A Review on the Study of Cyanotoxins in Paleolimnological Research: Current Knowledge and Future Needs
Abstract
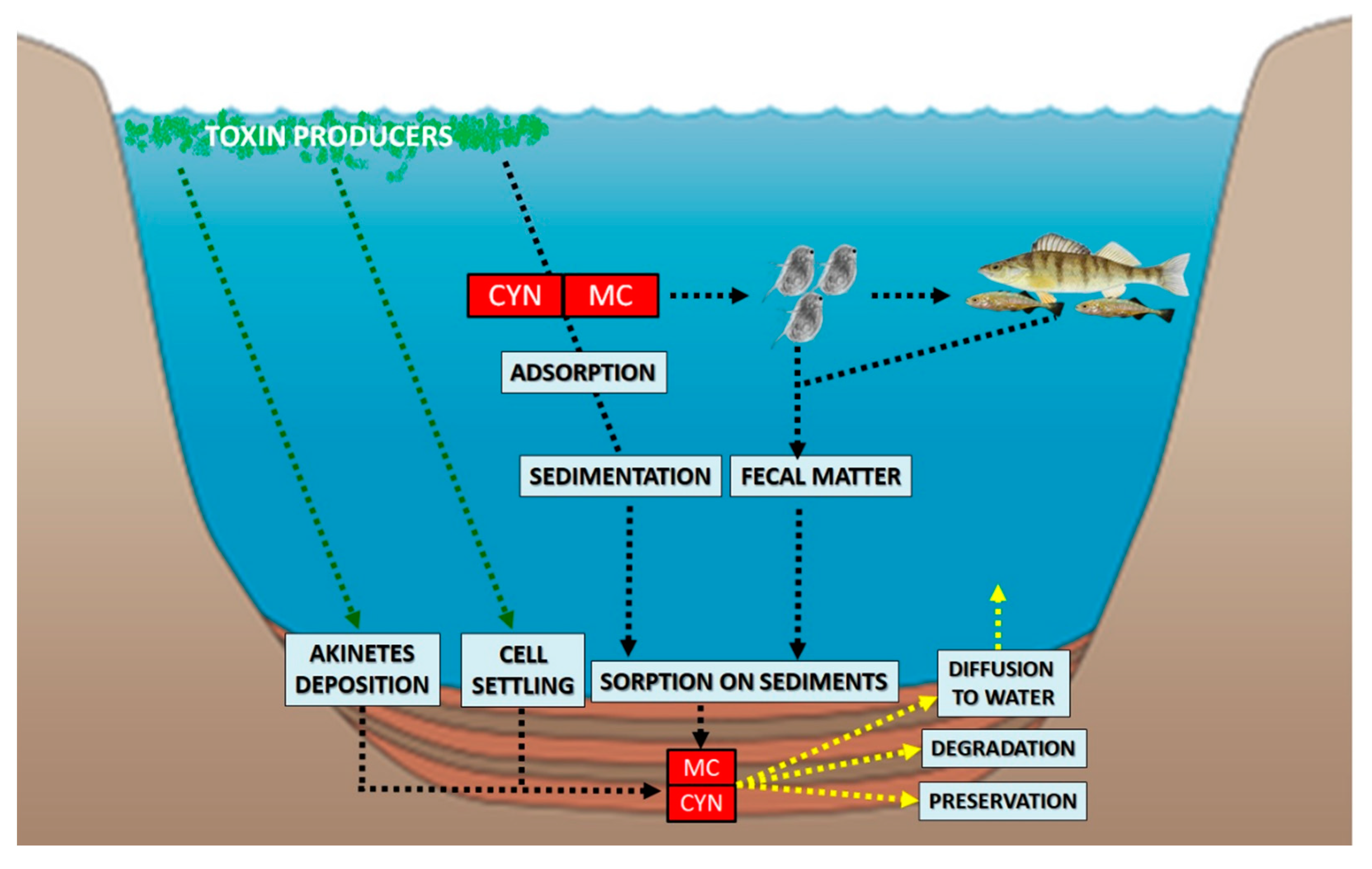
:1. Introduction
2. Determination of Cyanotoxins in Sediment Cores
2.1. Microcystins (MCs)
2.2. Cylindrospermopsin (CYN)
3. Establishment of Cyanotoxins as a Paleolimnological Tool
4. Future Research Prospects
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Holland, A.; Kinnear, S. Interpreting the possible ecological role(s) of cyanotoxins: Compounds for competitive advantage and/or physiological aide? Mar. Drugs 2013, 11, 2239–2258. [Google Scholar] [CrossRef] [Green Version]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar]
- Rzymski, P.; Poniedzialek, B. In search of environmental role of cylindrospermopsin: A review on global distribution and ecology of its producers. Water Res. 2014, 66, 320–337. [Google Scholar] [CrossRef]
- Cartmell, C.; Evans, D.M.; Elwood, J.M.L.; Fituri, H.S.; Murphy, P.J.; Caspari, T.; Poniedzialek, B.; Rzymski, P. Synthetic analogues of cyanobacterial alkaloid cylindrospermopsin and their toxicological activity. Toxicol. In Vitro 2017, 44, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: Current status and future prospects. Front. Microbiol. 2017, 8, 515. [Google Scholar] [CrossRef] [Green Version]
- Pearson, L.; Mihali, T.; Moffitt, M.; Kellmann, R.; Neilan, B. On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopsin. Mar. Drugs 2010, 8, 1650–1680. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Liu, H.; Yuan, L.; Wang, Y.; Ma, Y.; Wang, R.; Chen, X.; Losiewicz, M.D.; Guo, H.; Zhang, H. The diversity of cyanobacterial toxins on structural characterization, distribution and identification: A systematic review. Toxins 2019, 11, 530. [Google Scholar] [CrossRef] [Green Version]
- DeMott, W.R.; Qing-Xue, Z.; Carmichael, W.W. Effects of toxic cyanobacteria and purified toxins on the survival and feeding of a copepod and three species of Daphnia. Limnol. Oceanogr. 1991, 36, 1346–1357. [Google Scholar] [CrossRef] [Green Version]
- DeMott, W.R.; Moxter, F. Foraging cyanobacteria by copepods: Responses to chemical defense and resource abundance. Ecology 1991, 72, 1820–1834. [Google Scholar] [CrossRef]
- Heckman, D.S.; Geiser, D.M.; Eidell, B.R.; Stauffer, R.L.; Kardos, N.L.; Hedges, S.B. Molecular evidence for the early colonization of land by fungi and plants. Science 2001, 293, 1129–1133. [Google Scholar] [CrossRef] [Green Version]
- Murray, S.A.; Mihali, T.K.; Neilan, B.A. Extraordinary conservation, gene loss, and positive selection in the evolution of an ancient neurotoxin. Mol. Biol. Evol. 2011, 8, 1173–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rzymski, P.; Poniedziałek, B.; Kokociński, M.; Jurczak, T.; Lipski, D.; Wiktorowicz, W. Interspecific allelopathy in cyanobacteria: Cylindrospermopsin and Cylindrospermopsis raciborskii effect on the growth and metabolism of Microcystis aeruginosa. Harmful Algae 2014, 35, 1–8. [Google Scholar] [CrossRef]
- Omidi, A.; Esterhuizen-Londt, M.; Pflugmacher, S. Still challenging: The ecological function of the cyanobacterial toxin microcystin—What we know so far. Toxin Rev. 2018, 37, 87–105. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Rzymski, P. Programmed Cell Death-Like and Accompanying Release of Microcystin in Freshwater Bloom-Forming Cyanobacterium Microcystis: From Identification to Ecological Relevance. Toxins 2019, 11, 706. [Google Scholar] [CrossRef] [Green Version]
- Dolman, A.M.J.; Rücker, F.; Pick, R.; Fastner, J.; Rohrlack, T.; Mischke, U.; Wiedner, C. Cyanobacteria and cyanotoxins: The influence of nitrogen versus phosphorus. PLoS ONE 2012, 7, e38757. [Google Scholar] [CrossRef]
- Utkilen, H.; Gjølme, N. Iron-stimulated toxin production in Microcystis aeruginosa. Appl. Enviorn. Microbiol. 1995, 61, 797–800. [Google Scholar]
- Griffiths, D.J.; Saker, M.L. The Palm Island mystery disease 20 years on: A review of research on the cyanotoxin cylindrospermopsin. Environ. Toxicol. 2003, 18, 78–93. [Google Scholar] [CrossRef]
- Dyble, J.; Tester, P.A.; Litaker, R.W. Effects of light intensity on cylindrospermopsin production in the cyanobacterial HAB species Cylindrospermopsis raciborskii. Afr. J. Mar. Sci. 2006, 28, 309–312. [Google Scholar] [CrossRef]
- Burge, D.R.L.; Edlund, M.B.; Frisch, D. Paleolimnology and resurrection ecology: The future of reconstructing the past. Evol. Appl. 2017, 11, 42–59. [Google Scholar] [CrossRef]
- Leavitt, P.R.; Hodgson, D.A. Sedimentary pigments. In Tracking Environmental Change Using Lake Sediments; Volume 3: Terrestrial, Algal, and Siliceous Indicators; Smol, J.P., Birks, H.J.B., Last, W.M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 295–325. [Google Scholar]
- Räsänen, J.; Kauppila, T.; Vuori., K. Sediment and phytoplankton records of the cyanobacterial genus Anabaena in boreal Lake Pyhäjärvi. Hydrobiologia 2006, 568, 455–465. [Google Scholar]
- Pal, S.; Gregory-Eaves, I.; Pick, F.R. Temporal trends in cyanobacteria revealed through DNA and pigments analyses of temperate lake sediment cores. J. Paleolimnol. 2015, 54, 87–101. [Google Scholar] [CrossRef]
- Monchamp, M.E.; Walser, J.C.; Pomati, F.; Spaak, P. Sedimentary DNA reveals cyanobacterial community diversity over 200 years in two perialpine Lakes. Appl. Environ. Microbiol. 2016, 82, 6472–6482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domaizon, I.; Winegardner, A.; Capo, E.; Gauthier, J.; Gregory-Eaves, I. DNA-based methods in paleolimnology: New opportunities for investigating long-term dynamics of lacustrine biodiversity. J. Paleolimnol. 2017, 58, 1–21. [Google Scholar] [CrossRef]
- Lindahl, T. Instability and decay of the primary structure of DNA. Nature 1993, 362, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Hofreiter, M.; Serre, D.; Poinar, H.N.; Kuch, M.; Pääbo, S. Ancient DNA. Nat. Rev. Genet. 2011, 2, 353–360. [Google Scholar] [CrossRef]
- Anderson-Carpenter, L.L.; McLachlan, J.S.; Jackson, S.T.; Kuch, M.; Lumibao, C.Y.; Poinar, H.N. Ancient DNA from lake sediments: Bridging the gap between paleoecology and genetics. BMC Evol. Biol. 2011, 11, 30. [Google Scholar] [CrossRef] [Green Version]
- Coolen, M.J.L.; Orsi, W.D.; Balkema, C.; Quince, C.; Harris, K.; Sylva, S.P.; Filipova-Marinova, M.; Giosan, L. Evolution of the plankton paleome in the Black Sea from the Deglacial to Anthropocene. Proc. Natl. Acad. Sci. USA 2013, 110, 8609–8614. [Google Scholar] [CrossRef] [Green Version]
- Kyle, M.; Haande, S.; Sønstebø, J.; Rohrlack, T. Amplification of DNA in sediment cores to detect historic Planktothrix occurrence in three Norwegian lakes. J. Paleolimnol. 2015, 53, 61–72. [Google Scholar] [CrossRef]
- Capo, E.; Debroas, D.; Arnaud, F.; Guillemot, T.; Bichet, V.; Millet, L.; Gauthier, E.; Massa, C.; Develle, A.L.; Pignol, C.; et al. Long-term dynamics in microbial eukaryotes communities: A palaeolimnological view based on sedimentary DNA. Mol. Ecol. 2016, 25, 5925–5943. [Google Scholar] [CrossRef]
- Chiswell, R.K.; Shaw, G.R.; Eaglesham, G.K.; Smith, M.J.; Norris, R.L.; Seawright, A.A.; Moore, M.R. Stability of cylindrospermopsin, the toxin from the cyanobacterium Cylindrospermopsis raciborskii, effect of pH, temperature, and sunlight on decomposition. Environ. Toxicol. 1999, 14, 155–165. [Google Scholar] [CrossRef]
- Mazur, H.; Plinski, M. Stability of cyanotoxins, microcystin-LR, microcystin-RR and nodularin in seawater and BG-11 medium of different salinity. Oceanologia 2011, 43, 329–339. [Google Scholar]
- Waters, M.N. A 4700-year history of cyanobacteria toxin production in a shallow subtropical lake. Ecosystems 2016, 19, 426–436. [Google Scholar] [CrossRef]
- Mashile, G.P.; Nomngongo, P.N. Recent application of solid phase based techniques for extraction and preconcentration of cyanotoxins in environmental matrices. Crit. Rev. Anal. Chem. 2017, 47, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.J.; Critchley, M.M.; Hutson, J.; Fallowfield, H.J. Theadsorption of cyanobacterial hepatotoxins from water onto soilduring batch experiments. Water Res. 2001, 35, 1461–1468. [Google Scholar] [CrossRef]
- Klitzke, S.; Beusch, C.; Fastner, J. Sorption of the cyanobacterial toxins cylindrospermopsin and anatoxin-a to sediments. Water Res. 2011, 45, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Q.; Xiao, B.D.; Li, R.H.; Wang, C.B.; Huang, J.T.; Wang, Z. Mechanisms and factors affecting sorption of microcystins onto natural sediments. Environ. Sci. Technol. 2011, 45, 2641–2647. [Google Scholar] [CrossRef]
- Liu, Y.L.; Walker, H.W.; Lenhart, J.J. The effect of natural organic matter on the adsorption of microcystin-LR onto clay minerals. Colloids Surf. A Physicochem. Eng. Asp. 2019. [Google Scholar] [CrossRef]
- Brunberg, A.K.; Blomqvist, P. Benthic overwintering of microcystis colonies under different environmental conditions. J. Plankton Res. 2002, 24, 1247–1252. [Google Scholar] [CrossRef]
- Misson, B.; Sabarta, M.; Amblard, C.; Latour, D. Benthic survival of Microcystis: Long-term viability and ability to transcribe microcystin genes. Harmful Algae 2012, 13, 20–25. [Google Scholar] [CrossRef]
- Bormans, M.; Lengronne, M.; Brient, L.; Duval, C. Cylindrospermopsin accumulation and release by the benthic cyanobacterium Oscillatoria sp. PCC 6506 under different light conditions and growth phases. Bull. Environ. Contam. Toxicol. 2014, 92, 243–247. [Google Scholar] [CrossRef] [Green Version]
- Magonono, M.; Oberholster, P.J.; Addmore, S.; Stanley, M.; Gumbo, J.R. The presence of toxic and non-toxic cyanobacteria in the sediments of the Limpopo River Basin: Implications for human health. Toxins 2018, 10, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legrand, B.; Le Jeune, A.H.; Colombet, J.; Thouvenot, A.; Latour, D. Akinetes may be representative of past Nostocalean blooms: A case study of their benthic spatiotemporal distribution and potential for germination in a Eutrophic lake. Appl. Environ. Microbiol. 2017, 83, e01571-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietrich, D.; Hoeger, S. Guidance values for microcystins in water and cyanobacterial supplement products (blue-green algal supplements): A reasonable or misguided approach? Toxicol. Appl. Pharmacol. 2005, 203, 273–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Figueiredo, D.R.; Azeiteiro, U.M.; Esteves, S.M.; Gonçalves, F.J.M.; Pereira, M.J. Microcystin-producing blooms—A serious global public health issue. Ecotoxicol. Environ. Saf. 2004, 59, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Antoniou, M.G.; He, X.; Dionysiou, D.D.; de la Cruz, A.A.; Tsimeli, K.; Triantis, T.; Hiskia, A.; Kaloudis, T.; Williams, C.; et al. Sources and occurrence of cyanotoxins worldwide. In Xenobiotics in the Urban Water Cycle: Mass Flows, Environmental Processes, Mitigation and Treatment Strategies; Fatta-Kassinos, D., Ed.; Springer: Dordrecht, The Netherlands; Berlin/Heidelberg, Germany; London, UK; New York, NY, USA, 2010. [Google Scholar]
- Nishizawa, T.; Ueda, A.; Asayama, M.; Fujii, K.; Harada, K.; Ochi, K.; Shirai, M. Polyketide synthase gene coupled to the peptide synthetase module involved in the biosynthesis of the cyclic heptapeptide microcystin. J. Biochem. 2000, 127, 779–789. [Google Scholar] [CrossRef] [Green Version]
- Tillett, D.; Dittmann, E.; Erhard, M.; von Dohren, H.; Borner, T.; Neilan, B.A. Structural organization of microcystin biosynthesis in microcystis aeruginosa pcc7806: An integrated peptide-polyketide synthetase system. Chem. Biol. 2000, 7, 753–764. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, A.; Harel, M.; Kaplan-Levy, R.N.; Hadas, O.; Sukenik, A.; Dittmann, E. The languages spoken in the water body (or the biological role of cyanobacterial toxins). Front. Microbiol. 2012, 3, 138. [Google Scholar] [CrossRef] [Green Version]
- Makower, A.K.; Schuurmans, J.M.; Groth, D.; Zilliges, Y.; Matthijs, H.C.P.; Dittmann, E. Transcriptomics-aided dissection of the intracellular and extracellular roles of microcystin in microcystis aeruginosa pcc 7806. Appl. Environ. Microbiol. 2015, 81, 544–554. [Google Scholar] [CrossRef] [Green Version]
- Gan, N.; Wei, N.; Song, L. Recent progress in research of the biological function of microcystins. J. Lake Sci. 2017, 29, 8. [Google Scholar]
- International Agency for Research on Cancer (IARC). Ingested Nitrate and Nitrite and Cyanobacterial Peptide Toxins: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; WHO Press: Geneva, Switzerland, 2010; Volume 94. [Google Scholar]
- Waters, M.N.; Piehler, M.F.; Smoak, J.M.; Rodriguez, A.B.; Bianchi, T.S. Shallow lake trophic status linked to Holocene climate and human impacts. J. Paleolimnol. 2009, 42, 51–64. [Google Scholar] [CrossRef]
- Waters, M.N.; Piehler, M.F.; Smoak, J.M.; Bianchi, T.S. Algal community responses to shallow lake dystrophication. Can. J. Fish. Aquat. Sci. 2012, 69, 1433–1443. [Google Scholar] [CrossRef]
- Waters, M.N.; Schelske, C.L.; Brenner, M. Cyanobacterial dynamics in shallow Lake Apopka (Florida, U.S.A.) before and after the shift from a macrophyte-dominated to a phytoplankton-dominated state. Freshw. Biol. 2015, 60, 1571–1580. [Google Scholar] [CrossRef]
- Legrand, B.; Miras, Y.; Beauger, A.; Dussauze, M.; Latour, D. Akinetes and ancient DNA reveal toxic cyanobacterial recurrences and their potential for resurrection in a 6700-year-old core from a eutrophic lake. Sci. Total Environ. 2019, 687, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Babica, P.; Kohoutek, J.; Bláha, L.; Adamovský, O.; Marsalek, B. Evaluation of extraction approaches linked to ELISA and HPLC for analyses of microcystin-LR, -RR and -YR in freshwater sediments with different organic material contents. Anal. Bioanal. Chem. 2006, 385, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, L.; Gan, N.; Song, L. Optimization of an effective extraction procedure for the analysis of microcystins in soils and lake sediments. Environ. Pollut. 2006, 143, 241–246. [Google Scholar] [CrossRef]
- Holst, T.; Jørgensen, N.O.; Jørgensen, C.; Johansen, A. Degradation of microcystin in sediments at oxic and anoxic, denitrifying conditions. Water Res. 2003, 37, 4748–4760. [Google Scholar] [CrossRef]
- Zastepa, A.; Pick, F.R.; Blais, J.M. Distribution and flux of microcystin congeners in lake sediments. Lake Reserv. Manag. 2017, 33, 444–451. [Google Scholar] [CrossRef] [Green Version]
- Pawlik-Skowronska, B.; Kornijow, R.; Pirszel, J. Sedimentary imprint of cyanobacterial blooms—A new tool for insight into recent history of lakes. Pol. J. Ecol. 2010, 58, 663–670. [Google Scholar]
- Efting, A.A.; Snow, D.D.; Fritz, S.C. Cyanobacteria and microcystin in the Nebraska (USA) Sand Hills Lakes before and after modern agriculture. J. Paleolimnol. 2011, 46, 17–27. [Google Scholar] [CrossRef]
- Zastepa, A.; Taranu, Z.E.; Kimpe, L.E.; Blais, J.M.; Gregory-Eaves, I.; Zurawell, R.W.; Pick, F.R. Reconstructing a long-term record of microcystins from the analysis of lake sediments. Sci. Total Environ. 2017, 579, 893–901. [Google Scholar] [CrossRef]
- Kaczorowska, A.; Kornijow, R. Paleoecological evidence for changes over the past 200 years in chironomid communities of a shallow lake exposed to cyanobacterial toxins. Aquat. Ecol. 2012, 46, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Lürling, M.; van Oosterhout, F.; Faassen, E.J. Eutrophication and warming boost cyanobacterial biomass and microcystins. Toxins 2017, 9, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, S.; Wang, Y.; Wang, D.; Li, Y.Y.; Gong, M.; Zhang, C. In situ, high-resolution evidence for iron-coupled mobilization of phosphorus in sediments. Sci. Rep. 2016, 6, 24341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, J.L.; Loftin, K.A.; Meyer, M.T.; Ziegler, A.C. Cyanotoxin mixtures and taste-and-odor compounds in cyanobacterial blooms from the midwestern United States. Environ. Sci. Technol. 2010, 44, 7361–7368. [Google Scholar] [CrossRef]
- Beversdorf, L.J.; Weirich, C.A.; Bartlett, S.L.; Miller, T.R. Variable cyanobacterial toxin and metabolite profiles across six eutrophic lakes of differing physiochemical characteristics. Toxins 2017, 9, 62. [Google Scholar] [CrossRef] [Green Version]
- Newcombe, G.; Cook, D.; Brooke, S.; Ho, L.; Sylman, N. Treatment options for microcystin toxins: Similarities and differences between variants. Environ. Technol. 2003, 24, 299–308. [Google Scholar] [CrossRef]
- Zastepa, A.; Pick, F.R.; Blais, J.M. Fate and persistence of particulate and dissolved microcystin-LA from Microcystis blooms. Hum. Ecol. Risk Assess. 2014, 20, 1670–1686. [Google Scholar] [CrossRef]
- Ohtani, I.; Moore, R.E.; Runnegar, M.T. Cylindrospermopsin: A potent hepatotoxin from the blue-green Alga Cylindrospermopsis raciborskii. J. Am. Chem. Soc. 1992, 114, 7941–7942. [Google Scholar] [CrossRef]
- Bohunická, M.; Mareš, J.; Hrouzek, P.; Urajová, P.; Lukeš, M.; Šmarda, J.; Komárek, J.; Gaysina, L.A.; Strunecký, O. A combined morphological, ultrastructural, molecular, and biochemical study of the peculiar family Gomontiellaceae (Oscillatoriales) reveals a new cylindrospermopsin-producing clade of cyanobacteria. J. Phycol. 2015, 51, 1040–1054. [Google Scholar] [CrossRef]
- Rzymski, P.; Poniedziałek, B. The surprising world of cyanobacteria: Cylindrospermopsin has a soil face. J. Phycol. 2015, 51, 1037–1039. [Google Scholar] [CrossRef]
- Wimmer, K.M.; Strangman, W.K.; Wright, J.L.C. 7-Deoxy-desulfo-cylindrospermopsin and 7-deoxy-desulfo-12-acetylcylindrospermopsin: Two new cylindrospermopsin analogs isolated from a Thai strain of Cylindrospermopsis raciborskii. Harmful Algae 2014, 37, 203–206. [Google Scholar] [CrossRef]
- Evans, D.M.; Hughes, J.; Jones, L.F.; Murphy, P.J.; Falfushynska, H.; Horyn, O.; Sokolova, I.M.; Christensen, J.; Coles, S.J.; Rzymski, P. Elucidating cylindrospermopsin toxicity via synthetic analogues: An in vitro approach. Chemosphere 2019, 234, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Bar-Yosef, Y.; Sukenik, A.; Hadas, O.; Viner-Mozzini, Y.; Kaplan, A. Enslavement in the water body by toxic Aphanizomenon ovalisporum, inducing alkaline phosphatase in phytoplanktons. Curr. Biol. 2010, 20, 1557–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobronoki, D.; B-Béres, V.; Vasas, G.; Gonda, S.; Nagy, S.A.; Basci, I. Potential role of the cellular matrix of Aphanizomenon strains in the effects of cylindrospermopsin - an experimental study. J. Appl. Phycol. 2018. [Google Scholar] [CrossRef] [Green Version]
- Mihali, T.K.; Kellmann, R.; Muenchhoff, J.; Barrow, K.D.; Neilan, B.A. Characterization of the gene cluster responsible for cylindrospermopsin biosynthesis. Appl. Environ. Microbiol. 2008, 74, 716–722. [Google Scholar] [CrossRef] [Green Version]
- Rzymski, P.; Poniedziałek, B.; Mankiewicz-Boczek, J.; Faassen, E.J.; Jurczak, T.; Gągała-Borowska, I.; Ballot, A.; Lürling, M.; Kokociński, M. Polyphasic toxicological screening of Cylindrospermopsis raciborskii and Aphanizomenon gracile isolated in Poland. Algal Res. 2017, 24, 72–80. [Google Scholar] [CrossRef]
- Mazmouz, R.; Chapuis-Hugon, F.; Mann, S.; Pichon, V.; Mejean, A.; Ploux, O. Biosynthesis of cylindrospermopsin and 7-epicylindrospermopsin in Oscillatoria sp. strain PCC 6506: Identification of the cyr gene cluster and toxin analysis. Appl. Environ. Microbiol. 2010, 76, 4943–4949. [Google Scholar] [CrossRef] [Green Version]
- Kleinteich, J.; Hildebrand, F.; Wood, S.A. Diversity of toxin and non-toxin containing cyanobacterial mats of meltwater ponds on the Antarctic Peninsula: A pyrosequencing approach. Antarct. Sci. 2014, 26, 521–532. [Google Scholar] [CrossRef] [Green Version]
- Fadel, A.; Atoui, A.; Lemaire, B.; Vinçon-Leite, B.; Slim, K. Dynamics of the toxin cylindrospermopsin and the cyanobacterium Chrysosporum (Aphanizomenon) ovalisporum in a Mediterranean eutrophic reservoir. Toxins 2014, 6, 3041–3057. [Google Scholar] [CrossRef] [Green Version]
- Tundisi, J.G.; Matsumura-Tundisi, T.; Tundisi, J.E.M.; Blanco, F.P.; Abe, D.S.; Contri Campanelli, L.; Sidagis-Galli, G.A.; Silva, V.; Lima, C.P.P. A bloom of cyanobacteria (Cylindrospermopsis raciborskii) in UHE Carlos Botelho (Lobo/Broa) reservoir: A consequence of global change? Braz. J. Biol. 2015, 75, 507–508. [Google Scholar] [CrossRef] [Green Version]
- Poniedzialek, B.; Rzymski, P.; Kokocinski, M. Cylindrospermopsin: Water-linked potential threat to human health in Europe. Environ. Toxicol. Pharmacol. 2012, 34, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, S.M.; Carmichael, W.W.; Jochimsen, E.M.; Rinehart, K.L.; Lau, S.; Shaw, G.R.; Eaglesham, G.K. Human intoxication by microcystins during renal dialysis treatment in Caruaru-Brazil. Toxicology 2002, 181, 441–446. [Google Scholar] [CrossRef]
- Adamski, M.; Żmudzki, P.; Chrapusta, E.; Bober, B.; Kaminski, A.; Zabaglo, K.; Latkowska, E.; Bialczyk, J. Effect of pH and temperature on the stability of cylindrospermopsin. Characterization of decomposition products. Algal Res. 2016, 15, 129–134. [Google Scholar] [CrossRef]
- Wörmer, L.; Huerta-Fontela, M.; Cirés, S.; Carrasco, D.; Quesada, A. Natural Photodegradation of the Cyanobacterial Toxins Microcystins and Cylindrospermopsin. Environ. Sci. Technol. 2010, 44, 3002–3007. [Google Scholar] [CrossRef]
- Klitzke, S.; Fastner, J. Cylindrospermopsin degradation in sediments: The role of temperature, redox conditions, and dissolved carbon. Water Res. 2012, 46, 1549–1555. [Google Scholar] [CrossRef]
- Klitzke, S.; Apelt, S.; Weiler, C. Retention and degradation of the cyanobacterial toxin cylindrospermopsin in sediments: The role of sediment preconditioning and DOM composition. Toxicon 2010, 55, 999–1007. [Google Scholar] [CrossRef]
- Preußel, K.; Chorus, I.; Fastner, J. Nitrogen limitation promotes accumulation and suppresses release of cylindrospermopsins in cells of Planktothrix sp. Toxins 2014, 6, 2932. [Google Scholar] [CrossRef] [Green Version]
- Bácsi, I.; Vasas, G.; Surányi, G.; M-Hamvas, M.; Máthé, C.; Tóth, E.; Grigorszky, I.; Gáspár, A.; Tóth, S.; Borbely, G. Alteration of cylindrospermopsin production in sulfate- or phosphate-starved cyanobacterium Aphanizomenon ovalisporum. FEMS Microbiol. Lett. 2006, 259, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Smol, J.P. Pollution of Lakes and Rivers: A Paleoenvironmental Perspective, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2008. [Google Scholar]
- Metcalf, J.S.; Young, F.M.; Codd, G.A. Performance assessment of a cylindrospermopsin ELISA with purified compounds and cyanobacterial extracts. Environ. Forensics 2017, 18, 147–152. [Google Scholar] [CrossRef]
- Zervou, S.K.; Christophoridis, C.; Kaloudis, T.; Triantis, T.M.; Hiskia, A. New SPE-LC-MS/MS method for simultaneous determination of multi-class cyanobacterial and algal toxins. J. Hazard. Mater. 2017, 323, 56–66. [Google Scholar] [CrossRef]
- Díez-Quijada, L.; Guzmán-Guillén, R.; Prieto Ortega, A.I.; Llana-Ruíz-Cabello, M.; Campos, A.; Vasconcelos, V.; Jos, Á.; Cameán, A.M. New Method for Simultaneous Determination of Microcystins and Cylindrospermopsin in Vegetable Matrices by SPE-UPLC-MS/MS. Toxins 2018, 10, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meriluoto, J.; Spoof, L.; Codd, G. Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; John Wiley & Sons, Ltd.: Chichester, UK, 2017. [Google Scholar]
- Jokela, J.; Heinila, L.M.P.; Shishido, T.K.; Wahlsten, M.; Fewer, D.P.; Fiore, M.F. Production of high amounts of hepatotoxin nodularin and new protease inhibitors pseudospumigins by the Brazilian benthic Nostoc sp. CENA543. Front. Microbiol. 2017, 8, 1963. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Marzec, H.; Tyminska, A.; Szafranek, J.; Plinski, M. Accumulation of nodularin in sediments, mussels, and fish from the Gulf of Gdansk, southern Baltic Sea. Environ. Toxicol. 2007, 22, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Kiviranta, J.; Sivonen, K.; Lahti, K.; Luukkainen, R.; Niemelae, S.I. Production and biodegradation of cyanobacterial toxins—A laboratory study. Arch. Hydrobiol. 1991, 121, 281–294. [Google Scholar]
- Kaminski, A.; Bober, B.; Lechowski, Z.; Bialczyk, J. Determination of anatoxin-a stability under certain abiotic factors. Harmful Algae 2013, 28, 83–87. [Google Scholar] [CrossRef]
- Roy-Lachapelle, A.; Solliec, M.; Bouchard, M.F.; Sauvé, S. Detection of cyanotoxins in algae dietary supplements. Toxins 2017, 9, 76. [Google Scholar] [CrossRef] [Green Version]
- Rumsby, P.; Hall, T.; Pitchers, R. Risk Assessment of BMAA; WRc: Swindon, UK, 2008. [Google Scholar]
- Jiang, L.; Eriksson, J.; Lage, S.; Jonasson, S.; Shams, S.; Mehine, M.; Ilag, L.L.; Rasmussen, U. Diatoms: A novel source for the neurotoxin BMAA in aquatic environments. PLoS ONE 2014, 9, e84578. [Google Scholar] [CrossRef]
- Violi, J.P.; Facey, J.A.; Mitrovic, S.M.; Colville, A.; Rodgers, K.J. Production of β-methylamino-L-alanine (BMAA) and Its Isomers by Freshwater Diatoms. Toxins 2019, 11, 512. [Google Scholar] [CrossRef] [Green Version]
- Burns, J.M.; Hall, S.; Ferry, J.L. The adsorption of saxitoxin to clays and sediments in fresh and saline waters. Water Res. 2009, 43, 1899–1904. [Google Scholar] [CrossRef]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic Alkaloids: Saxitoxin and Its Analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [Green Version]
- Donovan, C.J.; Ku, J.C.; Quilliam, M.A.; Gill, T.A. Bacterial degradation of paralytic shellfish toxins. Toxicon 2008, 52, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.S.; Zimba, P.V. Cyanobacterial bioactive metabolites—A review of their chemistry and biology. Harmful Algae 2019, 83, 42–94. [Google Scholar] [CrossRef] [PubMed]
- Jaja-Chimedza, A.; Saez, C.; Sanchez, K.; Gantar, M.; Berry, J.P. Identification of teratogenic polymethoxy-1-alkenes from Cylindrospermopsis raciborskii, and taxonomically diverse freshwater cyanobacteria and green algae. Harmful Algae 2015, 49, 156–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rzymski, P.; Evans, D.M.; Murphy, P.J.; Kokociński, M. A study of polymethoxy-1-alkenes in Raphidiopsis (Cylindrospermopsis) raciborskii and Aphanizomenon gracile isolated in Poland. Toxicon 2019, 171, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Bucas, G.; Saliot, A. Sea transport of animal and vegetable oils and its environmental consequences. Mar. Pollut. Bull. 2002, 44, 1388–1396. [Google Scholar] [CrossRef]
- Legrand, B.; Lamarque, A.; Sabart, M.; Latour, D. Characterization of akinetes from cyanobacterial strains and lake sediment: A study of their resistance and toxic potential. Harmful Algae 2016, 59, 42–50. [Google Scholar] [CrossRef]
| Toxin | Location | Core Length 1 | Oldest Date 2 | Detection Method | Extraction | Reference |
|---|---|---|---|---|---|---|
| MC (8 congeners) | Canada, Lake Baptiste | 50 | 1824 AD | LC-MS | 75% MeOH, SPE | [63] |
| MC-Total | Poland, Lake Glębokie | 40 | n.a. | GC-MS | 75% MeOH | [61] |
| MC-Total | Poland, Lake Syczyskie | 50 | n.a. | GC-MS | 75% MeOH | [61] |
| MC (8 congeners) | Canada/USA, Lake of the Woods | 7 | 2000 AD | LC-MS | 75% MeOH, SPE | [60] |
| MC-Total | Poland, Lake Syczyńskie | 50 | 1800s? | GC-MS | 75% MeOH | [64] |
| MC-LR | USA (Nebraska), Two Mile Lake | 18 | 1866 AD | LC-MS | EDTA, Na4P2O7, MeOH, SPE | [62] |
| MC-LR | USA (Nebraska), Lake Dewey | 40 | 1945 AD | LC-MS | EDTA, Na4P2O7, MeOH, SPE | [62] |
| MC-LR | USA (Nebraska), Island Lake | 20 | 1832 AD | LC-MS | EDTA, Na4P2O7, MeOH, SPE | [62] |
| CYN | USA (Florida), Lake Griffin | 300 | 4732 BP | ELISA | 50% MeOH | [33] |
| Cyanotoxins Reported | Units | Max Value Post-1980 AD | Max Value Pre-1980 AD | Method of Detection | Reference |
|---|---|---|---|---|---|
| CYN | ng g−1 org. matter | 7 | 4 | ELISA | [33] |
| MC-LR | µg cm−2 yr−1 | 0.045 | 0.015 | LC-MS | [62] |
| MC (8 congeners) | ng g−1 dry weight | >1000 | 70 | LC-MS | [63] |
| MC - total | µg g−1 dry weight | 900 | 100 | GC-MS | [61] |
| Required Developments |
|
| Unique Applications |
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henao, E.; Rzymski, P.; Waters, M.N. A Review on the Study of Cyanotoxins in Paleolimnological Research: Current Knowledge and Future Needs. Toxins 2020, 12, 6. https://doi.org/10.3390/toxins12010006
Henao E, Rzymski P, Waters MN. A Review on the Study of Cyanotoxins in Paleolimnological Research: Current Knowledge and Future Needs. Toxins. 2020; 12(1):6. https://doi.org/10.3390/toxins12010006
Chicago/Turabian StyleHenao, Eliana, Piotr Rzymski, and Matthew N. Waters. 2020. "A Review on the Study of Cyanotoxins in Paleolimnological Research: Current Knowledge and Future Needs" Toxins 12, no. 1: 6. https://doi.org/10.3390/toxins12010006
APA StyleHenao, E., Rzymski, P., & Waters, M. N. (2020). A Review on the Study of Cyanotoxins in Paleolimnological Research: Current Knowledge and Future Needs. Toxins, 12(1), 6. https://doi.org/10.3390/toxins12010006






