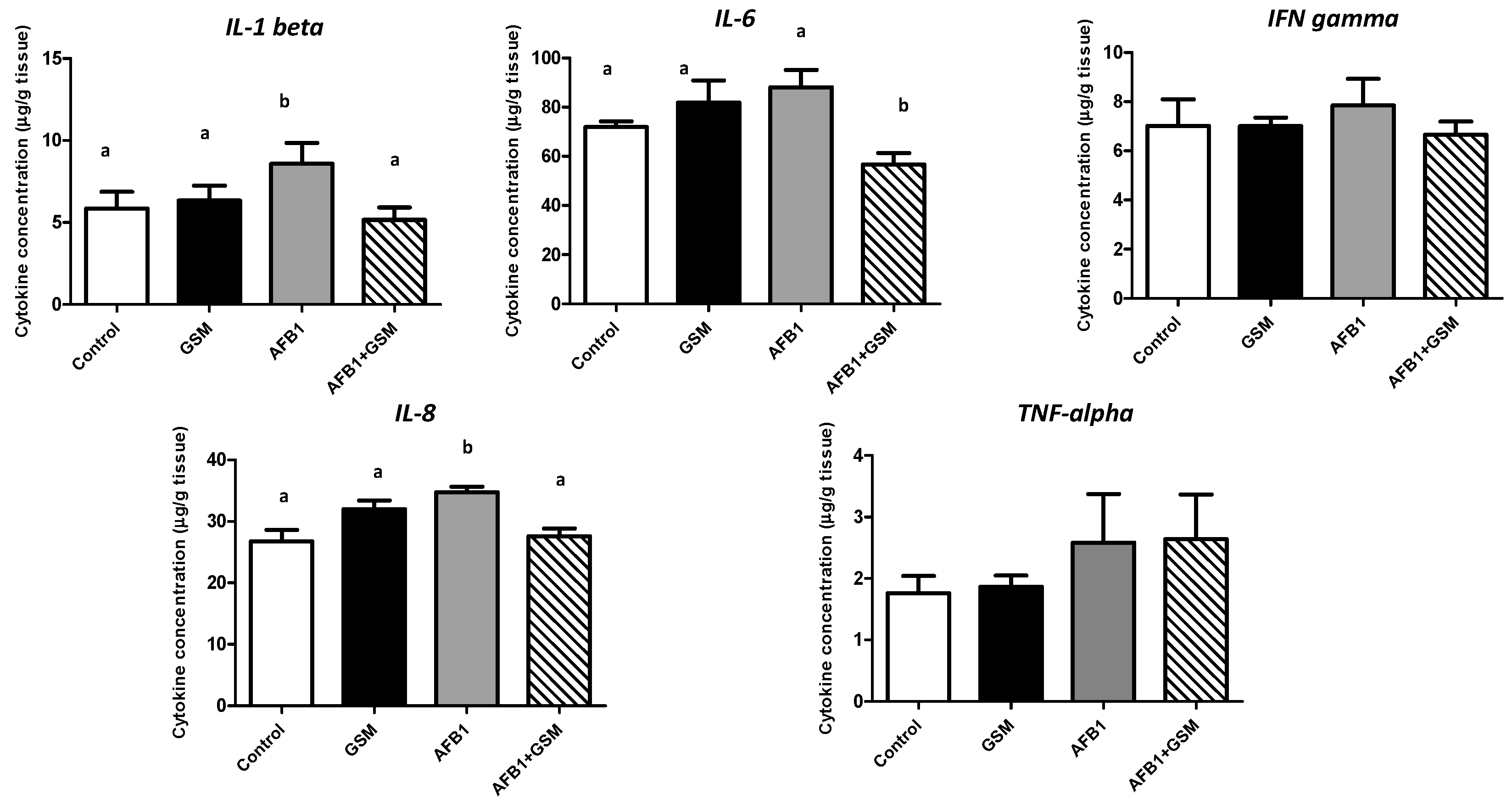
2.2. Effect of Grape Seed Meal and Aflatoxin B1 on Inflammatory Cytokine Synthesis in Mesenteric Lymph Nodes
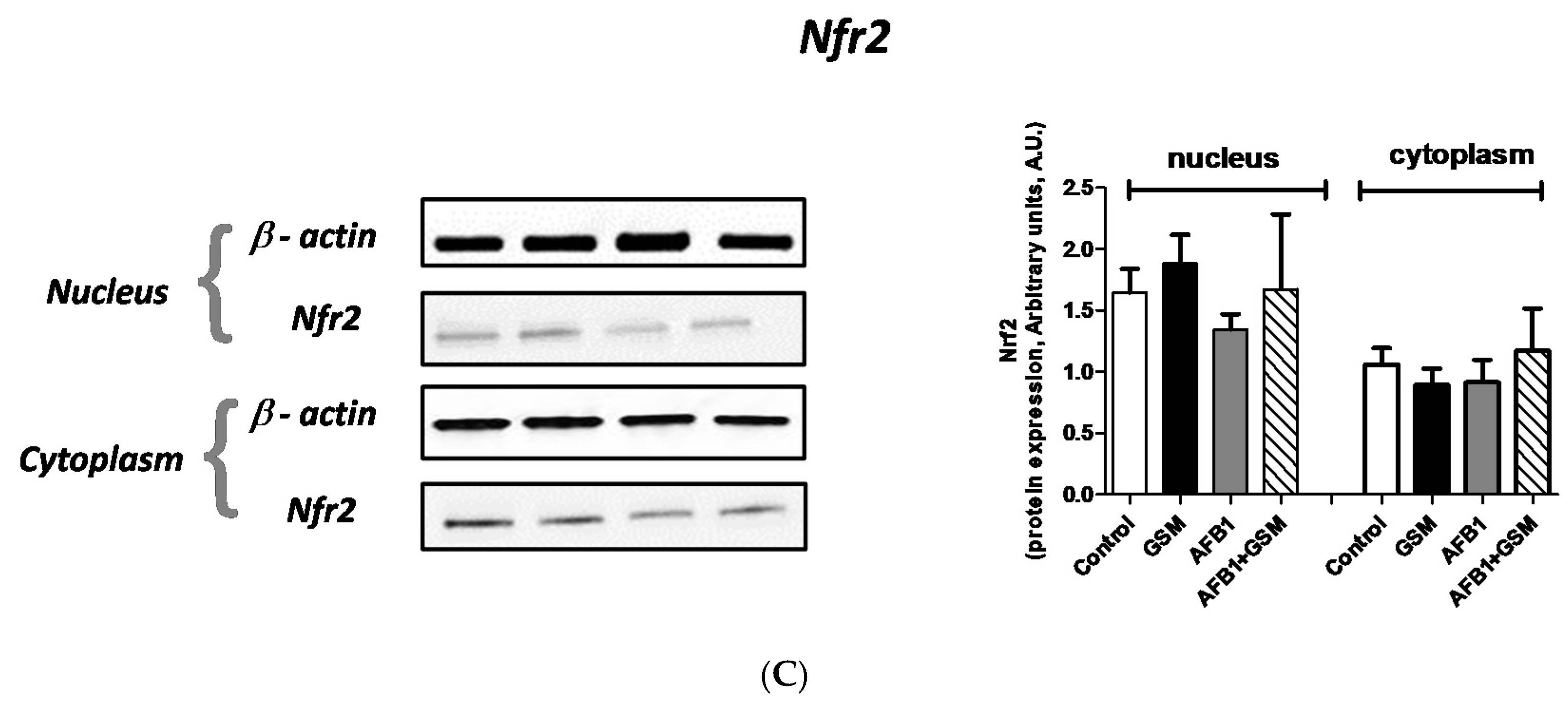
2.3. Effect of Grape Seed Meal and Aflatoxin B1 on Cell Signaling Pathways in Mesenteric Lymph Nodes
2.4. Effect of Grape Seed Meal and Aflatoxin B1 on mRNA Gene Expression Involved in Inflammation
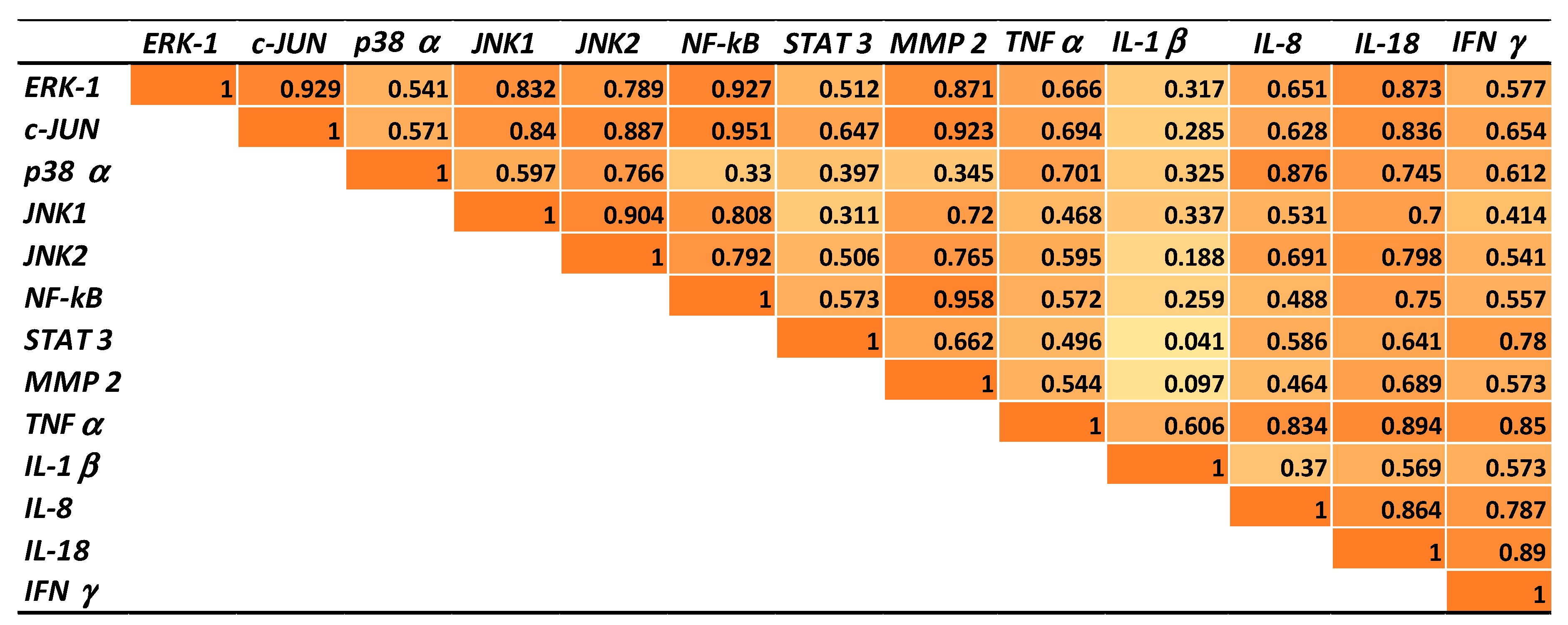
2.5. Correlations between Gene Expressions of Transcription Factors, MAP Kinases, Metalloproteinases, and Cytokines in Pigs Fed AFB1 and AFB1 + GSM Diets
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals and Dietary Treatments
5.2. Composition of the Grape Seed Meal
5.3. Toxin
5.4. Determination of Total Antioxidant Status
5.5. TBARS Assessment
5.6. Enzyme Activity Assessment
5.7. Cytokine Measurement
5.8. Extraction of Total RNA and cDNA Synthesis
5.9. Detection of Inflammatory and Signalling Gene Expression by qPCR Array
5.10. Western Blot Analysis
5.11. Statistical Analyses
Author Contributions
Funding
Conflicts of Interest
References
- Grenier, B.; Applegate, T.J. Modulation of intestinal functions following mycotoxin ingestion: Meta-analysis of published experiments in animals. Toxins 2013, 5, 396–430. [Google Scholar] [CrossRef]
- Sarma, U.P.; Bhetaria, P.J.; Devi, P.; Varma, A. Aflatoxins: Implications on health. Indian J. Clin. Biochem. 2017, 32, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin b1 and m1: Biological properties and their involvement in cancer development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Taniwaki, M.H.; Pitt, J.I.; Magan, N. Aspergillus species and mycotoxins: Occurrence and importance in major food commodities. Curr. Opin. Food Sci. 2018, 23, 38–43. [Google Scholar] [CrossRef]
- Liew, W.-P.-P.; Mohd-Redzwan, S. Mycotoxin: Its impact on gut health and microbiota. Front. Cell Infect. Microbiol. 2018, 8, 60. [Google Scholar] [CrossRef]
- Yin, H.; Jiang, M.; Peng, X.; Cui, H.; Zhou, Y.; He, M.; Zuo, Z.; Ouyang, P.; Fan, J.; Fang, J. The molecular mechanism of g2m cell cycle arrest induced by afb1 in the jejunum. Oncotarget 2016, 7, 35592–35606. [Google Scholar] [CrossRef]
- Peng, X.; Chen, K.; Chen, J.; Fang, J.; Cui, H.; Zuo, Z.; Deng, J.; Chen, Z.; Geng, Y.; Lai, W. Aflatoxin b1 affects apoptosis and expression of bax, bcl-2, and caspase-3 in thymus and bursa of fabricius in broiler chickens. Environ. Toxicol. 2016, 31, 1113–1120. [Google Scholar] [CrossRef]
- Yang, X.; Liu, L.; Chen, J.; Xiao, A. Response of intestinal bacterial flora to the long-term feeding of aflatoxin b1 (afb1) in mice. Toxins 2017, 9, 317. [Google Scholar] [CrossRef]
- Akbari, P.; Braber, S.; Varasteh, S.; Alizadeh, A.; Garssen, J.; Fink-Gremmels, J. The intestinal barrier as an emerging target in the toxicological assessment of mycotoxins. Arch. Toxicol. 2017, 91, 1007–1029. [Google Scholar] [CrossRef]
- Romero, A.; Ares, I.; Ramos, E.; Castellano, V.; Martínez, M.; Martínez-Larrañaga, M.R.; Anadón, A.; Martínez, M.A. Mycotoxins modify the barrier function of caco-2 cells through differential gene expression of specific claudin isoforms: Protective effect of illite mineral clay. Toxicology 2016, 353–354, 21–33. [Google Scholar] [CrossRef]
- Kohan, A.B.; Yoder, S.M.; Tso, P. Using the lymphatics to study nutrient absorption and the secretion of gastrointestinal hormones. Physiol. Behav. 2011, 105, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.S.; Ganta, V.C.; Jordan, P.A.; Witte, M.H. Gastrointestinal lymphatics in health and disease. Pathophysiology 2010, 17, 315–335. [Google Scholar] [CrossRef]
- Worbs, T.; Bode, U.; Yan, S.; Hoffmann, M.W.; Hintzen, G.; Bernhardt, G.; Förster, R.; Pabst, O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J. Exp. Med. 2006, 203, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of mycotoxin on immune response and consequences for pig health. Anim. Nutr. 2016, 2, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Meissonnier, G.; Pinton, P.; Laffitte, J.; Cossalter, A.-M.; Gong, Y.Y.; Wild, C.; Bertin, G.; Galtier, P.; Oswald, I. Immunotoxicity of aflatoxin b1: Impairment of the cell-mediated response to vaccine antigen and modulation of cytokine expression. Toxicol. Appl. Pharmacol. 2008, 231, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Turcu, R.; Margareta, O.; Criste, R.D.; Mariana, R.; Tatiana, P.; Șoica, C.; Drăgotoiu, D. The effect of using grape seeds meal as natural antioxidant in broiler diets enriched in fatty acids, on meat quality. J. Hyg. Eng. Des. 2019, 35, 14–20. [Google Scholar]
- Chedea, V.S.; Palade, L.M.; Marin, D.E.; Pelmus, R.S.; Habeanu, M.; Rotar, M.C.; Gras, M.A.; Pistol, G.C.; Taranu, I. Intestinal absorption and antioxidant activity of grape pomace polyphenols. Nutrients 2018, 10, 588. [Google Scholar] [CrossRef]
- Veskoukis, A.S.; Kyparos, A.; Nikolaidis, M.G.; Stagos, D.; Aligiannis, N.; Halabalaki, M.; Chronis, K.; Goutzourelas, N.; Skaltsounis, L.; Kouretas, D. The antioxidant effects of a polyphenol-rich grape pomace extract in vitro do not correspond in vivo using exercise as an oxidant stimulus. Oxid. Med. Cell Longev. 2012, 2012, 185867. [Google Scholar] [CrossRef]
- Taranu, I.; Habeanu, M.; Gras, M.A.; Pistol, G.C.; Lefter, N.; Palade, M.; Ropota, M.; Sanda Chedea, V.; Marin, D.E. Assessment of the effect of grape seed cake inclusion in the diet of healthy fattening-finishing pigs. J. Anim. Physiol. Anim. Nutr. 2018, 102, e30–e42. [Google Scholar] [CrossRef]
- Abdel-Wahhab, M.A.; El-Nekeety, A.A.; Hathout, A.S.; Salman, A.S.; Abdel-Aziem, S.H.; Sabry, B.A.; Hassan, N.S.; Abdel-Aziz, M.S.; Aly, S.E.; Jaswir, I. Bioactive compounds from aspergillus niger extract enhance the antioxidant activity and prevent the genotoxicity in aflatoxin b1-treated rats. Toxicon 2020, 181, 57–68. [Google Scholar] [CrossRef]
- Oskoueian, E.; Abdullah, N.; Zulkifli, I.; Ebrahimi, M.; Karimi, E.; Goh, Y.M.; Oskoueian, A.; Shakeri, M. Cytoprotective effect of palm kernel cake phenolics against aflatoxin b1-induced cell damage and its underlying mechanism of action. BMC Complement. Altern. Med. 2015, 15, 392. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Liu, F.; Zhu, Q.; Zhang, M.; Li, T.; Chen, J.; Huang, Y.; Wang, X.; Sheng, J. Aflatoxin b1 can be complexed with oxidised tea polyphenols and the absorption of the complexed aflatoxin b1 is inhibited in rats. J. Sci. Food Agric. 2017, 97, 1910–1915. [Google Scholar] [CrossRef] [PubMed]
- Ali Rajput, S.; Sun, L.; Zhang, N.; Mohamed Khalil, M.; Gao, X.; Ling, Z.; Zhu, L.; Khan, F.A.; Zhang, J.; Qi, D. Ameliorative effects of grape seed proanthocyanidin extract on growth performance, immune function, antioxidant capacity, biochemical constituents, liver histopathology and aflatoxin residues in broilers exposed to aflatoxin b1. Toxins 2017, 9, 371. [Google Scholar] [CrossRef] [PubMed]
- Taranu, I.; Hermenean, A.; Bulgaru, C.; Pistol, G.C.; Ciceu, A.; Grosu, I.A.; Marin, D.E. Diet containing grape seed meal by-product counteracts afb1 toxicity in liver of pig after weaning. Ecotoxicol. Environ. Saf. 2020, 203, 110899. [Google Scholar] [CrossRef]
- Grosu, I.A.; Pistol, G.C.; Taranu, I.; Marin, D.E. The impact of dietary grape seed meal on healthy and aflatoxin b1 afflicted microbiota of pigs after weaning. Toxins 2019, 11, 25. [Google Scholar] [CrossRef]
- Whitlow, L. Evaluation of mycotoxin binders. In Proceedings of the 4th Mid-Atlantic Nutrition Conference, Timonium, MD, USA, 29–30 March 2006. [Google Scholar]
- Avantaggiato, G.; Solfrizzo, M.; Visconti, A. Recent advances on the use of adsorbent materials for detoxification of fusarium mycotoxins. Food Addit. Contam. 2005, 22, 379–388. [Google Scholar] [CrossRef]
- Sabater-Vilar, M.; Malekinejad, H.; Selman, M.H.; van der Doelen, M.A.; Fink-Gremmels, J. In vitro assessment of adsorbents aiming to prevent deoxynivalenol and zearalenone mycotoxicoses. Mycopathologia 2007, 163, 81–90. [Google Scholar] [CrossRef]
- Bočarov-Stančić, A.; Adamović, M.; Salma, N.; Bodroža-Solarov, M.; Vučković-Đisalov, J.; Pantić, V. In vitro efficacy of mycotoxins adsorption by natural mineral adsorbents. Biotechnol. Anim. Husb. 2011, 27, 1241–1251. [Google Scholar] [CrossRef]
- Huwig, A.; Freimund, S.; Käppeli, O.; Dutler, H. Mycotoxin detoxication of animal feed by different adsorbents. Toxicol. Lett. 2001, 122, 179–188. [Google Scholar] [CrossRef]
- Di Gregorio, M.C.; Neeff, D.V.D.; Jager, A.V.; Corassin, C.H.; Carão, Á.C.D.P.; Albuquerque, R.D.; Azevedo, A.C.D.; Oliveira, C.A.F. Mineral adsorbents for prevention of mycotoxins in animal feeds. Toxin Rev. 2014, 33, 125–135. [Google Scholar] [CrossRef]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.-Y.; Zhao, C.-N.; Liu, Q.; Feng, X.-L.; Xu, X.-Y.; Cao, S.-Y.; Meng, X.; Li, S.; Gan, R.-Y.; Li, H.-B. Potential of grape wastes as a natural source of bioactive compounds. Molecules 2018, 23, 2598. [Google Scholar] [CrossRef] [PubMed]
- Chedea, V.S.; Pelmus, R.S.; Lazar, C.; Pistol, G.C.; Calin, L.G.; Toma, S.M.; Dragomir, C.; Taranu, I. Effects of a diet containing dried grape pomace on blood metabolites and milk composition of dairy cows. J. Sci. Food Agric. 2017, 97, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Avantaggiato, G.; Greco, D.; Damascelli, A.; Solfrizzo, M.; Visconti, A. Assessment of multi-mycotoxin adsorption efficacy of grape pomace. J. Agric. Food Chem. 2014, 62, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Gambacorta, L.; Pinton, P.; Avantaggiato, G.; Oswald, I.P.; Solfrizzo, M. Grape pomace, an agricultural byproduct reducing mycotoxin absorption: In vivo assessment in pig using urinary biomarkers. J. Agric. Food Chem. 2016, 64, 6762–6771. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.E.; Taranu, I. Overview on aflatoxins and oxidative stress. Toxin Rev. 2012, 31, 32–43. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Marin, D.E.; Pistol, G.C.; Gras, M.; Palade, M.; Taranu, I. A comparison between the effects of ochratoxin a and aristolochic acid on the inflammation and oxidative stress in the liver and kidney of weanling piglets. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 1147–1156. [Google Scholar] [CrossRef]
- Marin, D.E.; Pistol, G.C.; Gras, M.A.; Palade, M.L.; Taranu, I. Comparative effect of ochratoxin a on inflammation and oxidative stress parameters in gut and kidney of piglets. Regul. Toxicol. Pharmacol. RTP 2017, 89, 224–231. [Google Scholar] [CrossRef]
- Yunus, A.W.; Razzazi-Fazeli, E.; Bohm, J. Aflatoxin b(1) in affecting broiler’s performance, immunity, and gastrointestinal tract: A review of history and contemporary issues. Toxins 2011, 3, 566–590. [Google Scholar] [CrossRef]
- Benkerroum, N. Chronic and acute toxicities of aflatoxins: Mechanisms of action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef]
- Mehrzad, J.; Malvandi, A.M.; Alipour, M.; Hosseinkhani, S. Environmentally relevant level of aflatoxin b(1) elicits toxic pro-inflammatory response in murine cns-derived cells. Toxicol. Lett. 2017, 279, 96–106. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Dell’Agli, M. Special issue: Anti-inflammatory activity of plant polyphenols. Biomedicines 2020, 8, 64. [Google Scholar] [CrossRef]
- Calder, P.C. Immunoregulatory and anti-inflammatory effects of n-3 polyunsaturated fatty acids. Braz. J. Med. Biol. Res. 1998, 31, 467–490. [Google Scholar] [CrossRef] [PubMed]
- Holanda, D.M.; Kim, S.W. Efficacy of mycotoxin detoxifiers on health and growth of newly-weaned pigs under chronic dietary challenge of deoxynivalenol. Toxins 2020, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Van Le Thanh, B.; Lessard, M.; Chorfi, Y.; Guay, F. The efficacy of anti-mycotoxin feed additives in preventing the adverse effects of wheat naturally contaminated with fusarium mycotoxins on performance, intestinal barrier function and nutrient digestibility and retention in weanling pigs. Can. J. Anim. Sci. 2015, 95, 197–209. [Google Scholar] [CrossRef]
- Solis-Cruz, B.; Hernandez-Patlan, D.; Petrone, V.M.; Pontin, K.P.; Latorre, J.D.; Beyssac, E.; Hernandez-Velasco, X.; Merino-Guzman, R.; Owens, C.; Hargis, B.M.; et al. Evaluation of cellulosic polymers and curcumin to reduce aflatoxin b1 toxic effects on performance, biochemical, and immunological parameters of broiler chickens. Toxins 2019, 11, 121. [Google Scholar] [CrossRef]
- Xu, F.; Wang, P.; Yao, Q.; Shao, B.; Yu, H.; Yu, K.; Li, Y. Lycopene alleviates afb(1)-induced immunosuppression by inhibiting oxidative stress and apoptosis in the spleen of mice. Food Funct. 2019, 10, 3868–3879. [Google Scholar] [CrossRef]
- Taranu, I.; Marin, D.E.; Palade, M.; Pistol, G.C.; Chedea, V.S.; Gras, M.A.; Rotar, C. Assessment of the efficacy of a grape seed waste in counteracting the changes induced by aflatoxin b1 contaminated diet on performance, plasma, liver and intestinal tissues of pigs after weaning. Toxicon Off. J. Int. Soc. Toxinol. 2019, 162, 24–31. [Google Scholar] [CrossRef]
- Taranu, I.; Gras, M.; Pistol, G.C.; Motiu, M.; Marin, D.E.; Lefter, N.; Ropota, M.; Habeanu, M. Omega-3 pufa rich camelina oil by-products improve the systemic metabolism and spleen cell functions in fattening pigs. PLoS ONE 2014, 9, e110186. [Google Scholar] [CrossRef]
- Palade, L.M.; Habeanu, M.; Marin, D.E.; Chedea, V.S.; Pistol, G.C.; Grosu, I.A.; Gheorghe, A.; Ropota, M.; Taranu, I. Effect of dietary hemp seed on oxidative status in sows during late gestation and lactation and their offspring. Anim. Open Access J. 2019, 9, 194. [Google Scholar] [CrossRef]
- Pistol, G.C.; Braicu, C.; Motiu, M.; Gras, M.A.; Marin, D.E.; Stancu, M.; Calin, L.; Israel-Roming, F.; Berindan-Neagoe, I.; Taranu, I. Zearalenone mycotoxin affects immune mediators, mapk signalling molecules, nuclear receptors and genome-wide gene expression in pig spleen. PLoS ONE 2015, 10, e0127503. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.E.; Pistol, G.C.; Neagoe, I.V.; Calin, L.; Taranu, I. Effects of zearalenone on oxidative stress and inflammation in weanling piglets. Food Chem. Toxicol. 2013, 58, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Pistol, G.C.; Gras, M.A.; Marin, D.E.; Israel-Roming, F.; Stancu, M.; Taranu, I. Natural feed contaminant zearalenone decreases the expressions of important pro- and anti-inflammatory mediators and mitogen-activated protein kinase/nf-kappab signalling molecules in pigs. Br. J. Nutr. 2014, 111, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.E.; Motiu, M.; Pistol, G.C.; Gras, M.A.; Israel-Roming, F.; Calin, L.; Stancu, M.; Taranu, I. Diet contaminated with ochratoxin a at the highest level allowed by eu recommendation disturbs liver metabolism in weaned piglets. World Mycotoxin J. 2016, 9, 587–596. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


| Gene Expression | Control | GSM | AFB1 | GSM + AFB1 | p Value | |
|---|---|---|---|---|---|---|
| AFB1 Effect | AFB1 × GSM Effect | |||||
| ERK-1 | 1.0 ± 0.73 | 3.53 ± 0.63 | 34.6 ± 0.63 | 1.96 ± 0.73 | 0.039 | 0.006 |
| c-JUN | 1.0 ± 0.28 | 0.94 ± 0.35 | 6.83 ± 0.62 | 1.07 ± 0.13 | 0.013 | 0.028 |
| p38 a | 1.0 ± 0.68 | 0.86 ± 0.03 | 2.21 ± 0.64 | 0.47 ± 0.24 | 0.108 | 0.782 |
| JNK1 | 1.0 ± 0.59 | 0.56 ± 0.48 | 2.53 ± 0.57 | 0.78 ± 0.15 | 0.211 | 0.543 |
| JNK2 | 1.0 ± 0.28 | 0.62 ± 0.51 | 3.12 ± 0.69 | 0.73 ± 0.25 | 0.170 | 0.297 |
| NF-kB p65 | 1.0 ± 0.36 | 0.80 ± 0.66 | 7.97 ± 0.42 | 1.57 ± 0.08 | 0.004 | 0.112 |
| STAT 3 | 1.0 ± 0.84 | 1.25 ± 0.62 | 17.4 ± 0.71 | 2.93 ± 0.17 | 0.011 | 0.681 |
| MMP 2 | 1.0 ± 0.45 | 0.75 ± 0.58 | 12.5 ± 0.66 | 2.34 ± 0.10 | 0.001 | 0.168 |
| TNF α | 1.0 ± 0.10 | 0.95± 0.05 | 7.75 ± 0.49 | 3.22 ± 0.07 | 0.187 | 0.107 |
| IL-1 β | 1.0 ± 0.15 | 0.76 ± 0.34 | 15.3 ± 0.71 | 0.66 ± 0.10 | 0.023 | 0.073 |
| IL-8 | 1.0 ± 0.49 | 0.94 ± 0.05 | 17.5 ± 0.37 | 0.87 ± 0.46 | 0.001 | 0.168 |
| IL-18 | 1.0 ± 0.28 | 0.96 ± 0.61 | 26.8 ± 0.22 | 0.35 ± 0.14 | 0.076 | 0.002 |
| IFN γ | 1.0 ± 0.25 | 1.68 ± 0.82 | 14.2 ± 0.29 | 0.37 ± 0.14 | 0.326 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin, D.E.; Bulgaru, C.V.; Anghel, C.A.; Pistol, G.C.; Dore, M.I.; Palade, M.L.; Taranu, I. Grape Seed Waste Counteracts Aflatoxin B1 Toxicity in Piglet Mesenteric Lymph Nodes. Toxins 2020, 12, 800. https://doi.org/10.3390/toxins12120800
Marin DE, Bulgaru CV, Anghel CA, Pistol GC, Dore MI, Palade ML, Taranu I. Grape Seed Waste Counteracts Aflatoxin B1 Toxicity in Piglet Mesenteric Lymph Nodes. Toxins. 2020; 12(12):800. https://doi.org/10.3390/toxins12120800
Chicago/Turabian StyleMarin, Daniela Eliza, Cristina Valeria Bulgaru, Cristian Andrei Anghel, Gina Cecilia Pistol, Madalina Ioana Dore, Mihai Laurentiu Palade, and Ionelia Taranu. 2020. "Grape Seed Waste Counteracts Aflatoxin B1 Toxicity in Piglet Mesenteric Lymph Nodes" Toxins 12, no. 12: 800. https://doi.org/10.3390/toxins12120800
APA StyleMarin, D. E., Bulgaru, C. V., Anghel, C. A., Pistol, G. C., Dore, M. I., Palade, M. L., & Taranu, I. (2020). Grape Seed Waste Counteracts Aflatoxin B1 Toxicity in Piglet Mesenteric Lymph Nodes. Toxins, 12(12), 800. https://doi.org/10.3390/toxins12120800









