Camelid VHH Antibodies that Neutralize Botulinum Neurotoxin Serotype E Intoxication or Protease Function
Abstract
1. Introduction
2. Results
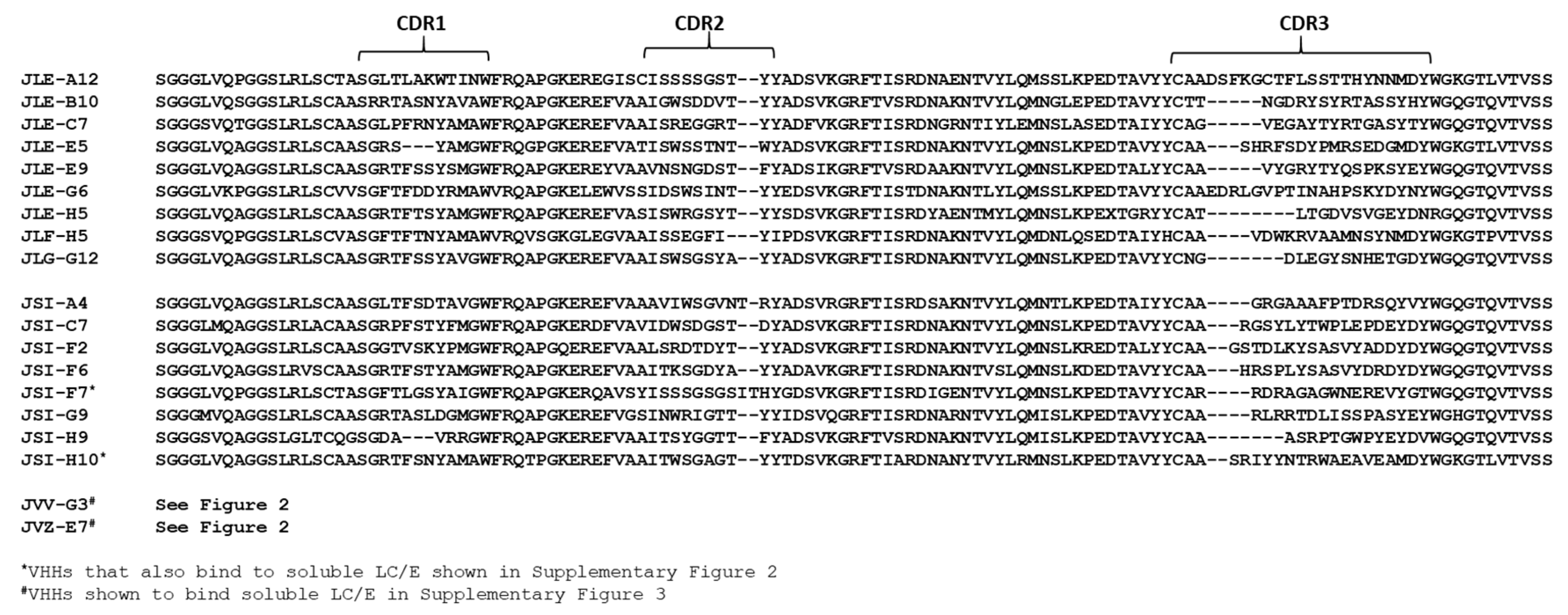
2.1. Discovery and Characterization of BoNT/E and LC/E Binding VHHs
2.1.1. Selection of VHHs Binding ciBoNTE or LC/E Adsorbed to Plastic
2.1.2. Selection of VHHs Recognizing Antibody-Captured ciBoNTE
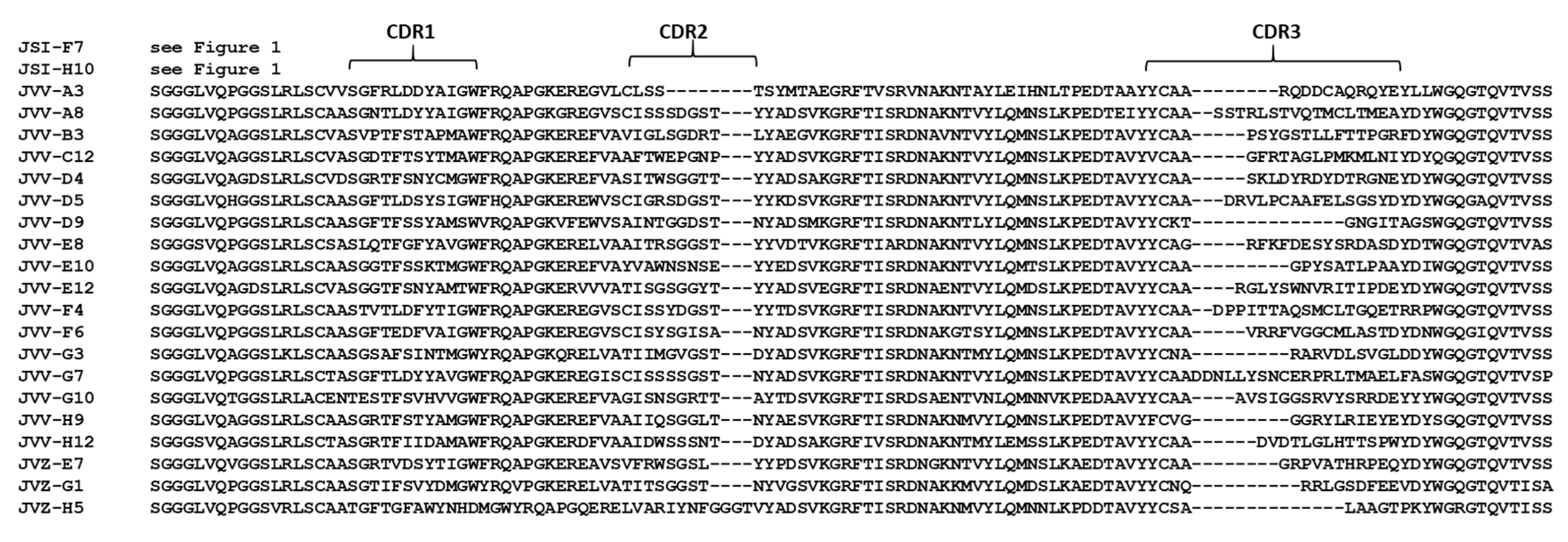
2.1.3. Selection of VHHs Recognizing VHH-Captured LC/E
2.2. VHH and VNA Neutralization of BoNT/E Intoxication
2.2.1. VHH Monomer Neutralization of BoNT/E in Neuronal Cultures
2.2.2. VHH Monomer Neutralization of BoNT/E in Mice
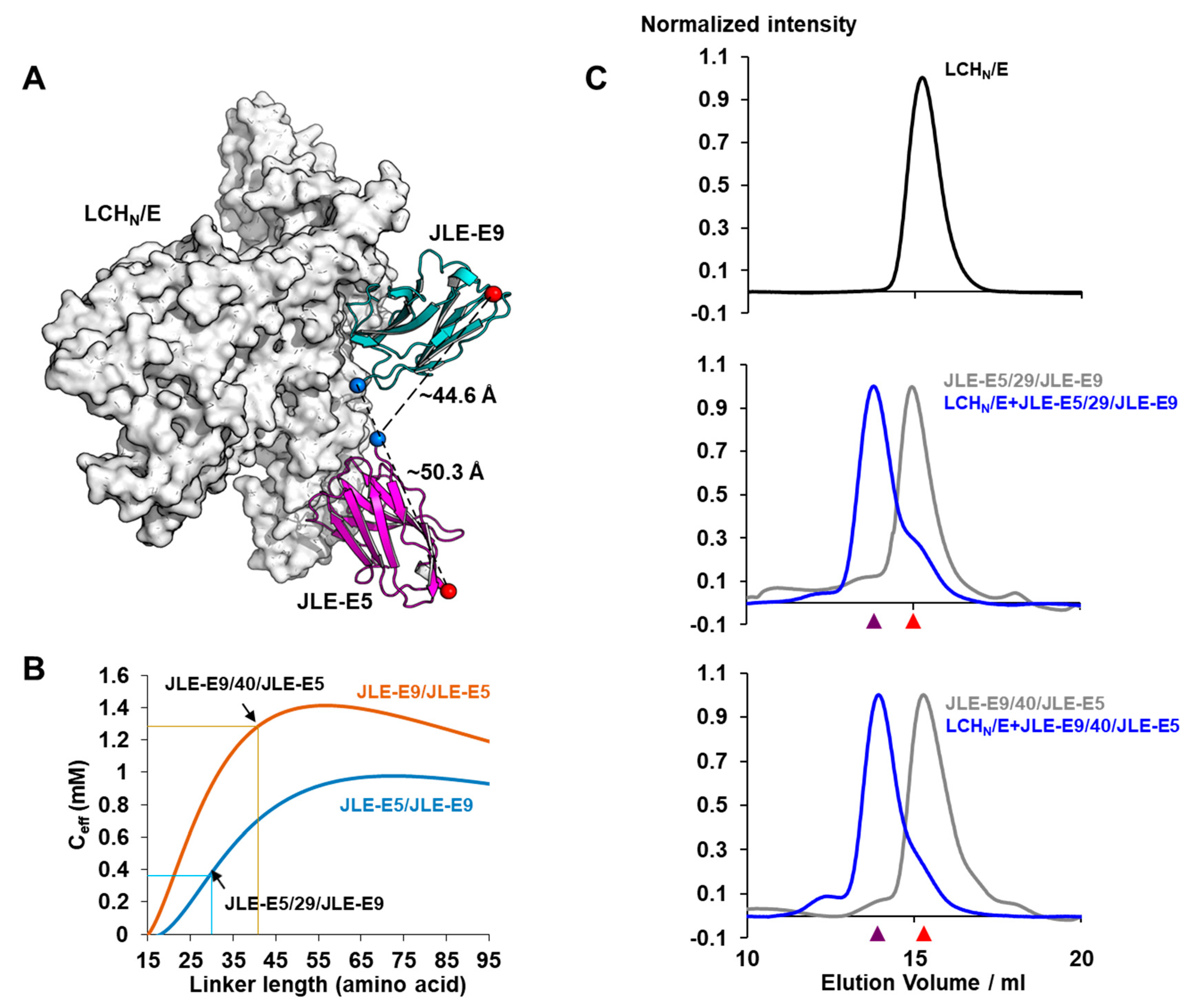
2.2.3. VHH Heterodimer Neutralization of BoNT/E in Mice
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Ethics Statement and Disclaimers
5.2. Toxins and Reagents
5.2.1. BoNT/E Toxin and ciBoNTE Immunogen
5.2.2. LC/E Protein
5.3. Alpaca Immunization, VHH Discovery and Purification of Recombinant Proteins
5.4. Dilution ELISAs
5.5. Competition Assays
5.6. Cell-Based BoNT/E Neutralization Assays
5.7. LC/E Protease Inhibition Assay
5.8. Standard Mouse Toxin Lethality Assay
5.9. Gel Filtration Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dong, M.; Masuyer, G.; Stenmark, P. Botulinum and Tetanus Neurotoxins. Annu. Rev. Biochem. 2019, 88, 811–837. [Google Scholar] [CrossRef] [PubMed]
- Arnon, S.S. Creation and development of the public service orphan drug Human Botulism Immune Globulin. Pediatrics 2007, 119, 785–789. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Investigational heptavalent botulinum antitoxin (HBAT) to replace licensed botulinum antitoxin AB and investigational botulinum antitoxin E. Morb. Mortal. Wkly. Rep. 2010, 59, 299. [Google Scholar]
- Nayak, S.U.; Griffiss, J.M.; McKenzie, R.; Fuchs, E.J.; Jurao, R.A.; An, A.T. Safety and pharmacokinetics of XOMA 3AB, a novel mixture of three monoclonal antibodies against botulinum toxin A. Antimicrob. Agents Chemother. 2014, 58, 5047–5053. [Google Scholar] [CrossRef]
- Snow, D.M.; Riling, K.; Kimbler, A.; Espinoza, Y.; Wong, D.; Pham, K. Safety and Pharmacokinetics of a Four Monoclonal Antibody Combination Against Botulinum C and D Neurotoxins. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Dock, M.; Ben Ali, A.; Karras, A.; Misset, B.; Garrouste-Orgeas, M.; Deletie, E. [Treatment of severe botulism with 3,4-diaminopyridine]. La Presse Med. 2002, 31, 601–602. [Google Scholar]
- Vazquez-Cintron, E.J.; Beske, P.H.; Tenezaca, L.; Tran, B.Q.; Oyler, J.M.; Glotfelty, E.J. Engineering Botulinum Neurotoxin C1 as a Molecular Vehicle for Intra-Neuronal Drug Delivery. Sci. Rep. 2017, 7, 42923. [Google Scholar] [CrossRef]
- Thanongsaksrikul, J.; Chaicumpa, W. Botulinum neurotoxins and botulism: A novel therapeutic approach. Toxins 2011, 3, 469–488. [Google Scholar] [CrossRef]
- Alirahimi, E.; Kazemi-Lomedasht, F.; Shahbazzadeh, D.; Habibi-Anbouhi, M.; Hosseininejad, C.M.; Sotoudeh, N. Nanobodies as novel therapeutic agents in envenomation. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2955–2965. [Google Scholar] [CrossRef]
- Muyldermans, S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef]
- Moayeri, M.; Leysath, C.E.; Tremblay, J.M.; Vrentas, C.; Crown, D.; Leppla, S.H. A Heterodimer of a VHH Antibody that Inhibits Anthrax Toxin Cell Binding Linked to a VHH that Blocks Oligomer Formation is Highly Protective in an Anthrax Spore Challenge Model. J. Biol. Chem. 2015. [Google Scholar] [CrossRef]
- Mukherjee, J.; Dmitriev, I.; Debatis, M.; Tremblay, J.M.; Beamer, G.; Kashentseva, E.A. Prolonged prophylactic protection from botulism with a single adenovirus treatment promoting serum expression of a VHH-based antitoxin protein. PLoS ONE 2014, 9, e106422. [Google Scholar] [CrossRef]
- Vance, D.J.; Tremblay, J.M.; Mantis, N.J.; Shoemaker, C.B. Stepwise engineering of heterodimeric single domain camelid VHH antibodies that passively protect mice from ricin toxin. J. Biol. Chem. 2013, 288, 36538–36547. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.J.; Beamer, G.; Tremblay, J.M.; Steele, J.A.; Kim, H.B.; Wang, Y. A Tetraspecific VHH-based Neutralizing Antibody Modifies Disease Outcome in Three Animal Models of Clostridium difficile Infection. Clin. Vaccine Immunol. 2016, 23, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Sheoran, A.S.; Dmitriev, I.P.; Kashentseva, E.A.; Cohen, O.; Mukherjee, J.; Debatis, M. Adenovirus vector expressing Stx1/Stx2-neutralizing agent protects piglets infected with Escherichia coli O157:H7 against fatal systemic intoxication. Infect. Immun. 2015, 83, 286–291. [Google Scholar] [CrossRef]
- Tremblay, J.M.; Mukherjee, J.; Leysath, C.E.; Debatis, M.; Ofori, K.; Baldwin, K. A single VHH-based toxin-neutralizing agent and an effector antibody protect mice against challenge with Shiga toxins 1 and 2. Infect. Immun. 2013, 81, 4592–4603. [Google Scholar] [CrossRef] [PubMed]
- Vrentas, C.E.; Moayeri, M.; Keefer, A.B.; Greaney, A.J.; Tremblay, J.; O’Mard, D. A diverse set of single-domain antibodies (VHHs) against the anthrax toxin lethal and edema factors provides a basis for construction of a bispecific agent that protects against anthrax infection. J. Biol. Chem. 2016, 291, 21596–21606. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Schmidt, D.; Liu, W.; Li, S.; Shi, L.; Sheng, J. A novel multivalent, single-domain antibody targeting TcdA and TcdB prevents fulminant Clostridium difficile infection in mice. J. Infect. Dis. 2014, 210, 964–972. [Google Scholar] [CrossRef]
- Sepulveda, J.; Mukherjee, J.; Tzipori, S.; Simpson, L.L.; Shoemaker, C.B. Efficient serum clearance of botulinum neurotoxin achieved using a pool of small antitoxin binding agents. Infect. Immun. 2010, 78, 756–763. [Google Scholar] [CrossRef]
- Thran, M.; Mukherjee, J.; Ponisch, M.; Fiedler, K.; Thess, A.; Mui, B.L. mRNA mediates passive vaccination against infectious agents, toxins, and tumors. EMBO Mol. Med. 2017, 9, 1434–1447. [Google Scholar] [CrossRef]
- Mukherjee, J.; Tremblay, J.M.; Leysath, C.E.; Ofori, K.; Baldwin, K.; Feng, X. A novel strategy for development of recombinant antitoxin therapeutics tested in a mouse botulism model. PLoS ONE 2012, 7, e29941. [Google Scholar] [CrossRef]
- Tremblay, J.M.; Kuo, C.L.; Abeijon, C.; Sepulveda, J.; Oyler, G.; Hu, X. Camelid single domain antibodies (VHHs) as neuronal cell intrabody binding agents and inhibitors of Clostridium botulinum neurotoxin (BoNT) proteases. Toxicon 2010, 56, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Godakova, S.A.; Noskov, A.N.; Vinogradova, I.D.; Ugriumova, G.A.; Solovyev, A.I.; Esmagambetov, I.B. Camelid VHHs Fused to Human Fc Fragments Provide Long Term Protection Against Botulinum Neurotoxin A in Mice. Toxins 2019, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Thompson, A.A.; Fan, Y.; Lou, J.; Conrad, F.; Ho, M. A single-domain llama antibody potently inhibits the enzymatic activity of botulinum neurotoxin by binding to the non-catalytic alpha-exosite binding region. J. Mol. Biol. 2010, 397, 1106–1118. [Google Scholar] [CrossRef]
- Lam, K.H.; Tremblay, J.M.; Vazquez-Cintron, E.; Perry, K.; Ondeck, C.; Webb, R.P. Structural Insights into Rational Design of Single-Domain Antibody-Based Antitoxins against Botulinum Neurotoxins. Cell Rep. 2020, 30, 2526–2539.e6. [Google Scholar] [CrossRef]
- Bakherad, H.; Gargari, S.L.M.; Rasooli, I.; Rajabibazl, M.; Mohammadi, M.; Ebrahimizadeh, W. In vivo neutralization of botulinum neurotoxins serotype E with heavy-chain camelid antibodies (VHH). Mol. Biotechnol. 2013, 55, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.J.; Pishesha, N.; Mukherjee, J.; Zhang, S.; Deshycka, R.; Sudaryo, V. Genetically engineered red cells expressing single domain camelid antibodies confer long-term protection against botulinum neurotoxin. Nat. Commun. 2017, 8, 423. [Google Scholar] [CrossRef]
- Lam, K.H.; Perry, K.; Shoemaker, C.B.; Jin, R. Two VHH antibodies neutralize botulinum neurotoxin E1 by blocking its membrane translocation in host cells. Toxins 2020, in press. [Google Scholar]
- Webb, R.P.; Smith, T.J.; Smith, L.A.; Wright, P.M.; Guernieri, R.L.; Brown, J.L. Recombinant Botulinum Neurotoxin Hc Subunit (BoNT Hc) and Catalytically Inactive Clostridium botulinum Holoproteins (ciBoNT HPs) as Vaccine Candidates for the Prevention of Botulism. Toxins 2017, 9, 269. [Google Scholar] [CrossRef]
- Lerman, M.J.; Lembong, J.; Muramoto, S.; Gillen, G.; Fisher, J.P. The Evolution of Polystyrene as a Cell Culture Material. Tissue Eng. Part B Rev. 2018, 24, 359–372. [Google Scholar] [CrossRef]
- Beske, P.H.; Bradford, A.B.; Grynovicki, J.O.; Glotfelty, E.J.; Hoffman, K.M.; Hubbard, K.S. Botulinum and Tetanus Neurotoxin-Induced Blockade of Synaptic Transmission in Networked Cultures of Human and Rodent Neurons. Toxicol. Sci. 2016, 149, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.X. Quantitative account of the enhanced affinity of two linked scFvs specific for different epitopes on the same antigen. J. Mol. Biol. 2003, 329, 1–8. [Google Scholar] [CrossRef]
- Webb, R.P.; Smith, T.J.; Wright, P.; Brown, J.; Smith, L.A. Production of catalytically inactive BoNT/A1 holoprotein and comparison with BoNT/A1 subunit vaccines against toxin subtypes A1, A2, and A3. Vaccine 2009, 27, 4490–4497. [Google Scholar] [CrossRef] [PubMed]
- Webb, R.; Wright, P.M.; Brown, J.L.; Skerry, J.C.; Guernieri, R.L.; Smith, T.J. Potency and stability of a trivalent, catalytically inactive vaccine against botulinum neurotoxin serotypes C, E and F (triCEF). Toxicon 2020, 176, 67–76. [Google Scholar] [CrossRef]
- Vance, D.J.; Tremblay, J.M.; Rong, Y.; Angalakurthi, S.K.; Volkin, D.B.; Middaugh, C.R. High-Resolution Epitope Positioning of a Large Collection of Neutralizing and Nonneutralizing Single-Domain Antibodies on the Enzymatic and Binding Subunits of Ricin Toxin. Clin. Vaccine Immunol. 2017, 24. [Google Scholar] [CrossRef]
- Nizak, C.; Moutel, S.; Goud, B.; Perez, F. Selection and application of recombinant antibodies as sensors of rab protein conformation. Methods Enzymol. 2005, 403, 135–153. [Google Scholar] [CrossRef]
- Pardon, E.; Laeremans, T.; Triest, S.; Rasmussen, S.G.; Wohlkonig, A.; Ruf, A. A general protocol for the generation of Nanobodies for structural biology. Nat. Protoc. 2014, 9, 674–693. [Google Scholar] [CrossRef]
- Chen, S.; Barbieri, J.T. Solubility of the catalytic domains of Botulinum neurotoxin serotype E subtypes. Protein Expr. Purif. 2016, 118, 18–24. [Google Scholar] [CrossRef]
- Puhar, A.; Johnson, E.A.; Rossetto, O.; Montecucco, C. Comparison of the pH-induced conformational change of different clostridial neurotoxins. Biochem. Biophys. Res. Commun. 2004, 319, 66–71. [Google Scholar] [CrossRef]
- Cai, S.; Kukreja, R.; Shoesmith, S.; Chang, T.W.; Singh, B.R. Botulinum neurotoxin light chain refolds at endosomal pH for its translocation. Protein J. 2006, 25, 455–462. [Google Scholar] [CrossRef]
- Kumar, R.; Kukreja, R.V.; Cai, S.; Singh, B.R. Differential role of molten globule and protein folding in distinguishing unique features of botulinum neurotoxin. Biochim. Biophys. Acta 2014, 1844, 1145–1152. [Google Scholar] [CrossRef]
- Chen, C.; Przedpelski, A.; Tepp, W.H.; Pellett, S.; Johnson, E.A.; Barbieri, J.T. Heat-Labile Enterotoxin IIa, a Platform To Deliver Heterologous Proteins into Neurons. mBio 2015, 6, e00734. [Google Scholar] [CrossRef]
- Bade, S.; Rummel, A.; Reisinger, C.; Karnath, T.; Ahnert-Hilger, G.; Bigalke, H. Botulinum neurotoxin type D enables cytosolic delivery of enzymatically active cargo proteins to neurones via unfolded translocation intermediates. J. Neurochem. 2004, 91, 1461–1472. [Google Scholar] [CrossRef]
- Council, N.R. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011; 246p. [Google Scholar]
- Pearce, L.B.; Borodic, G.E.; First, E.R.; MacCallum, R.D. Measurement of botulinum toxin activity: Evaluation of the lethality assay. Toxicol. Appl. Pharmacol. 1994, 128, 69–77. [Google Scholar] [CrossRef]
- Studier, F.W. Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 2005, 41, 207–234. [Google Scholar] [CrossRef]
- Eubanks, L.M.; Silhar, P.; Salzameda, N.T.; Zakhari, J.S.; Xiaochuan, F.; Barbieri, J.T. Identification of a Natural Product Antagonist against the Botulinum Neurotoxin Light Chain Protease. ACS Med. Chem. Lett. 2010, 1, 268–272. [Google Scholar] [CrossRef]
- Gut, I.M.; Beske, P.H.; Hubbard, K.S.; Lyman, M.E.; Hamilton, T.A.; McNutt, P.M. Novel application of stem cell-derived neurons to evaluate the time- and dose-dependent progression of excitotoxic injury. PLoS ONE 2013, 8, e64423. [Google Scholar] [CrossRef]
- Vazquez-Cintron, E.; Machamer, J.; Ondeck, C.; Pagarigan, K.; Winner, B.; Bodner, P. Symptomatic treatment of botulism with a clinically approved small molecule. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed]
| VHH Name | ciBoNTE-Binding EC50, nM Nunc A | ciBoNTE-Binding EC50, nM Costar A | HNE-Binding EC50, nM Costar B | HCE-Binding EC50, nM Costar B | LC/E-Binding A EC50, nM Nunc A | BoNT/E Neutralization C | Competition Group D |
|---|---|---|---|---|---|---|---|
| JLE-A12 | 1 | 1 | NB | NB | NB | - | 1 |
| JLE-B10 | 3 | 10 | NB | NB | NB | - | 1 |
| JLE-C7 | 3 | 1 | NB | NB | NB | - | 1 |
| JLE-E5 | 1 | 1 | NB | NB | NB | + | 2 |
| JLE-E9 | 1 | 1 | NB | NB | NB | + | 1 |
| JLE-G6 | 1 | 20 | NB | 3 | NB | + | 3 |
| JLE-H5 | 10 | >50 | 20 | NB | NB | - | 1 |
| JLF-H5 | 20 | >100 | NB | NB | 10 | - | 4 |
| JLG-G12 | 3 | >100 | NB | NB | 2 | - | 4 |
| VHH Name | ciBoNTE-Binding EC50, nM Captured A | LC/E-Binding EC50, nM Captured A | HNE-Binding EC50, nM Costar B | HCE-Binding EC50, nM Costar B | BoNT/E Neutralization C | Competition Group D |
|---|---|---|---|---|---|---|
| JSI-A4 | 10 | NB | NB | NB | + | 5 |
| JSI-C7 | 0.8 | NB | NB | NB | + | 6 |
| JSI-F2 | 0.5 | NB | NB | 2 | + | 7 |
| JSI-F6 | 1 | NB | NB | 3 | - | 8 |
| JSI-F7 | 0.3 | 2 | NB | NB | + | 9 |
| JSI-G9 | 0.3 | NB | 5 | NB | + | 10 |
| JSI-H9 | 0.6 | NB | >100 | NB | - | 6 |
| JSI-H10 | 0.2 | 0.4 | NB | NB | - | 6 |
| JVV-G3 E | 2 | 0.6 | ND | ND | - | 11 |
| JVZ-E7 E | 3 | 0.4 | ND | ND | - | 11 |
| VHH Name | LC/E-Binding EC50 (nM) Nunc A | LC/E-Binding EC50 (nM) Costar A | LC/E-Binding EC50 (nM) JSI-F7-Captured A | LC/E Protease Inhibition |
|---|---|---|---|---|
| JSI-F7 B | >25 | >25 | >25 * | - |
| JSI-H10 B | >25 | >25 | 0.03 | - |
| JVV-A3 | >25 | >25 | 0.4 | ++ |
| JVV-A8 | >25 | >25 | 0.8 | ++ |
| JVV-B3 | >25 | 10 | 0.4 | ++ |
| JVV-C12 | >25 | 10 | 0.2 | - |
| JVV-D4 | >25 | >25 | 0.4 | - |
| JVV-D5 | >25 | >25 | 0.2 | - |
| JVV-D9 | >25 | 5 | 0.2 | - |
| JVV-E8 | >25 | >25 | 0.2 | - |
| JVV-E10 | >25 | >25 | 0.4 | ++ |
| JVV-E12 | >25 | >25 | 0.8 | - |
| JVV-F4 | >25 | >25 | 0.2 | - |
| JVV-F6 | >25 | 2 | 0.4 | - |
| JVV-G3 B | >25 | >25 | 0.6 | + |
| JVV-G7 | >25 | >25 | 0.4 | ++ |
| JVV-G10 | >25 | >25 | 0.4 | - |
| JVV-H9 | >25 | >25 | 1 | - |
| JVV-H12 | >25 | 10 | 0.4 | ++ |
| JVZ-E7 B | >25 | >25 | 0.4 | + |
| JVZ-G1 | >25 | >25 | 2 | - |
| JVZ-H5 | >25 | >25 | 0.4 | ++ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tremblay, J.M.; Vazquez-Cintron, E.; Lam, K.-H.; Mukherjee, J.; Bedenice, D.; Ondeck, C.A.; Conroy, M.T.; Bodt, S.M.L.; Winner, B.M.; Webb, R.P.; et al. Camelid VHH Antibodies that Neutralize Botulinum Neurotoxin Serotype E Intoxication or Protease Function. Toxins 2020, 12, 611. https://doi.org/10.3390/toxins12100611
Tremblay JM, Vazquez-Cintron E, Lam K-H, Mukherjee J, Bedenice D, Ondeck CA, Conroy MT, Bodt SML, Winner BM, Webb RP, et al. Camelid VHH Antibodies that Neutralize Botulinum Neurotoxin Serotype E Intoxication or Protease Function. Toxins. 2020; 12(10):611. https://doi.org/10.3390/toxins12100611
Chicago/Turabian StyleTremblay, Jacqueline M., Edwin Vazquez-Cintron, Kwok-Ho Lam, Jean Mukherjee, Daniela Bedenice, Celinia A. Ondeck, Matthieu T. Conroy, Skylar M. L. Bodt, Brittany M. Winner, Robert P. Webb, and et al. 2020. "Camelid VHH Antibodies that Neutralize Botulinum Neurotoxin Serotype E Intoxication or Protease Function" Toxins 12, no. 10: 611. https://doi.org/10.3390/toxins12100611
APA StyleTremblay, J. M., Vazquez-Cintron, E., Lam, K.-H., Mukherjee, J., Bedenice, D., Ondeck, C. A., Conroy, M. T., Bodt, S. M. L., Winner, B. M., Webb, R. P., Ichtchenko, K., Jin, R., McNutt, P. M., & Shoemaker, C. B. (2020). Camelid VHH Antibodies that Neutralize Botulinum Neurotoxin Serotype E Intoxication or Protease Function. Toxins, 12(10), 611. https://doi.org/10.3390/toxins12100611








