Suspect and Target Screening of Natural Toxins in the Ter River Catchment Area in NE Spain and Prioritisation by Their Toxicity
Abstract
1. Introduction
2. Results and Discussion
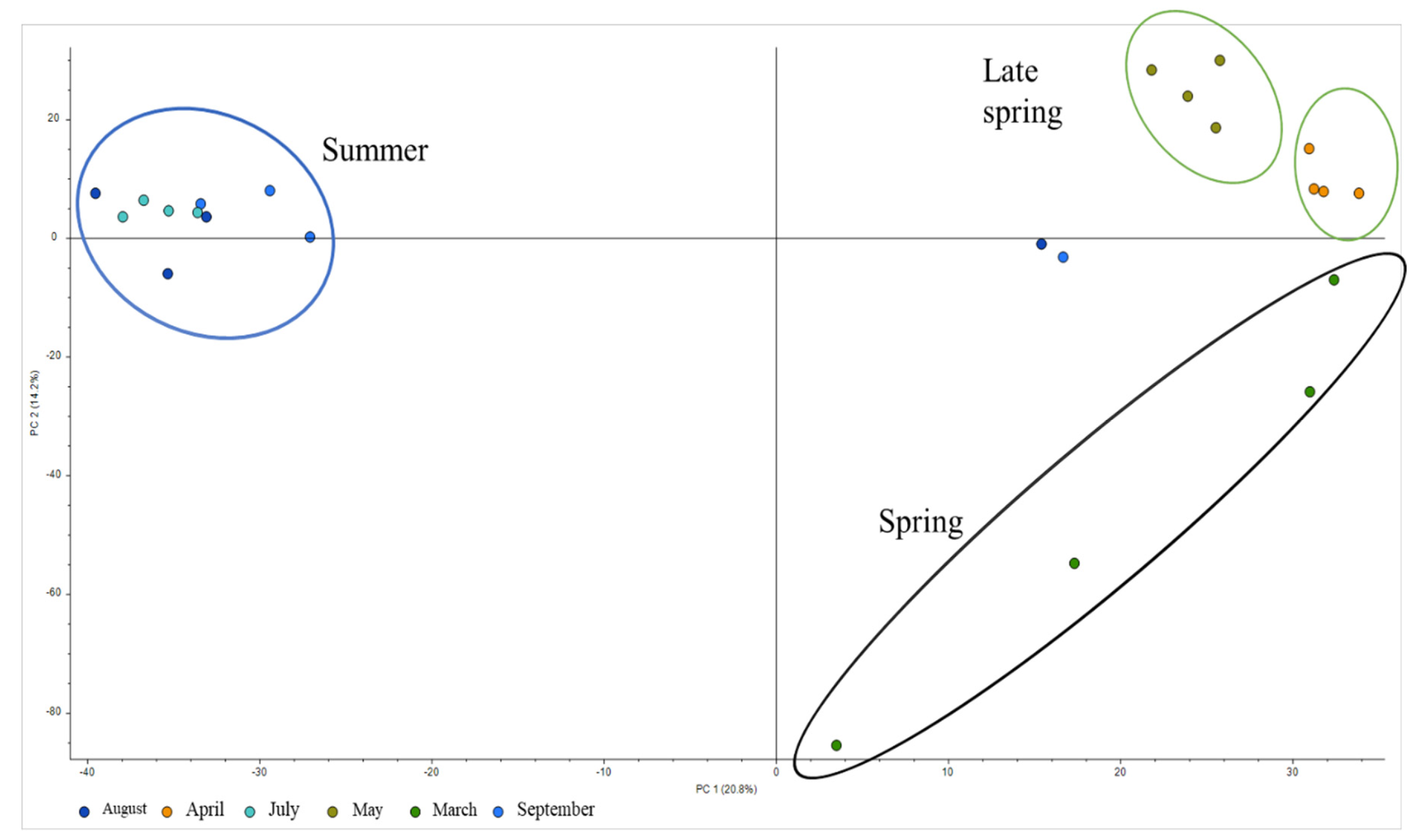
2.1. Tentatively Identified Compounds
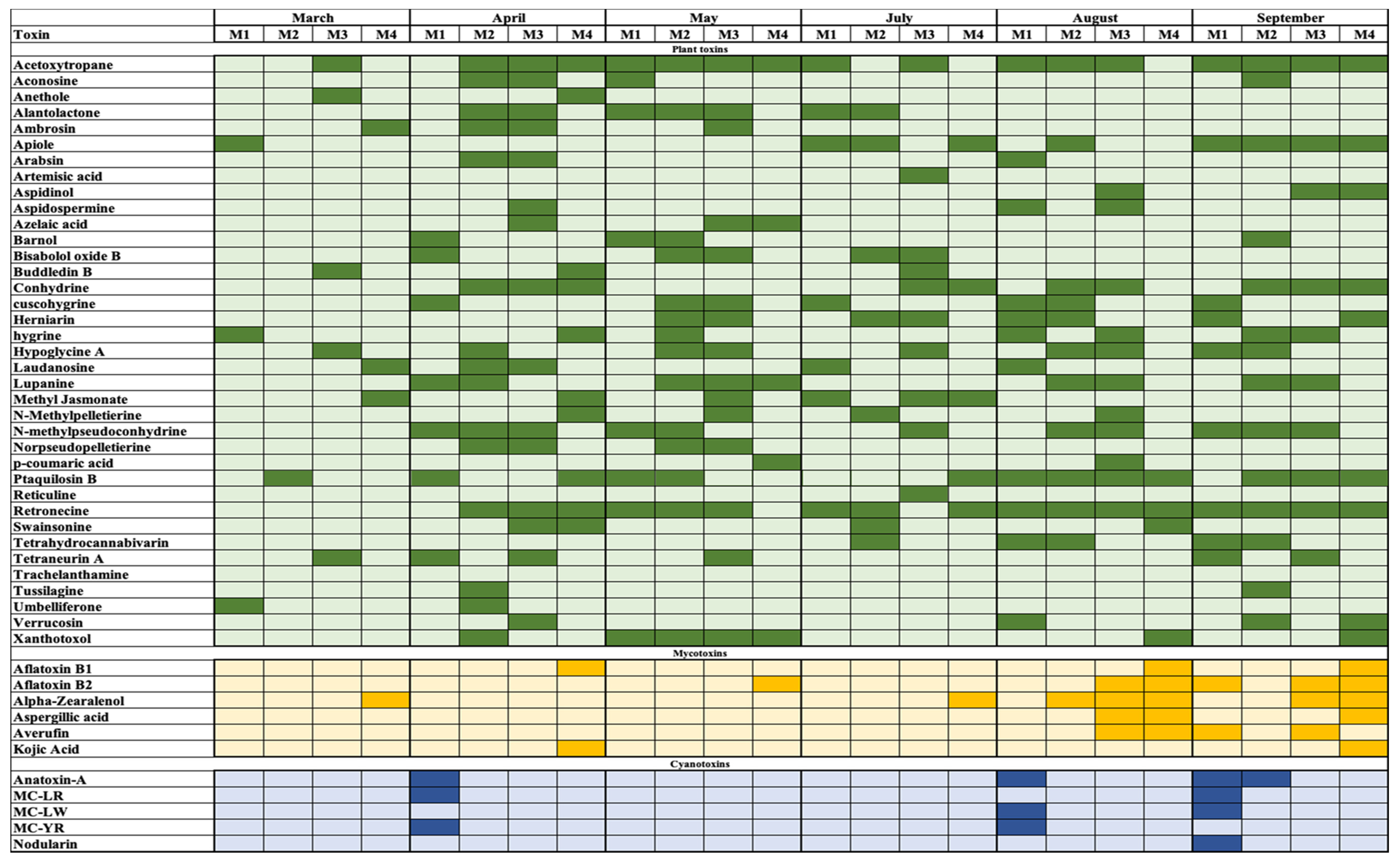
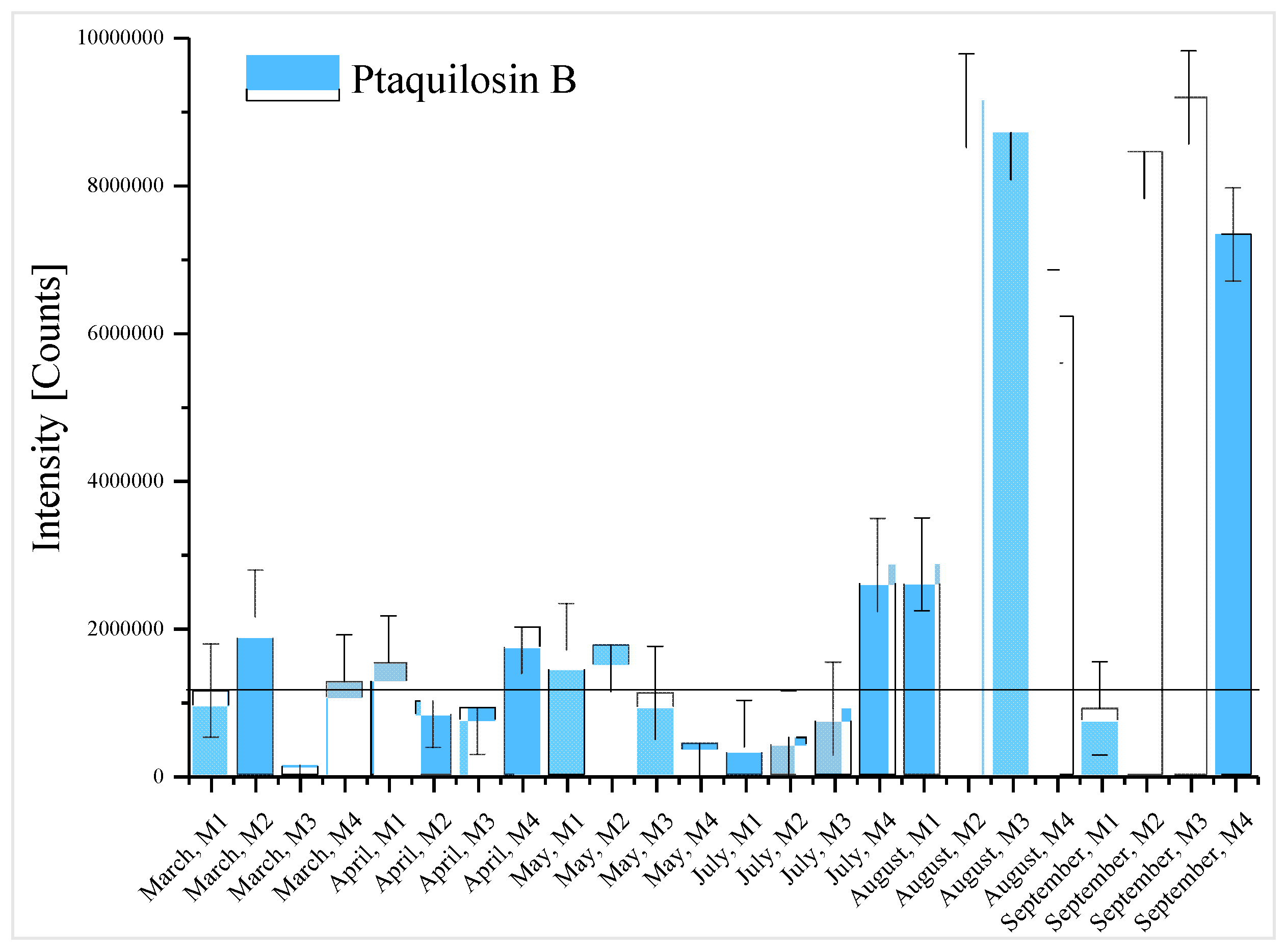
2.2. Target Analysis
2.3. Prioritisation
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Samples and Sampling Sites
4.3. Sample Pre-Treatment
4.4. Liquid Chromatography Coupled with High-Resolution Mass Spectrometry
4.5. Data Processing: Suspect Screening of Natural Toxins
4.6. Accuracy, Precision, Limits of Detection, and Quantification
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Toxin | Toxic Group | Chemical Formula | Exact Mass | Purity (%) | Supplied by |
|---|---|---|---|---|---|
| Microcystin LA | Cyanotoxin | C46H67N7O12 | 909.4847 | >95 | Cyano (Cyanobiotech GmbH, Berlin, Germany) |
| Microcystin LF | Cyanotoxin | C52H71N7O12 | 985.5160 | >95 | Cyano (Cyanobiotech GmbH, Berlin, Germany) |
| Microcystin LR | Cyanotoxin | C49H74N10O12 | 994.5488 | >95 | Cyano (Cyanobiotech GmbH, Berlin, Germany) |
| Microcystin LY | Cyanotoxin | C52H71N7O13 | 1001.5109 | >95 | Cyano (Cyanobiotech GmbH, Berlin, Germany) |
| Microcystin LW | Cyanotoxin | C54H72N8O12 | 1024.5269 | >95 | Cyano (Cyanobiotech GmbH, Berlin, Germany) |
| Microcystin YR | Cyanotoxin | C52H72N10O13 | 1044.5353 | >95 | Cyano (Cyanobiotech GmbH, Berlin, Germany) |
| Nodularin | Cyanotoxin | C41H60N8O10 | 824.4432 | >95 | Cyano (Cyanobiotech GmbH, Berlin, Germany) |
| Anatoxin-a | Cyanotoxin | C10H15NO | 165.2320 | >98 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| Cylindrospermopsin | Cyanotoxin | C15H21N5O7S | 399.1219 | 99 | BOCSci (BOC Sciences, Ramsey Road Shirley, NY, USA) |
| Aflatoxin B1 | Mycotoxin | C17H12O6 | 312.0632 | >98 | Merck (Darmstadt, Germany) |
| Ochratoxin-A | Mycotoxin | C20H18ClNO6 | 403.0823 | >98 | Merck (Darmstadt, Germany) |
| Baicalein | Phytotoxin | C15H10O5 | 270.0528 | 98 | Merck (Darmstadt, Germany) |
| Genistein | Phytotoxin | C15H10O5 | 270.0528 | >98 | Merck (Darmstadt, Germany) |
| Amygdalin | Phytotoxin | C20H27NO11 | 457.158 | >99 | Merck (Darmstadt, Germany) |
| Scopolamine | Phytotoxin | C17H21NO4 | 303.147 | >98 | Merck (Darmstadt, Germany) |
| Cinchonine | Phytotoxin | C19H22N2O | 294.1732 | >98 | Merck (Darmstadt, Germany) |
| Atropine | Phytotoxin | C17H23NO3 | 289.1682 | >99 | Merck (Darmstadt, Germany) |
| Kojic Acid | Mycotoxin | C6H6O4 | 142.0274 | >98 | Merck (Darmstadt, Germany) |
| b-Asarone | Phytotoxin | C12H16O3 | 208.1099 | 70 | Merck (Darmstadt, Germany) |
| p-Coumaric acid | Phytotoxin | C9H8O3 | 164.0471 | >98 | Merck (Darmstadt, Germany) |
| Abietic acid | Phytotoxin | C20H30O2 | 302.2256 | >95 | Merck (Darmstadt, Germany) |
| 7-Ethoxyoumarin | Phytotoxin | C11H10O3 | 190.0634 | ≥97% | Merck (Darmstadt, Germany) |
| 7-Metoxycoumarin | Phytotoxin | C10H8O3 | 176.0479 | >98 | Merck (Darmstadt, Germany) |
| Arbutin | Phytotoxin | C12H16O7 | 272.0986 | >98 | Merck (Darmstadt, Germany) |
| Umbelliferone | Phytotoxin | C9H6O3 | 162.0327 | >99 | Merck (Darmstadt, Germany) |
| Thujone | Phytotoxin | C10H16O | 152.1235 | >99 | Merck (Darmstadt, Germany) |
| Cotinine | Phytotoxin | C10H12N2O | 176.0956 | >99 | Merck (Darmstadt, Germany) |
| Mass [M + H]+ | Formula [M] | CE | Toxin and Possible Isomers |
|---|---|---|---|
| 239.1542 | C16H18N2 | 35 | (−)-Agroclavine |
| 180.1019 | C10H13NO2 | 35 | (−)-Salsolinol, Fusaric acid |
| 398.0961 | C18H24BrNO4 | 35 | (−)-Scopolamin bromide |
| 128.1433 | C8H17N | 35 | (+)-Coniine |
| 142.1226 | C8H15NO | 35 | (+)-Hygrine |
| 249.1961 | C15H24N2O | 35 | (+)-Lupanine |
| 333.2060 | C20 H28 O4 | 35 | 20-Deoxyingenol |
| 184.1332 | C10 H17 N O2 | 35 | 3-Acetoxytropane |
| 197.1536 | C12H20O2 | 35 | 3-Thujyl acetate |
| 646.3221 | C34H47NO11 | 35 | Aconitine |
| 313.0706 | C17 H12 O6 | 70 | Aflatoxin B1 |
| 315.0863 | C17 H14 O6 | 35 | Aflatoxin B2 |
| 329.065 | C17 H12 O7 | 35 | Aflatoxin G1 |
| 331.0812 | C17H14O7 | 35 | Aflatoxin G2 |
| 502.2951 | C32H39NO4 | 35 | Aflatrem |
| 159.0513 | C4 H6 N4 O3 | 35 | Allantoin |
| 924.4951 | C47H73NO17 | 35 | Amphotericin Bh |
| 458.1656 | C20H27NO11 | 60 | Amygdalin |
| 456.1511 | C20H27NO11 | 35 | Amygdalin negative |
| 166.1226 | C10 H15 N O | 45 | Anatoxin-A |
| 187.03897 | C11H6O3 | 35 | Angelicin (Isopsoralen) |
| 504.343 | C28H45N3O5 | 35 | Antillatoxin |
| 624.3755 | C34H49N5O6 | 35 | Apicidin |
| 271.0601 | C15H10O5 | 35 | Apigenin |
| 283.1540 | C15H22O5 | 35 | Artemisinin |
| 189.1121 | C9 H16 O4 | 35 | Aspionene |
| 290.1751 | C17H23NO3 | 50 | Atropine |
| 369.0968 | C20H16O7 | 35 | Averufin |
| 321.1696 | C18H24O5 | 35 | a-Zearalenol |
| 261.1597 | C15H20N2O2 | 35 | Baptifoline |
| 784.4167 | C45H57N3O9 | 35 | Beauvericin |
| 641.2891 | C34H44N2O8S | 35 | Belladonnine |
| 209.1172 | C12H16O3 | 50 | beta-Asarone |
| 285.0757 | C16H12O5 | 35 | Biochanin A (BIO) |
| 438.2638 | C27H35NO4 | 35 | b-Paxitriol |
| 281.1747 | C16 H24 O4 | 35 | Brefeldin A |
| 235.1692 | C15 H22 O2 | 35 | Buddledin B |
| 317.2111 | C20H28O3 | 35 | Cafestol |
| 195.0876 | C8H10N4O2 | 35 | Caffeine |
| 153.1273 | C10H16O | 35 | Carveol |
| 261.1849 | C17H24O2 | 35 | Cicudiol |
| 259.1692 | C17 H22 O2 | 35 | Cicutoxin |
| 1111.5836 | C60H86O19 | 35 | Ciguatoxin |
| 295.1804 | C19H22N2O | 35 | Cinchonine |
| 279.0863 | C14H14O6 | 35 | Citreoisocoumarin |
| 403.2115 | C23H30O6 | 35 | Citreoviridin |
| 400.1754 | C22H25NO6 | 35 | Colchicine |
| 144.1382 | C8H17NO | 35 | Conhydrine |
| 127.0389 | C6H6O3 | 35 | Coumarin |
| 300.2169 | C16 H29 N O4 | 35 | Curassavine |
| 225.1961 | C13H24N2O | 35 | Cuscohygrine |
| 416.1234 | C15H21N5O7S | 45 | Cylindrospermopsin |
| 255.0651 | C15H10O4 | 35 | Daidzein (DAI) |
| 417.1180 | C21H20O9 | 35 | Daidzin |
| 589.1915 | C29H32O13 | 35 | Dalbin |
| 427.1387 | C23H22O8 | 35 | Dalbinol |
| 249.1485 | C15H20O3 | 35 | Damsin |
| 291.1227 | C16H18O5 | 35 | Dehydrocurvularin |
| 355.1176 | C20H18O6 | 35 | Deoxynivalenol |
| 411.1074 | C22H18O8 | 35 | Desertorin A |
| 367.1751 | C19H26O7 | 35 | Diacetoxyscirpenol |
| 765.4419 | C41H64O13 | 35 | Digitoxin |
| 415.3206 | C27H42O3 | 35 | Diosgenin |
| 295.1903 | C17 H26 O4 | 50 | Embelin |
| 271.0601 | C15H10O5 | 35 | Emodin |
| 1095.5662 | C60H74N10O10 | 35 | Ergoclavin |
| 350.1598 | C18H23NO6 | 35 | Erucifoline |
| 269.0808 | C16H12O4 | 35 | Formononetin (FOR) |
| 209.0444 | C10H8O5 | 35 | Fraxetin |
| 271.0601 | C15H10O5 | 50 | Genistein or baicalein |
| 155.1430 | C10H18O | 35 | Geraniol |
| 781.4368 | C41H64O14 | 35 | Gitoxin |
| 156.1019 | C8 H13 N O2 | 35 | Heliotridine |
| 304.1543 | C17H21NO4 | 35 | Hyoscine |
| 143.0338 | C6H6O4 | 35 | Kojic acid |
| 541.3887 | C34 H52 O5 | 35 | Lantadene D |
| 358.2012 | C21 H27 N O4 | 35 | Laudanosine |
| 910.4920 | C46H67N7O12 | 35 | MC-LA |
| 995.5560 | C49H74N10O12 | 35 | MC-LR |
| 1025.5344 | C54H72N8O12 | 35 | MC-LW |
| 1045.5353 | C52H72N10O13 | 35 | MC-YR |
| 192.0781 | C11H12O3 | 35 | Myristicin |
| 825.4505 | C41 H60 N8 O10 | 35 | Nodularin |
| 128.1069 | C7H13NO | 35 | Norhygrine |
| 152.0566 | C5H5N5O | 35 | Nostocine |
| 404.0895 | C20H18ClNO6 | 70 | Ochratoxin-a |
| 215.1277 | C11H18O4 | 35 | Pestalotin |
| 165.0658 | C8H8N2O2 | 35 | Ricinine |
| 194.1175 | C11H15NO2 | 35 | Salsoline |
| 868.5053 | C45H73NO15 | 35 | Solanine |
| 746.4837 | C42H67NO10 | 35 | Spirolide |
| 183.0288 | C8H6O5 | 35 | Stipitatic acid |
| 174.11247 | C8H15NO3 | 35 | Swainsonine |
| 153.1273 | C10H16O | 35 | Thujone |
| 115.0389 | C5H6O3 | 35 | Tulipalin B |
| 163.0389 | C9H6O3 | 35 | Umbelliferone |
| 355.2380 | C22 H30 N2 O2 | 35 | Vincaminorein (Aspidospermine) |
| 203.0338 | C11 H6 O4 | 35 | Xanthotoxol |
| Toxins | Molecular Formula | [M+H]+ | Recovery% | RSD% | LOD µg/L | LOQ µg/L | R2 |
|---|---|---|---|---|---|---|---|
| Ana | C10H15NO | 166.1234 | 84 | 8.0 | 0.2 | 0.5 | 0.989 |
| AflB1 | C17H12O6 | 416.1242 | 86 | 9.9 | 0.2 | 0.7 | 0.999 |
| MC-LR | C49H74N10O12 | 995.5568 | 78 | 3.3 | 0.2 | 0.5 | 0.995 |
| MC-LW | C54H72N8O12 | 1025.5342 | 55 | 5.8 | 0.1 | 0.5 | 0.991 |
| Nod | C41H60N8O10 | 825.4512 | 94 | 16.2 | 0.2 | 0.8 | 0.992 |
| MC-YR | C54H72N8O12 | 1045.5361 | 84 | 16.9 | 0.4 | 1.5 | 0.943 |
| Kja | C12H16O3 | 208.1093 | 85 | 6.4 | 0.02 | 0.08 | 0.990 |
| 7-methoxycoumarin | C10H8O3 | 177.0546 | 82 | 7 | 0.002 | 0.007 | 0.999 |
| Umbelliferone | C9H6O3 | 163.0389 | 79 | 11.2 | 0.009 | 0.03 | 0.998 |
References
- Picardo, M.; Filatova, D.; Nuñez, O.; Farré, M. Recent advances in the detection of natural toxins in freshwater environments. Trends Anal. Chem. 2019, 112, 75–86. [Google Scholar] [CrossRef]
- Ferrão-Filho, A.d.S.; Kozlowsky-Suzuki, B. Cyanotoxins: Bioaccumulation and effects on aquatic animals. Mar. Drugs 2011, 9, 2729–2772. [Google Scholar] [CrossRef] [PubMed]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef] [PubMed]
- Neilan, B.A.; Pearson, L.A.; Muenchhoff, J.; Moffitt, M.C.; Dittmann, E. Environmental conditions that influence toxin biosynthesis in cyanobacteria. Environ. Microbiol. 2013, 15, 1239–1253. [Google Scholar] [CrossRef]
- Guidelines for Drinking-Water Quality, 2nd ed.; Addendum to Vol. 2. Health Criteria and Other Supporting Information; World Health Organization: Geneva, Switzerland, 1998.
- Zinedine, A.; Mañes, J. Occurrence and legislation of mycotoxins in food and feed from Morocco. Food Control. 2009, 20, 334–344. [Google Scholar] [CrossRef]
- Miller, J.D. Mycotoxins in food and feed: A Challenge for the twenty-first century. In Biology of Microfungi; Li, D.-W., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 469–493. [Google Scholar] [CrossRef]
- Oliveira, B.R.; Mata, A.T.; Ferreira, J.P.; Barreto Crespo, M.T.; Pereira, V.J.; Bronze, M.R. Production of mycotoxins by filamentous fungi in untreated surface water. Environ. Sci. Pollut. Res. 2018, 25, 17519–17528. [Google Scholar] [CrossRef]
- Mata, A.T.; Ferreira, J.; Oliveira, B.R.; Batoreu, C.; Crespo, M.T.; Pereira, V.; Bronze, M. Bottled water: Analysis of mycotoxins by LC-MS/MS. Food Chem. 2015, 176, 455–464. [Google Scholar] [CrossRef]
- Novak Babič, M.; Gunde-Cimerman, N.; Vargha, M.; Tischner, Z.; Magyar, D.; Veríssimo, C.; Sabino, R.; Viegas, C.; Meyer, W.; Brandão, J. Fungal contaminants in drinking water regulation? A tale of ecology, exposure, purification and clinical relevance. Int. J. Environ. Res. Public Health 2017, 14, 636. [Google Scholar] [CrossRef]
- Bucheli, T.D. Phytotoxins: Environmental micropollutants of concern? Environ. Sci. Technol. 2014, 48, 13027–13033. [Google Scholar] [CrossRef]
- Yamane, H.; Konno, K.; Sabelis, M.; Takabayashi, J.; Sassa, T.; Oikawa, H. 4.08—Chemical defence and toxins of plants. In Comprehensive Natural Products II; Liu, H.-W., Mander, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 339–385. [Google Scholar] [CrossRef]
- Clauson-Kaas, F.; Hansen, H.C.B.; Strobel, B.W. UPLC-MS/MS determination of ptaquiloside and pterosin B in preserved natural water. Anal. Bioanal. Chem. 2016, 408, 7981–7990. [Google Scholar] [CrossRef]
- Günthardt, B.F.; Hollender, J.; Hungerbühler, K.; Scheringer, M.; Bucheli, T.D. Comprehensive toxic plants–phytotoxins database and its application in assessing aquatic micropollution potential. J. Agric. Food Chem. 2018, 66, 7577–7588. [Google Scholar] [CrossRef] [PubMed]
- Virgilio, A.; Sinisi, A.; Russo, V.; Gerardo, S.; Santoro, A.; Galeone, A.; Taglialatela-Scafati, O.; Roperto, F. Ptaquiloside, the major carcinogen of bracken fern, in the pooled raw milk of healthy sheep and goats: An underestimated, global concern of food safety. J. Agric. Food Chem. 2015, 63, 4886–4892. [Google Scholar] [CrossRef] [PubMed]
- Hoerger, C.; Schenzel, J.; Strobel, B.; Bucheli, T. Analysis of selected phytotoxins and mycotoxins in environmental samples. Anal. Bioanal. Chem. 2009, 395, 1261–1289. [Google Scholar] [CrossRef] [PubMed]
- Griffith, A.W.; Gobler, C.J. Harmful algal blooms: A climate change co-stressor in marine and freshwater ecosystems. Harmful Algae 2020, 91, 101590. [Google Scholar] [CrossRef]
- Turner, A.D.; Dhanji-Rapkova, M.; O’Neill, A.; Coates, L.; Lewis, A.; Lewis, K. Analysis of microcystins in cyanobacterial blooms from freshwater bodies in England. Toxins 2018, 10, 39. [Google Scholar] [CrossRef]
- Sanseverino, I.; António, D.C.; Loos, R.; Lettieri, T. Cyanotoxins: Methods and Approaches for Their Analysis and Detection; EUR 28624; Publications Office of the European Union: Luxembourg, 2017. [Google Scholar] [CrossRef]
- Picardo, M.; Sanchís, J.; Núñez, O.; Farré, M. Suspect screening of natural toxins in surface and drinking water by high performance liquid chromatography and high-resolution mass spectrometry. Chemosphere 2020, 261, 127888. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Ninot, J.; Ferré, A.; Grau, O.; Castell, X.; Pérez-Haase, A.; Carrillo, E. Environmental drivers and plant species diversity in the Catalan and Andorran Pyrenees. Lazaroa 2013, 34, 89–105. [Google Scholar] [CrossRef]
- Mohanraj, S.; Subramanian, P.; Culvenor, C.; Edgar, J.; Frahn, J.; Smith, L.; Cockrum, P. Curassavine, an alkaloid from Heliotropium curassavicum Linn. with a C8 necic acid skeleton. J. Chem. Soc. Chem. Commun. 1978. [Google Scholar] [CrossRef]
- Moreno-Saiz, J.C.; Pataro, L.; Pajarón Sotomayor, S. Atlas de los pteridófitos de la Península Ibérica e Islas Baleares. Acta Bot. Malacit. 2015, 40, 5–55. [Google Scholar] [CrossRef]
- Ribeiro, D.D.S.F.; Keller, K.M.; Soto-Blanco, B. Ptaquiloside and pterosin B levels in mature green fronds and sprouts of Pteridium arachnoideum. Toxins 2020, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Agnew, M.P.; Lauren, D.R. Determination of ptaquiloside in bracken fern (Pteridium esculentum). J. Chromatogr. A 1991, 538, 462–468. [Google Scholar] [CrossRef]
- Marcé, R.; Armengol, J.; Dolz, J. Els efectes als embassaments i la seva rellevància en la quantitat i la qualitat de l’aigua per a la garantia del recurs. 2009. Available online: https://aca-web.gencat.cat/aca/documents/ca/publicacions/impactes_sobre_ecosistemes/capitol17_lowress.pdf. (accessed on 5 October 2020).
- Fortuño, P.; Bonada, N.; Prat, N.; Acosta, R.; Cañedo-Argüelles, M.; Castro, D.; Cid, N.; Múrria, C.; Pineda, D.; Rocha, K.; et al. Efectes del Canvi Ambiental en les comunitats d’organismes dels RIus MEDiterranis (CARIMED). Informe 2017. Diputació de Barcelona. Àrea d’Espais Naturals (Estudis de la Qualitat Ecològica dels Rius; 27). p 80. 2018. Available online: http://www.ub.edu/barcelonarius/web/index.php/informe-2017 (accessed on 5 October 2020).
- Jara, V.l.S. Comunidades de Cianobacterias Bentónicas, Producción y Liberación de Microcistinas en el río Muga (NE Península Ibérica). Ph.D. Thesis, Universitat de Girona, Girona, Spain, 2010. [Google Scholar]
- Sivonen, K.; Carmichael, W.; Namikoshi, M.; Rinehart, K.; Dahlem, A.; Niemelä, S. Isolation and characterization of hepatotoxic microcystin homologs from the filamentous freshwater cyanobacterium Nostoc sp. strain 152. Appl. Environ. Microbiol. 1990, 56, 2650–2657. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, J.H.; Kim, K.; Mun, H.; Park, N.; Jeon, J. Identification, quantification, and prioritization of new emerging pollutants in domestic and industrial effluents, Korea: Application of LC-HRMS based suspect and non-target screening. J. Hazard. Mater. 2020, 402. [Google Scholar] [CrossRef]
- Soni, P.; Siddiqui, A.A.; Dwivedi, J.; Soni, V. Pharmacological properties of Datura stramonium L. as a potential medicinal tree: An overview. Asian Pac. J. Trop. Biomed. 2012, 2, 1002–1008. [Google Scholar] [CrossRef]
- Shmuel, Y. Dictionary of Food Compounds with CD-ROM: Additives, Flavors, and Ingredients; Chapman & Hall/CRC: Boca Raton, FL, USA, 2004. [Google Scholar]
- U.S. Environmental Protection Agency. Chemistry Dashboard. p-Hydroxycinnamic Acid. Available online: https://comptox.epa.gov/dashboard/DTXSID6064660 (accessed on 3 April 2020).
- Fernández, H.; Kumar, A.; Revilla, M.A. Working with Ferns: Issues and Applications; Springer: New York, NY, USA, 2010. [Google Scholar]
- National Center for Biotechnology Information. PubChem Database. Reticuline, CID=439653. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Reticuline (accessed on 3 April 2020).
- Khalil, M.N.A.; Choucry, M.A.; El Senousy, A.S.; Hassan, A.; El-Marasy, S.A.; El Awdan, S.A.; Omar, F.A. Ambrosin, a potent NF-κβ inhibitor, ameliorates lipopolysaccharide induced memory impairment, comparison to curcumin. PLoS ONE 2019, 14, e0219378. [Google Scholar] [CrossRef]
- Mulder, P.P.J.; López, P.; Castelari, M.; Bodi, D.; Ronczka, S.; Preiss-Weigert, A.; These, A. Occurrence of pyrrolizidine alkaloids in animal- and plant-derived food: Results of a survey across Europe. Food Addit. Contam. Part A 2018, 35, 118–133. [Google Scholar] [CrossRef]
- Sethi, J.K.; Vidal-Puig, A.J. Thematic review series: Adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J. Lipid Res. 2007, 48, 1253–1262. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.; Jung, E.; Cho, J.Y.; Park, D. Artemisinic acid inhibits melanogenesis through downregulation of C/EBP α-dependent expression of HMG-CoA reductase gene. Food Chem. Toxicol. 2013, 51, 225–230. [Google Scholar] [CrossRef]
- Hua, X.; Yang, Q.; Zhang, W.; Dong, Z.; Yu, S.; Schwarz, S.; Liu, S. Antibacterial activity and mechanism of action of aspidinol against multi-drug-resistant methicillin-resistant staphylococcus aureus. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Chemistry Dashboard. Aspidospermine. Available online: https://comptox.epa.gov/dashboard/DTXSID70196883 (accessed on 3 April 2020).
- Fugh-Berman, A. The 5-Minute Herb and Dietary Supplement Consult; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003. [Google Scholar]
- Yoshida, T.; Nobuhara, J.; Uchida, M.; Okuda, T. Buddledin A, B and C, piscicidal sesquiterpenes from Buddleja davidii Franch. Tetrahedron Lett. 1976, 17, 3717–3720. [Google Scholar] [CrossRef]
- Furbee, B. Neurotoxic plants. Clin. Neurotoxicol. Syndr. Subst. Environ. 2009, 523–542. [Google Scholar] [CrossRef]
- Rodríguez, A.; Rodríguez, M.; Martín, A.; Nuñez, F.; Córdoba, J.J.J.F.C. Evaluation of hazard of aflatoxin B1, ochratoxin A and patulin production in dry-cured ham and early detection of producing moulds by qPCR. Food Control 2012, 27, 118–126. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 10748, 7-Methoxycoumarin. 2020. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/7-Methoxycoumarin (accessed on 28 October 2020).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 440933, Hygrine. 2020. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Hygrine (accessed on 28 October 2020).
- Dutcher, J.D. Aspergillic acid: An antibiotic substance produced by Aspergillus flavus i. general properties; formation of desoxyaspergillic acid; structural conclusions. J. Biol. Chem. 1947, 171, 321–339. [Google Scholar]
- Katz, Y.; Weizman, A.; Pick, C.G.; Pasternak, G.W.; Liu, L.; Fonia, O.; Gavish, M. Interactions between laudanosine, GABA, and opioid subtype receptors: Implication for laudanosine seizure activity. Brain Res. 1994, 646, 235–241. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 91471, Lupanine. 2020. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Lupanine (accessed on 28 October 2020).
- U.S. Environmental Protection Agency. Chemistry Dashboard. Methyl jasmonate. Available online: https://comptox.epa.gov/dashboard/DTXSID3036731 (accessed on 1 April 2020).
- Saxton, J.E. The Alkaloids; Royal Society of Chemistry: London, UK, 2007. [Google Scholar]
- Sánchez-Lamar, A.; Fonseca, G.; Fuentes, J.L.; Cozzi, R.; Cundari, E.; Fiore, M.; Ricordy, R.; Perticone, P.; Degrassi, F.; De Salvia, R. Assessment of the genotoxic risk of Punica granatum L. (Punicaceae) whole fruit extracts. J. Ethnopharmacol. 2008, 115, 416–422. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Chemistry Dashboard. 1-(1-Methyl-2-pyrrolidinyl)-2-propanone. Available online: https://comptox.epa.gov/dashboard/DTXSID40894081 (accessed on 3 April 2020).
- Mosbach, K.; Ljungcrantz, I. Biosynthetic studies on barnol, a novel phenolic compound of penicillium baarnense. Physiol. Plant. 1965, 18, 1–3. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Baile, C.A.; Zhu, S.; Shoji, M. Bioactive flavonoid p-hydroxycinnamic acid stimulates osteoblastogenesis and suppresses adipogenesis in bone marrow culture. Cell Tissue Res. 2013, 354, 743–750. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Chemistry Dashboard. Swainsonine. Available online: https://comptox.epa.gov/dashboard/DTXSID5046356 (accessed on 3 April 2020).
- U.S. Environmental Protection Agency. Chemistry Dashboard. Tetrahydrocannabivarin. Available online: https://comptox.epa.gov/dashboard/DTXSID10893920 (accessed on 3 April 2020).
- Recio, M.C.; Giner, R.M.; Uriburu, L.; Máñez, S.; Cerdá, M.; De La Fuente, J.R.; Ríos, J.L. In vivo activity of pseudoguaianolide sesquiterpene lactones in acute and chronic inflammation. Life Sci. 2000, 66, 2509–2518. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Database. Trachelanthamine, CID=26477. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Trachelanthamine (accessed on 3 April 2020).
- Passreiter, C.; Willuhn, G.; Röder/Roeder, E. Tussilagine and isotussilagine: Two pyrrolizidine alkaloids in the genus arnica 1. Planta Med. 1993, 58, 556–557. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Chemistry Dashboard. Umbelliferone. Available online: https://comptox.epa.gov/dashboard/DTXSID5052626 (accessed on 3 April 2020).
- U.S. Environmental Protection Agency. Chemistry Dashboard. Xanthotoxol. Available online: https://comptox.epa.gov/dashboard/DTXSID50173910 (accessed on 3 April 2020).
- U.S. Environmental Protection Agency. Chemistry Dashboard. Aflatoxin B1. Available online: https://comptox.epa.gov/dashboard/DTXSID9020035 (accessed on 3 April 2020).
- U.S. Environmental Protection Agency. Chemistry Dashboard. Aflatoxin B2. Available online: https://comptox.epa.gov/dashboard/DTXSID70222535 (accessed on 3 April 2020).
- U.S. Environmental Protection Agency. Chemistry Dashboard. alpha-Zearalenol. Available online: https://comptox.epa.gov/dashboard/DTXSID8022402 (accessed on 3 April 2020).
- U.S. Environmental Protection Agency. Chemistry Dashboard. ASPERGILLIC ACID ANALOG. Available online: https://comptox.epa.gov/dashboard/DTXSID20420024 (accessed on 3 April 2020).
- U.S. Environmental Protection Agency. Chemistry Dashboard. Averufin. Available online: https://comptox.epa.gov/dashboard/DTXSID10891789 (accessed on 3 April 2020).
- U.S. Environmental Protection Agency. Chemistry Dashboard. Kojic Acid. Available online: https://comptox.epa.gov/dashboard/DTXSID2040236 (accessed on 3 April 2020).
- U.S. Environmental Protection Agency. Chemistry Dashboard. Azelaic Acid, Potassium Salt. Available online: https://comptox.epa.gov/dashboard/DTXSID50173293 (accessed on 3 April 2020).
- U.S. Environmental Protection Agency. Chemistry Dashboard. Anatoxin a. Available online: https://comptox.epa.gov/dashboard/DTXSID50867064 (accessed on 3 April 2020).
- U.S. Environmental Protection Agency. Chemistry Dashboard. Microcystin LR. Available online: https://comptox.epa.gov/dashboard/DTXSID3031654 (accessed on 3 April 2020).
- U.S. Environmental Protection Agency. Chemistry Dashboard. Microcystin LW. Available online: https://comptox.epa.gov/dashboard/DTXSID70891285 (accessed on 3 April 2020).
- U.S. Environmental Protection Agency. Chemistry Dashboard. Microcystin YR. Available online: https://comptox.epa.gov/dashboard/DTXSID00880086 (accessed on 3 April 2020).
- U.S. Environmental Protection Agency. Chemistry Dashboard. Nodularin. Available online: https://comptox.epa.gov/dashboard/DTXSID60880022 (accessed on 3 April 2020).
- Carrasco, D.; Moreno, E.; Sanchis, D.; Wörmer, L.; Paniagua, T.; Del Cueto, A.; Quesada, A. Cyanobacterial abundance and microcystin occurrence in Mediterranean water reservoirs in Central Spain: Microcystins in the Madrid area. Eur. J. Phycol. 2006, 41, 281–291. [Google Scholar] [CrossRef]
- Krauss, M.; Singer, H.; Hollender, J. LC-high resolution MS in environmental analysis: From target screening to the identification of unknowns. Anal. Bioanal. Chem. 2010, 397, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Lipschitz, D.L.; Michel, W.C. Amino acid odorants stimulate microvillar sensory neurons. Chem. Senses 2002, 27, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, B.; Örnemark, U. Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; 2014; ISBN 978-91-87461-59-0. Available online: http://www.eurachem.org (accessed on 5 October 2020).


| Toxins | Formula | [M + H] | Rt | MS2 (1) | [M-e]+ | MS2 (2) | [M-e]+ | MS2 (3) | [M-e]+ | MS2 (4) | [M-e]+ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant Toxins | |||||||||||
| Acetoxytropane | C10H17NO2 | 184.1332 | 9.1 | 123.0805 | C8H11O | 142.0864 | C7H12NO2 | 125.0599 | C7H9O2 | 165.0913 | C10H13O2 |
| Aconosine | C22H35NO4 | 378.2639 | 11.3 | 283.1701 | C19H23O2 | 269.1539 | C18H21O2 | 235.1324 | C14H19O3 | 137.0599 | C8H9O2 |
| Anethole | C10H12O | 149.0961 | 9.8 | 115.0544 | C9H7 | 103.0543 | C8H7 | 145.065 | C10H9O | 121.0649 | C8H9O |
| Ambrosin | C15H18O3 | 247.1332 | 8.5 | 229.1227 | C15H17 O2 | 201.1267 | C13H13O2 | 119.0857 | C9H11 | ||
| Apiol | C12H14O4 | 223.0965 | 11.9 | 105.07 | C8H9 | 119.0857 | C9H11 | 163.0755 | C10H11O2 | 149.0963 | C10H13O |
| Arabsin | C15H22O4 | 266.1521 | 10.8 | 249.1488 | C15H21O3 | 231.1384 | C15H19O2 | 221.1539 | C14H21O2 | ||
| Artemisic acid | C15H22O2 | 235.1702 | 14 | 179.1069 | C11H15O2 | 165.0901 | C10H13O2 | 119.0853 | C9H11 | ||
| Aspidinol | C12H16O4 | 225.1121 | 9.5 | 107.0492 | C7H7O | 137.0599 | C8H9O2 | 123.0441 | C7H7O2 | 109.0649 | C7H7O |
| Aspidospermine | C22H30N2O2 | 355.2380 | 12.5 | 107.0492 | C7H7O | 136.0759 | C8H10NO | 174.0915 | C11H12NO | 148.0759 | C9H10NO |
| Azelaic acid | C9H16O4 | 189.1121 | 11.0 | 107.0854 | C8H11 | 155.0704 | C8H11O3 | 111.0806 | C7H11O | 115.0391 | C5H7O3 |
| Barnol | C10H14O3 | 183.1016 | 10.8 | 119.0857 | C9H11 | 135.0806 | C9H11O | 163.0755 | C10H11O2 | 181.086 | C10H13O3 |
| Bisabolol oxide | C15H26O2 | 239.2006 | 12.4 | 133.1013 | C10H13 | 121.1013 | C9H13 | 149.1326 | C11H17 | 187.1483 | C14H19 |
| Buddledin B | C15H22O2 | 235.1693 | 12.9 | 113.0598 | C6H9O2 | 179.0106 | C11H15O2 | 193.1225 | C12H17O2 | 155.1067 | C9H15O2 |
| Conhydrine | C8H17NO | 144.1383 | 11.6 | 107.0856 | C8H11 | 125.0962 | C8H11O | 138.0915 | C8H12NO | ||
| Cuscohygrine | C13H24N2O | 225.1961 | 12.3 | 123.0805 | C8H11O | 109.0649 | C7H9O | 163.1118 | C11H15O | 150.0914 | C9H12NO |
| Curassavine | C16H29NO4 | 300.2169 | 12.6 | 155.0703 | C8H11O3 | 107.0856 | C8H11 | 123.0805 | C8H11O | 173.081 | C8H13O4 |
| Herniarin | C10H8O3 | 176.0477 | 11.8 | 121.0649 | C7H5O2 | 133.0653 | C9H9O | ||||
| Hydroxyarbusculin A | C15H22O4 | 267.1585 | 13.3 | 159.1169 | C12H15 | 123.0805 | C8H11O | ||||
| Hydroxycoumarin | C9H6O3 | 163.0390 | 15.1 | 121.0284 | C7H5O2 | 149.0233 | C8H5O3 | 163.0389 | C9H7O3 | 105.0335 | C7H5O |
| Hygrine | C8H15NO | 142.1226 | 10.9 | 109.065 | C7H9O | 124.0758 | C7H10NO | 111.0804 | C7H11O | 140.1069 | C8H14NO |
| Hypoglycine A | C7H11NO2 | 142.0862 | 2.34 | 97.0287 | C5H5O2 | 120.0444 | C7H6NO | 124.0757 | C7H10NO | ||
| Laudanosine | C21H27NO4 | 358.2013 | 13.2 | 121.0285 | C7H5O2 | 115.0543 | C9H7 | 159.088 | C11H11O | 147.0805 | C10H11O |
| Lupanine | C15H24N2O | 249.1961 | 5.3 | 110.0965 | C7H12N | 120.0808 | C8H10N | 122.0966 | C8H12N | 138.0915 | C8H12NO |
| Methyl Jasmonate | C13 H20 O3 | 225.1485 | 0.1 | 107.0855 | C8H11 | 121.1012 | C8H13 | 175.112 | C12H15O | 165.1275 | C11H17O |
| Methylpelletierine | C9H17NO | 156.1386 | 2.2 | 107.0705 | C8H11 | 140.105 | C8H14NO | ||||
| Methylpseudoconhydrine | C9H19NO | 158.1539 | 11.9 | 107.0856 | C8H11 | 114.0914 | C6H12NO | 123.0805 | C8H11O | 109.0649 | C7H9O |
| Norpseudopelletierine | C8 H13NO | 140.1070 | 9.1 | 109.0649 | C7H9O | 121.0649 | C8H9O | 138.0917 | C8H12NO | 123.0806 | C8H11O |
| p-Coumaric acid | C9 H8 O3 | 165.0546 | 12.5 | 105.07 | C8H9 | 123.0441 | C7H7O2 | 133.0649 | C9H9O | 125.0598 | C7H9O2 |
| Ptaquilosin B | C14 H20 O3 | 237.1485 | 11.2 | 119.0857 | C9H11 | 159.0807 | C11H11O | 145.1013 | C11H13 | 111.0442 | C6H7O2 |
| Reticuline | C19 H23 N O4 | 330.1700 | 13.2 | 115.0543 | C9H7 | 125.0597 | C7H9O2 | 145.0646 | C10H9O | 135.0441 | C8H7O2 |
| Retronecine | C8 H13 N O2 | 156.1019 | 1.9 | 152.0709 | C8H10NO2 | 118.0652 | C8H8N | 114.0916 | C6H12NO | 124.0758 | C7H10NO |
| Swainsonine | C8 H15 N O3 | 174.1125 | 8.1 | 140.0682 | C7H10NO2 | 114.0914 | C6H12NO | 125.0598 | C7H9O2 | 118.0652 | C8H8N |
| Tetrahydrocannabivarin | C19 H26 O2 | 287.2006 | 12.9 | 105.07 | C8H9 | 163.1118 | C11H15O | 175.0755 | C11H11O2 | 217.0123 | C14H17O2 |
| Tetraneurin A | C17 H22 O6 | 323.1489 | 12.6 | 281.0996 | C14H17O6 | 199.0968 | C19H15O4 | 155.0704 | C8H11O3 | 213.112 | C11H17O4 |
| Trachelanthamine | C15 H27 N O4 | 286.2013 | 12.5 | 155.0704 | C8H11O3 | 107.085 | C8H11 | 159.0655 | C7H11O4 | 215.1269 | C11H19O4 |
| Tussilagine | C10 H17 N O3 | 200.1281 | 10.6 | 180.1021 | C10H14NO2 | 165.0912 | C10H13O2 | 151.0756 | C9H11O2 | 134.0967 | C9H12N |
| Umbelliferone | C9 H6 O3 | 163.0390 | 11.1 | 147.0441 | C9H7O2 | 135.0442 | C8H7O2 | 111.0441 | C6H7O2 | 123.0441 | C7H7O2 |
| Verrucosin | C20 H24 O5 | 345.1697 | 13.0 | 301.143 | C18H21O4 | 121.0286 | C7H5O2 | 141.0548 | C7H9O3 | 247.1332 | C15H19O3 |
| Xanthotoxol | C11H6O4 | 203.0348 | 1.3 | 147.1173 | C9H10O2 | 177.0188 | C9H5O4 | 173.0239 | C10H5O3 | ||
| Mycotoxins | |||||||||||
| Aflatoxin B1 | C17H12O6 | 313.0707 | 11.2 | 213.0547 | C13H9O3 | 269.0444 | C15H9O5 | 285.0761 | C16H13O5 | 217.0497 | C12H9O4 |
| Aflatoxin B2 | C17 H14 O6 | 315.0863 | 11.6 | 273.0761 | C15H13O5 | 255.0654 | C15H1104 | 68.9979 | C3HO2 | ||
| Alpha-Zearalenol | C18H24O5 | 321.1674 | 14.8 | 149.133 | C11H17 | 121.1016 | C9H13 | 139.1123 | C9H15O | ||
| Aspergillic acid | C12 H20 N2 O2 | 225.1598 | 9.4 | 114.0915 | C6H12NO | 144.0889 | C6H12N2O2 | 150.0915 | C9H12NO | 128.07 | C6H10NO2 |
| Averufin | C20 H16 O7 | 369.0969 | 10.6 | 327.0853 | C18H15O6 | 299.0555 | C16H11O6 | 137.0236 | C7H5O3 | ||
| Kojic Acid | C6H6O4 | 143.0344 | 1.38 | 125.0239 | C6H5O3 | 97.02844 | C5H5O2 | 69.0335 | C4H5O | ||
| Cyanotoxins | |||||||||||
| ANA-a | C10H15NO | 166.1226 | 0.5 | 149.1 | C10H13O | 131.0859 | C10H11 | 107.0858 | C8H11 | ||
| MC-LR | C49H74N10O12 | 995.556 | 9 | 135.0807 | C9H11O | 213.087 | C9H13N2O4 | 375.1914 | C20H27N2O5 | ||
| MC-LW | C54H72N8O12 | 1025.5343 | 12 | 135.0807 | C9H11O | 376.1926 | C19H21N10 | 288.1354 | C17H20O4 | ||
| MC-YR | C52H72N10O13 | 1045.5317 | 8.9 | 135.0807 | C9H11O | 375.1935 | C19H21N9 | 213.0874 | C9H13N2O4 | ||
| NOD | C41H60N8O10 | 824.4446 | 8.6 | 135.0807 | C9H11O | 389.2079 | C21H29NO5 | 691.3795 | C34H53O10N5 | ||
| Toxin | Month | Sampling Point | Concentration (µg L−1) |
|---|---|---|---|
| Ana-a | April | M1 | 0.12 |
| August | M1 | 0.03 | |
| September | M1 | 0.06 | |
| September | M2 | 0.28 | |
| Afla B1 | September | M4 | 0.9 |
| Kja | April | M4 | 0.7 |
| Nod | September | M1 | 0.1 |
| MC-YR | April August | M1 M1 | 0.1 0.2 |
| MC-LW | August | M1 | 0.4 |
| September | M1 | 0.1 | |
| MC-LR | April | M1 | 0.2 |
| September | M1 | 0.7 | |
| Umbelliferone | May | M3 | <LOD |
| July | M2 M3 | <LOD 0.1 | |
| August | M2 M3 | <LOD <LOD | |
| 7-methoxycoumarin | May | M2 M3 | 0.17 0.008 |
| July | M2 M3 | 0.08 0.18 | |
| August | M2 M3 | 0.06 0.03 | |
| September | M1 | 0.04 |
| Detection Frequency | Biodegradability * | Log BAF * | EC50 (mg/kg) | Score |
|---|---|---|---|---|
| <5% | Days | <2 | >1000 | 0 |
| 5~30% | Weeks | 2~3 | 100~1000 | 25 |
| 30~55% | Weeks–Months | 3~4 | 10~100 | 50 |
| 55~80% | Months | 4~5 | 1~10 | 75 |
| >80% | Recalcitrant | >5 | <1 | 100 |
| Toxin | CAS No. | Frequency % | Log Kow | Biodegradation Frame * | Log BAF * | LD50 (Mouse) mg/Kg | Effects | Ref. | Smileys |
|---|---|---|---|---|---|---|---|---|---|
| Phytotoxins | |||||||||
| Acetoxytropane | 3423-26-5 | 71 | 1.5 | Week–Months | 1 | 1830 | Diarrhoea and hypoactivity after administration of 50 and 200 mg/kg | [32] | CC(=O)OC12CCCC(N1C)CC2 |
| Aconosine | 38839-95-1 | 17 | 1.2 | Months | 0.5 | 0.27 | [33] | CCN1CC2CCC(C34C2CC(C31)C5(CC(C6CC4C5C6O)OC)O)OC | |
| Anethole | 104-46-1 | 13 | 2.7 | Weeks | 2.31 | 2090 | Lethal oral toxicity in rats at 2 g/kg | [34] | CC=CC1=CC=C(C=C1)OC |
| Alantolactone | 546-43-0 | 29 | 3.47 | Week–Months | 2.06 | 1200 | Carcinogenic/anticarcinogenic potential; Cytotoxic in vitro | [35] | CC1CCCC2(C1=CC3C(C2)OC(=O)C3=C)C |
| Ambrosin | 509-93-3 | 17 | 1,03 | Week–Months | 0.21 | NF-κβ inhibitor | [36,37] | CC1CCC2C(C3(C1C=CC3=O)C)OC(=O)C2=C | |
| Apiole | 523-80-8 | 38 | 2.7 | Week–Months | 2.21 | 4200 | Acute oral LD50 in rats 3.96 g/kg, in mice 1.52 g/kg; acute dermal LD50 in rabbits > 5 g/kg | [38] | COC1=C2C(=C(C(=C1)CC=C)OC)OCO2 |
| Arabsin | 38412-44-1 | 13 | 0.76 | Weeks | −0.02 | [39] | CC1C2CCC3(C(CC(=O)C(C3C2OC1=O)C)O)C | ||
| Artemisic acid | 80286-58-4 | 4 | 3.8 | Week–Months | 4.39 | 50 | Cytotoxicity | [40] | CC1CCC(C2C1CCC(=C2)C)C(=C)C(=O)O |
| Aspidinol | 519-40-4 | 13 | 2.6 | Week–Months | 1.01 | 50 | anti-MRSA activity, with antibacterial effect. Inhibition of the formation of the ribosome | [41] | CCCC(=O)C1=C(C(=C(C=C1O)OC)C)O |
| Aspidospermine | 466-49-9 | 13 | 3.78 | Recalcitrant | 1.76 | 46.3 | Cytotoxicity against mouse NIH3T3 cells | [42] | CCC12CCCN3C1C4(CC3)C(CC2)N(C5=C4C=CC=C5OC)C(=O)C |
| Bisabolol oxide B | 26184-88-3 | 21 | 2.5 | Months | 2.63 | 633 | Skin reaction; hepatic toxicity | [43] | CC1=CCC(CC1)C2(CCC(O2)C(C)(C)O)C |
| Buddledin B | 62346-21-8 | 13 | 2.9 | Week–Months | 2.97 | Piscicidal activity | [44] | CC1=CCCC(=C)C2CC(C2C(C1=O)O)(C)C | |
| Conhydrine | 495-20-5 | 50 | 1.21 | Months | 0.39 | 11 | Activation and then blocking of nicotinic acetylcholine receptors | [45] | CN1CCC23C4C1CC5=C2C(=C(C=C5)OC)OC3C(CC4)O |
| Cuscohygrine | 454-14-8 | 29 | 1 | Months | 0 | 111 | Autonomic nervous system blockade | [46] | CN1CCC[C@@H]1CC(=O)C[C@@H]2CCCN2C |
| Herniarin | 531-59-9 | 29 | 1.74 | Weeks | 0.72 | 4300 | Inhibition of human carbonic anhydrase with a concentration of 2.4 µM | [47] | COC1=CC2=C(C=C1)C=CC(=O)O2 |
| Hygrine | 496-49-1 | 29 | 0.5 | Week–Months | −0.02 | 91 | [48] | CC(=O)C[C@H]1CCCN1C | |
| Hypoglycine A | 156-56-9 | 33 | -2.5 | Day–Weeks | −0.05 | 98 | Jamaican vomiting sickness; hypoglycaemia and death; encephalopathy | [49] | C=C1CC1CC(C(=O)O)N |
| Laudanosine | 2688-77-9 | 25 | 3.7 | Months | 1.59 | 410 | GABA receptors interaction glycine receptors, involved in epilepsy and other types of seizures | [50] | CN1CCC2=CC(=C(C=C2C1CC3=CC(=C(C=C3)OC)OC)OC)OC |
| Lupanine | 550-90-3 | 38 | 1.6 | Week–Months | 0.65 | 410 | Tremor, Muscle contraction and dyspnoea within mouse | [51] | C1CCN2CC3CC(C2C1)CN4C3CCCC4=O |
| Methyl-Jasmonate | 1211-29-6 | 25 | 2.76 | Weeks | 1.25 | 5000 | Anti-inflammatory activity in LPS-stimulation within mouse | [52] | CCC=CCC1C(CCC1=O)CC(=O)OC |
| Methylpelletierine | 40199-45-9 | 17 | 0.8 | Week–Months | 0.05 | 40 | Taenicide | [53,54] | CC(=O)CC1CCCCN1C |
| Methylpseudoconhydrine | 140-55-6 | 46 | 1.5 | Week–Months | 0.33 | 250 | Antinociceptive | [55] | CC(C(C1=CC=CC=C1)O)N(C)C |
| Norpseudopelletierine | 4390-39-0 | 17 | 0.2 | Weeks | 0.15 | Causes severe skin burns and eye damage; genotoxic in vitro + in vivo | [56] | C1CC2CC(=O)CC(C1)N2 | |
| p-Coumaric acid | 7400-08-0 | 8 | 1.46 | Day–Weeks | 1.81 | 1.2 | Reproductive toxicity | [57] | C1=CC(=CC=C1C=CC(=O)O)O |
| Ptaquilosin B | 87625-62-5 | 33 | ND | Months | 0.42 | Generation of carcinogenic ADN adducts | [35] | CC1CC2(C=C(C3(CC3)C(C2C1=O)(C)O)C)O | |
| Reticuline | 485-19-8 | 0 | 3 | Months | 0.61 | 56 | Ptosis, somnolence, convulsions. | [36] | CN1CCC2=CC(=C(C=C2C1CC3=CC(=C(C=C3)OC)O)O)OC |
| Retronecine | 480-85-3 | 71 | -0.56 | Weeks | −0.04 | 634 | Carcinogenic, pulmonary oedema, blood lymphoma, convulsions | [38] | C1CN2CC=C(C2C1O)CO |
| Swainsonine | 72741-87-8 | 17 | -1.3 | Weeks | −0.05 | 0.35 | Locoweed intoxication; It is a potent inhibitor of Golgi alpha-mannosidase II | [58] | C1CC(C2C(C(CN2C1)O)O)O |
| Tetrahydro-cannabivarin | 31262-37-0 | 21 | 5.76 | Months | 3.06 | 3 | Neurotoxicity | [59] | CCCC1=CC(=C2C3C=C(CCC3C(OC2=C1)(C)C)C)O |
| Tetraneurin A | 22621-72-3 | 29 | 0.6 | Week–Months | −0.04 | 42 | Antiviral activity; Ear thickness in rats; dermatitis | [60] | CC(=O)OCC1CCC2C(C3(C1(CCC3=O)O)C)OC(=O)C2=C |
| Trachelanthamine | 14140-18-2 | 0 | 1.4 | Week–Months | 0.69 | 1500 | Somnolence, tremor, muscle weakness | [61] | CC(C)C(C(C)O)(C(=O)OCC1CCN2C1CCC2)O |
| Tussilagine | 80151-77-5 | 8 | 0.6 | Week–Months | −0.04 | 28.8 | Carcinogenic in vivo | [43,62] | CC1(CN2CCCC2C1C(=O)OC)O |
| Umbelliferone | 93-35-6 | 21 | 1,58 | Weeks | 0.4 | 10000 | Inhibition of human carbonic anhydrase 9 catalytic domain | [63] | C1=CC(=CC2=C1C=CC(=O)O2)O |
| Xanthotoxol | 2009-24-7 | 29 | 1.16 | Weeks | 0.22 | 480 | Inhibitors of Secretory Acid Sphingomyelinase (S-ASM); | [64] | C1=CC(=O)OC2=C(C3=C(C=CO3)C=C21)O |
| Mycotoxins | |||||||||
| Aflatoxin B1 | 1162-65-8 | 13 | 1.45 | Week–Months | 0.1 | 3.2 | Carcinogenic, terathogenic | [65] | COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4C5C=COC5OC4=C1 |
| Aflatoxin B2 | 7220-81-7 | 25 | 0.855 | Week–Months | 0.18 | 100 | Carcinogenic, terathogenic; hepatotoxic | [66] | COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4C5CCOC5OC4=C1 |
| Alpha-Zearalenol | 36455-72-8 | 29 | 4 | Weeks | 1.41 | 0.010 | Chronic toxicity and carcinogenic | [67] | CC1CCCC(CCCC=CC2=C(C(=CC(=C2)O)O)C(=O)O1)O |
| Aspergillic acid | 2152-59-2 | 13 | 1.7 | Week–Months | 0.8 | 100 | Antibiotic substance; animal toxicity | [49,68] | CCC(C)C1=CN=C(C(=O)N1O)CC(C)C |
| Averufin | 14016-29-6 | 17 | 3 | Months | 1.09 | 20.64 | Inhibition of deaminase | [69] | CC12CCCC(O1)C3=C(O2)C=C4C(=C3O)C(=O)C5=C(C4=O)C=C(C=C5O)O |
| Kojic Acid | 501-30-4 | 8 | -0,64 | Weeks | −0.05 | 23.8 | Inhibition of human recombinant DAAO | [70] | C1=C(OC=C(C1=O)O)CO |
| Azelaic acid | 19619-43-3 | 13 | 1.55 | Day–Weeks | 0.64 | 5 | Irritant | [71] | C(CCCC(=O)O)CCCC(=O)O |
| Barnol | 2151-18-0 | 0 | 2.26 | Week–Months | 0.79 | [56,62] | CCC1=C(C(=C(C(=C1C)O)O)O)C | ||
| Cyanotoxins | |||||||||
| Anatoxin-a | 64285-06-9 | 17 | 0.8 | Weeks | 0.36 | 420 | Neurotoxicity; muscular fasciculation, respiratory paralysis. | [72] | CC(=O)C1=CCCC2CCC1N2 |
| MC-LR | 101043-37-2 | 8 | -1.2 | Recalcitrant | −0.01 | 5 | Hepatotoxicity; visual disturbance, respiratory irritation; vomiting, and muscle weakness | [73] | CC1C(NC(=O)C(NC(=O)C(C(NC(=O)C(NC(=O)C(NC(=O)C(=C)N(C(=O)CCC(NC1=O) C(=O)O)C)C)CC(C)C)C(=O)O)C)CCCN=C(N)N)C=CC(=CC(C)C(CC2=CC=CC=C2)OC)C |
| MC-LW | 157622-02-1 | 8 | 5.2 | Recalcitrant | 0.81 | 0.25-0.33 | Hepatotoxicity; visual disturbance, respiratory irritation; vomiting, and muscle weakness | [74] | CC1C(NC(=O)C(NC(=O)C(C(NC(=O)C(NC(=O)C(NC(=O)C(=C)N(C(=O)CCC(NC1=O)C(=O) O)C)C)CC(C)C)C(=O)O)C)CC2=CNC3=CC=CC=C32)C=CC(=CC(C)C(CC4=CC=CC=C4)OC)C |
| MC-YR | 101064-48-6 | 8 | -0.2 | Recalcitrant | −0.02 | 40 | Hepatotoxicity; visual disturbance, respiratory irritation; vomiting, and muscle weakness | [75] | CC1C(NC(=O)C(NC(=O)C(C(NC(=O)C(NC(=O)C(NC(=O)C(=C)N(C(=O)CCC(NC1=O)C (=O)O)C)C)CC2=CC=C(C=C2)O)C(=O)O)C)CCCN=C(N)N)C=CC(=CC(C)C(CC3=CC=CC=C3)OC)C |
| Nodularin | 118399-22-7 | 4 | 1.7 | Months | −0.04 | 0.060 | Hepatotoxicity; visual disturbance, respiratory irritation; vomiting, and muscle weakness | [76] | CC=C1C(=O)NC(C(C(=O)NC(C(=O)NC(C(C(=O)NC(CCC(=O)N1C)C(=O)O)C)C=CC(=CC(C)C (CC2=CC=CC=C2)OC)C)CCCN=C(N)N)C)C(=O)O |
| Ranking | Tentatively Identified Substance |
|---|---|
| 325 | Tetrahydrocannabivarin |
| 325 | MC-LW |
| 300 | Aconosine |
| 300 | MC-LR |
| 275 | MC-YR |
| 275 | Nodularin |
| 250 | Aflatoxin B1 |
| 250 | Alpha-Zearalenol |
| 225 | Ptaquilosin B |
| 225 | Retronecine |
| 225 | Tussilagine |
| 225 | Aflatoxin B2 |
| 200 | Aspidospermine |
| 175 | Artemisic acid |
| 175 | Conhydrine |
| 175 | Anatoxin-a |
| 150 | Bisabolol oxide B |
| 150 | Swainsonine |
| 150 | Averufin |
| 125 | Acetoxytropane |
| 125 | Apiole |
| 125 | Aspidinol |
| 125 | Cuscohygrine |
| 125 | Hygrine |
| 125 | Laudanosine |
| 125 | Lupanine |
| 125 | Methylpelletierine |
| 125 | Methylpseudoconhydrine |
| 125 | Reticuline |
| 125 | Tetraneurin A |
| 125 | Aspergillic acid |
| 100 | Alantolactone |
| 100 | Buddledin B |
| 100 | Hypoglycine A |
| 100 | p-Coumaric acid |
| 100 | Kojic Acid |
| 100 | Azelaic acid |
| 75 | Anethole |
| 75 | Ambrosin |
| 75 | Xanthotoxol |
| 50 | Arabsin |
| 50 | Herniarin |
| 50 | Methyl-Jasmonate |
| 50 | Norpseudopelletierine |
| 50 | Trachelanthamine |
| 50 | Umbelliferone |
| 50 | Barnol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picardo, M.; Núñez, O.; Farré, M. Suspect and Target Screening of Natural Toxins in the Ter River Catchment Area in NE Spain and Prioritisation by Their Toxicity. Toxins 2020, 12, 752. https://doi.org/10.3390/toxins12120752
Picardo M, Núñez O, Farré M. Suspect and Target Screening of Natural Toxins in the Ter River Catchment Area in NE Spain and Prioritisation by Their Toxicity. Toxins. 2020; 12(12):752. https://doi.org/10.3390/toxins12120752
Chicago/Turabian StylePicardo, Massimo, Oscar Núñez, and Marinella Farré. 2020. "Suspect and Target Screening of Natural Toxins in the Ter River Catchment Area in NE Spain and Prioritisation by Their Toxicity" Toxins 12, no. 12: 752. https://doi.org/10.3390/toxins12120752
APA StylePicardo, M., Núñez, O., & Farré, M. (2020). Suspect and Target Screening of Natural Toxins in the Ter River Catchment Area in NE Spain and Prioritisation by Their Toxicity. Toxins, 12(12), 752. https://doi.org/10.3390/toxins12120752






