Ciguatera Fish Poisoning: The Risk from an Aotearoa/New Zealand Perspective
Abstract
1. Introduction
2. Distribution and Habitat of Gambierdiscus and Fukuyoa
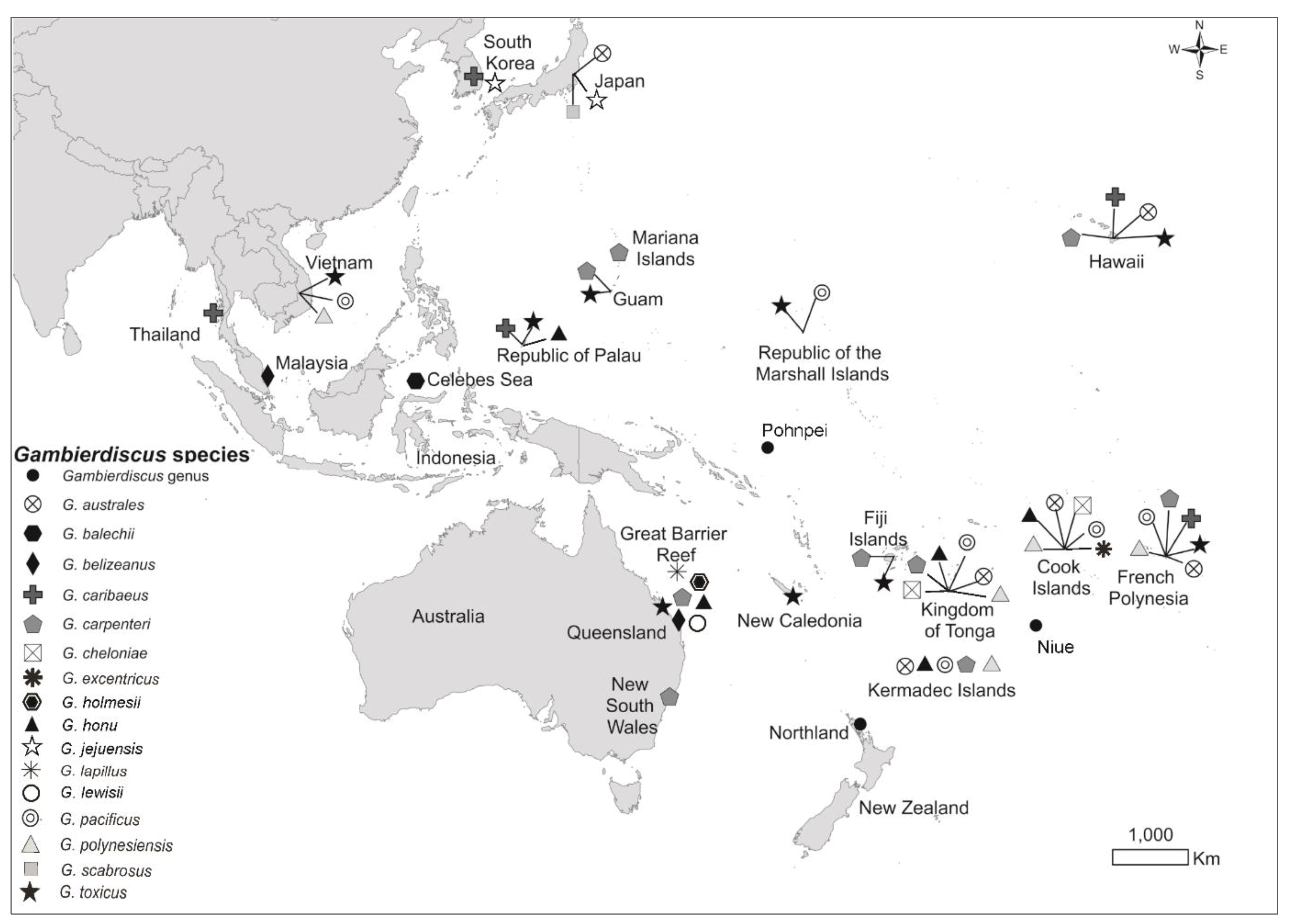
2.1. Global Distribution
2.2. Species of Gambierdiscus and Fukuyoa Isolated from New Zealand and Rangitāhua/Kermadec Islands
2.3. Macroalgae Substrates
3. Field Methods for Sampling Microalgae and CFP Toxins
3.1. Artificial Substrate Samplers for Microalgae
3.2. Sampling for Toxins In Situ
4. Molecular Methods for Detection and Quantification of Gambierdiscus and Fukuyoa
5. Toxin Production by Gambierdiscus and Fukuyoa Species Isolated from Aotearoa/New Zealand and Rangitāhua/Kermadec Islands
5.1. Ciguatera Fish Poisoning Related Toxins and Their Toxicity
5.2. Ciguatera Toxin Risk in New Zealand Waters
6. In Vivo Toxicity of Pure Compounds Involved in CFP
7. Potential Uptake of CTXs and MTXs by Fish
8. Potential Impacts of Climate Change
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rongo, T.; van Woesik, R. Socioeconomic consequences of ciguatera poisoning in Rarotonga, southern Cook Islands. Harmful Algae 2012, 20, 92–100. [Google Scholar] [CrossRef]
- Hamilton, B.; Whittle, N.; Shaw, G.; Eaglesham, G.; Moore, M.R.; Lewis, R.J. Human fatality associated with Pacific ciguatoxin contaminated fish. Toxicon 2010, 56, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Schep, L.J.; Slaughter, R.J.; Temple, W.A.; Beasley, D.M.G. Ciguatera poisoning: An interesting occurrence in New Zealand. N. Z. Med. J. 2010, 123, 100–102. [Google Scholar]
- Friedman, M.A.; Fernandez, M.; Backer, L.C.; Dickey, R.W.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.O.; Bienfang, P.; et al. An updated review of ciguatera fish poisoning: Clinical, epidemiological, environmental, and public health management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Tester, P.A.; Wickliffe, L.; Jossart, J.; Rhodes, L.; Envoldsen, H.; Adachi, M.; Nishimura, T.; Rodriguez, F.; Chinain, M.; Litaker, W. Global distribution of the genera Gambierdiscus and Fukuyoa. In Proceedings of the 18th International Conference on Harmful Algae, Nantes, France, 21–26 October 2018. in press. [Google Scholar]
- Darius, H.T.; Roué, M.; Sibat, M.; Viallon, J.; Gatti, C.M.I.; Vandersea, M.W.; Tester, P.A.; Litaker, R.W.; Amzil, Z.; Hess, P.; et al. Tectus niloticus (Tegulidae, Gastropod) as a novel vector of ciguatera poisoning: Detection of Pacific ciguatoxins in toxic samples from Nuku Hiva Island (French Polynesia). Toxins 2018, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Gatti, C.M.; Lonati, D.; Darius, H.T.; Zancan, A.; Roué, M.; Schicchi, A.; Locatelli, C.A.; Chinain, M. Tectus niloticus (Tegulidae, Gastropod) as a novel vector of ciguatera poisoning: Clinical characterization and follow-up of a mass poisoning event in Nuku Hiva Island (French Polynesia). Toxins 2018, 28, 102. [Google Scholar] [CrossRef]
- Chinain, M.; Gatti, C.M.; Roué, M.; Darius, H.T. Ciguatera poisoning in French Polynesia: Insights into the novel trends of an ancient disease. New Microbes New Infect. 2019, 31. [Google Scholar] [CrossRef]
- Silva, M.; Rodriguez, I.; Barreiro, A.; Kaufmann, M.; Neto, A.I.; Hassouani, M.; Sabour, B.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. First report of ciguatoxins in two starfish species: Ophidiaster ophidianus and Marthasterias glacialis. Toxins 2015, 7, 3740–3757. [Google Scholar] [CrossRef]
- Parsons, M.; Aligizaki, A.; Bottein, M.-Y.D.; Fraga, S.; Morton, S.; Penna, A.; Rhodes, L. Gambierdiscus and Ostreopsis: Reassessment of the state of knowledge of their taxonomy, geography, ecophysiology and toxicology. Harmful Algae 2012, 14, 107–129. [Google Scholar] [CrossRef]
- Rhodes, L.; Smith, K. A checklist of the benthic and epiphytic marine dinoflagellates of New Zealand, including Rangitāhua/Kermadec Islands. N. Z. J. Mar. Freshw. Res. 2019, 53, 258–277. [Google Scholar] [CrossRef]
- Rhodes, L.; Smith, K.F.; Murray, S.; Harwood, D.T.; Trnski, T.; Munday, R. The epiphytic genus Gambierdiscus (Dinophyceae) in the Kermadec Islands and Zealandia regions of the southwestern Pacific and the associated risk of ciguatera fish poisoning. Mar. Drugs 2017, 15, 219. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, L. Key Factors Influencing the Occurrence and Frequency of Ciguatera. Ph.D. Thesis, College of Science and Engineering, James Cook University, Townsville, Australia, 2018. [Google Scholar]
- Armstrong, P.; Murray, P.; Nesdale, A.; Peckler, B. Ciguatera fish poisoning. N. Z. Med. J. 2016, 129, 113–116. [Google Scholar]
- Lehane, L.; Lewis, R.J. Ciguatera: Recent advances but the risk remains. Int. J. Food Microbiol. 2000, 61, 91–125. [Google Scholar] [CrossRef]
- Stewart, I.; Eaglesham, G.K.; Poole, S.; Graham, G.; Paulo, C.; Wickramasinghe, W.; Sadler, R.; Shaw, G.R. Establishing a public health analytical service based on chemical methods for detecting and quantifying Pacific ciguatoxin in fish samples. Toxicon 2010, 56, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Farrell, H.; Zammit, A.; Harwood, D.T.; Murray, S. Is ciguatera moving south in Australia? Harmful Algae News 2016, 54, 5–6. [Google Scholar]
- Shellfish Toxin Update. Available online: https://www.seafoodnewzealand.org.nz (accessed on 13 January 2020).
- Chinain, M.; Darius, H.T.; Ung, A.; Cruchet, P.; Wang, Z.; Ponton, D.; Laurent, D.; Pauillac, S. Growth and toxin production in the ciguatera-causing dinoflagellate Gambierdiscus polynesiensis (Dinophyceae) in culture. Toxicon 2010, 56, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.; Richlen, M.L.; Erdner, D.; Smith, T.B.; Anderson, D.M.; Liefer, J.; Xu, Y.; McCarron, P.; Miles, C.; Parsons, M.L. Toxicity, chemistry, and implications of Gambierdiscus silvae: A ciguatoxin superbug in the Greater Caribbean region. In Proceedings of the 18th International Conference on Harmful Algae, Nantes, France, 21–26 October 2018. in press. [Google Scholar]
- Fraga, S.; Rodríguez, F.; Caillaud, A.; Diogène, J.; Raho, N.; Zapata, M. Gambierdiscus excentricus (Dinophyceae), a benthic toxic dinoflagellate from the Canary Islands (NE Atlantic Ocean). Harmful Algae 2011, 11, 10–22. [Google Scholar] [CrossRef]
- Litaker, R.W.; Holland, W.C.; Hardison, D.R.; Pisapia, F.; Hess, P.; Kibler, S.R.; Tester, P.A. Ciguatoxicity of Gambierdiscus and Fukuyoa species from the Caribbean and Gulf of Mexico. PLoS ONE 2017, 12, e0185776. [Google Scholar] [CrossRef]
- Pisapia, F.; Holland, W.C.; Hardison, D.R.; Litaker, R.W.; Fraga, S.; Nishimura, T.; Adachi, M.; Nguyen-Ngoc, L.; Séchet, V.; Amzil, Z.; et al. Toxicity screening of 13 Gambierdiscus strains using neuro-2 and erythrocyte lysis bioassays. Harmful Algae 2017, 63, 173–183. [Google Scholar] [CrossRef]
- Hoppenrath, M.; Kretzschmar, A.L.; Kaufmann, M.J.; Murray, S.A. Morphological and molecular phylogenetic identification and record verification of Gambierdiscus excentricus (Dinophyceae) from Madeira Island (NE Atlantic Ocean). Mar. Biodivers. Rec. 2019, 12, doi. [Google Scholar] [CrossRef]
- Kohli, G.S.; Murray, S.A.; Neilan, B.A.; Rhodes, L.L.; Harwood, D.T.; Smith, K.F.; Meyer, L.; Capper, A.; Brett, S.; Hallegraeff, G.M. High abundance of the potentially maitotoxic dinoflagellate Gambierdiscus carpenteri in temperate waters of New South Wales, Australia. Harmful Algae 2014, 39, 134–145. [Google Scholar] [CrossRef]
- Larsson, M.E.; Laczka, O.F.; Harwood, D.T.; Lewis, R.J.; Himaya, S.W.A.; Doblin, M.A. Toxicology of Gambierdiscus spp. (Dinophyceae) from tropical and temperate Australian waters. Mar. Drugs 2018, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Litaker, R.W.; Vandersea, M.W.; Faust, M.A.; Kibler, S.R.; Nau, A.W.; Holland, W.C.; Chinain, M.; Holmes, M.J.; Tester, P.A. Global distribution of ciguatera causing dinoflagellates in the genus Gambierdiscus. Toxicon 2010, 56, 711–730. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.; Smith, L.; Harwood, T.; Murray, S.; Biessy, L.; Argyle, P.; Munday, R. Is Gambierdiscus expanding its geographic range in the Pacific region? Harmful Algae News 2017, 56, 1–4. [Google Scholar]
- Salinger, M.J.; Renwick, J.; Behrens, E.; Mullan, A.B.; Diamond, H.J.; Sirguey, P.; Smith, R.O.; Trought, M.C.T.; Alexander, V.L.; Cullen, N.J.; et al. The unprecedented coupled ocean-atmosphere summer heatwave in the New Zealand region 2017/18: Drivers, mechanisms and impacts. Environ. Res. Lett. 2019, 14, 18. [Google Scholar] [CrossRef]
- Vergés, A.; Steinberg, P.D.; Hay, M.E.; Poore, A.G.B.; Campbell, A.H.; Ballesteros, E.; Heck, K.L., Jr.; Booth, D.J.; Coleman, M.A.; Feary, D.A.; et al. The tropicalization of temperate marine ecosystems: Climate-mediated changes in herbivory and community phase shifts. Proc. R. Soc. B 2014, 281, 20140846. [Google Scholar] [CrossRef]
- Trnski, T.; Schlumpf, H.A. (Eds.) Kermadec Biodiscovery Expedition. Bull Auckland Museum, 2011; Volume 20, Available online: http://www.aucklandmuseum.com/research/pub/bulletin/20/1 (accessed on 12 January 2020).
- Rhodes, L.; Gimenez Papiol, G.; Smith, K.; Harwood, T. Gambierdiscus cf. yasumotoi (Dinophyceae) isolated from New Zealand’s sub-tropical northern coastal waters. N. Z. J. Mar. Freshw. Res. 2014, 48, 303–310. [Google Scholar]
- Rhodes, L.; Smith, K.; Harwood, T.; Selwood, A.; Argyle, P.; Bedford, C.; Munday, R. Gambierdiscus and Ostreopsis from New Zealand, the Kermadec Islands and the Cook Islands and the risk of ciguatera fish poisoning to New Zealand. In Marine and Freshwater Algae 2014, Proceeding the 16th International Conference Harmful Algae, Wellington, New Zealand, 27–31 August 2014; Cawthron Institute: Nelson, New Zealand, 2014; pp. 180–183. [Google Scholar]
- Gómez, F.; Quit, D.; Lopes, R.M.; Lin, S. Fukuyoa paulensis gen. et sp. nov., a new genus for the globular species of the dinoflagellate Gambierdiscus (Dinophyceae). PLoS ONE 2015. [Google Scholar] [CrossRef]
- Smith, K.F.; Kohli, G.S.; Murray, S.A.; Rhodes, L.L. Assessment of the metabarcoding approach for community analysis of benthic-epiphytic dinoflagellates using mock communities. N. Z. J. Mar. Freshw. Res. 2017, 51, 555–576. [Google Scholar] [CrossRef]
- Litaker, R.W.; Vandersea, M.W.; Faust, M.A.; Kibler, S.R.; Chinain, M.; Holmes, M.J.; Holland, W.C.; Tester, P.A. Taxonomy of Gambierdiscus including four new species, Gambierdiscus caribaeus, Gambierdiscus carolinianus, Gambierdiscus carpenteri and Gambierdiscus ruetzleri (Gonyaulacales, Dinophyceae). Phycologia 2009, 48, 344–390. [Google Scholar] [CrossRef]
- Rhodes, L.L.; Smith, K.F.; Verma, A.; Murray, S.; Harwood, D.T.; Trnski, T. The dinoflagellate genera Gambierdiscus and Ostreopsis from sub-tropical Raoul Island and North Meyer Island, Kermadec Islands. N. Z. J. Mar. Freshw. Res. 2017, 51, 490–504. [Google Scholar] [CrossRef]
- Munday, R.; Murray, S.; Rhodes, L.; Larsson, M.; Harwood, D.T. Ciguatoxins and maitotoxins in extracts of sixteen Gambierdiscus isolates and one Fukuyoa isolate from the South Pacific and their toxicity to mice by intraperitoneal and oral administration. Mar. Drugs 2017, 7, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.L.; Smith, K.F.; Verma, A.; Curley, B.G.; Harwood, D.T.; Kohli, G.S.; Solomona, D.; Rongo, T.; Munday, R.; Murray, S. A new species of Gambierdiscus (Dinophyceae) from the south-west Pacific: Gambierdiscus honu sp. nov. Harmful Algae 2017, 65, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.; Murray, S.; Harwood, T.; Smith, K.; Nishimura, T. HABs in Paradise revisited. Harmful Algae News (IOC Newsl.) 2019, 62, 16–17. [Google Scholar]
- Parsons, M.L.; Settlemier, C.J.; Ballauer, J.M. An examination of the epiphytic nature of Gambierdiscus toxicus, a dinoflagellate involved in ciguatera fish poisoning. Harmful Algae 2011, 10, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Rains, L.; Parsons, M.L. Gambierdiscus species exhibit different epiphytic behaviors toward a variety of macroalgal hosts. Harmful Algae 2015, 49, 29–39. [Google Scholar] [CrossRef]
- Sparrow, L.; Momigliano, P.; Russ, G.R.; Heimann, K. Effects of temperature, salinity and composition of the dinoflagellate assemblage on the growth of Gambierdiscus carpenteri isolated from the Great Barrier Reef. Harmful Algae 2017, 65, 52–60. [Google Scholar] [CrossRef]
- Smith, K.F.; Rhodes, L.L.; Verma, A.; Curley, B.G.; Harwood, D.T.; Kohli, G.S.; Solomona, D.; Rongo, T.; Munday, R.; Murray, S. A new Gambierdiscus species (Dinophyceae) from Rarotonga, Cook Islands: Gambierdiscus cheloniae sp. nov. Harmful Algae 2016, 60, 45–56. [Google Scholar] [CrossRef]
- Rhodes, L.; Smith, K.; Munday, R.; Selwood, A.; McNabb, P.; Holland, P.; Bottein, M.-Y. Toxic dinoflagellates (Dinophyceae) from Rarotonga, Cook Islands. Toxicon 2010, 56, 751–758. [Google Scholar] [CrossRef]
- Rhodes, L.; Harwood, T.; Smith, K.; Adamson, J.; Argyle, P.; Munday, R. Production of ciguatoxin and maitotoxin by strains of Gambierdiscus australes, G. pacificus and G. polynesiensis (Dinophyceae) isolated from Rarotonga, Cook Islands. Harmful Algae 2014, 39, 185–190. [Google Scholar] [CrossRef]
- Mustapa, N.I.; Yong, H.L.; Lee, L.K.; Lim, Z.F.; Lim, H.C.; Teng, S.T.; Luo, Z.; Gu, H.; Leaw, C.P.; Lim, P.T. Growth and epiphytic behaviour of three Gambierdiscus species (Dinophyceae) associated with various macroalgal substrates. Harmful Algae 2019, 89, 101671. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, L.; Heimann, K. Key environmental factors in the management of ciguatera. J. Coast. Res. 2016, 75, 1007–1011. [Google Scholar] [CrossRef]
- Grzebyk, D.; Berland, B.; Thomassin, B.A.; Bosi, C.; Arnoux, A. Ecology of ciguateric dinoflagellates in the coral reef complex of Mayotte Island (S.W. Indian Ocean). J. Exp. Mar. Biol. Ecol. 1994, 178, 51–66. [Google Scholar] [CrossRef]
- Argyle, P. The Ecology and Toxin Production of Gambierdiscus and Fukuyoa Species from the Pacific. Ph.D. Thesis, School of Biological Sciences, University of Canterbury, Christchurch, New Zealand, 21 December 2018. [Google Scholar]
- Tester, P.A.; Kibler, S.R.; Holland, W.C.; Usup, G.; Vandersea, M.W.; Leaw, C.P.; Teen, L.P.; Larsen, J.; Mohammed-Noor, N.; Faust, M.A.; et al. Sampling harmful benthic dinoflagellates: Comparison of artificial and natural substrate methods. Harmful Algae 2014, 39, 8–25. [Google Scholar] [CrossRef]
- Parsons, M.L.; Brandt, A.L.; Ellsworth, A.; Leynse, A.K. Assessing the use of artificial substrates to monitor Gambierdiscus populations in the Florida Keys. Harmful Algae 2017, 68, 52–66. [Google Scholar] [CrossRef]
- Argyle, P.; Smith, K.; Halafihi, T.; Rhodes, L.; Harwood, T.; Marsden, I.; Halafihi, T. Ciguatera and related benthic HAB organisms and toxins. In Proceedings of the 18th International Conference on Harmful Algae, Nantes, France, 21–26 October 2018. in press. [Google Scholar]
- Fernandez-Zabala, J.; Tuya, F.; Amorim, A.; Soler-Onis, E. Benthic dinoflagellates: Testing the reliability of the artificial substrate method in the Macronesian region. Harmful Algae 2019, 86, 101634. [Google Scholar] [CrossRef]
- Mangialajo, L.; Fricke, A.; Perez-Gutierrez, G.; Catania, D.; Jauzein, C.; Lemee, R. Benthic Dinoflagellate Intergrator (BEDI): A new method for the quantification of benthic harmful algae blooms. Harmful Algae 2017, 64, 1–10. [Google Scholar] [CrossRef]
- MacKenzie, L.; Beuzenberg, V.; Holland, P.; McNabb, P.; Selwood, A. Solid phase adsorption toxin tracking (SPATT): A new monitoring tool that simulates the biotoxin contamination of filter feeding bivalves. Toxicon 2004, 44, 901–918. [Google Scholar] [CrossRef]
- Roué, M.; Darius, H.T.; Viallon, J.; Ung, A.; Gatti, C.; Harwood, D.T.; Chinain, M. Application of solid phase adsorption toxin tracking (SPATT) devices for the field detection of Gambierdiscus toxins. Harmful Algae 2018, 71, 40–49. [Google Scholar] [CrossRef]
- Smith, K.F.; Biessy, L.; Argyle, P.A.; Trnski, T.; Halafihi, T.; Rhodes, L.L. Molecular identification of Gambierdiscus and Fukuyoa (Dinophyceae) from environmental samples. Mar Drugs 2017, 15, 243. [Google Scholar] [CrossRef]
- Vandersea, M.W.; Kibler, S.R.; Holland, W.C.; Tester, P.A.; Schultz, T.F.; Faust, M.A.; Holmes, M.J.; Chinain, M.; Litaker, R.W. Development of semi-quantitative PCR assays for the detection and enumeration of Gambierdiscus species (Gonyaulacales, Dinophyceae) 1. J. Phycol. 2012, 48, 902–915. [Google Scholar] [CrossRef]
- Kretzschmar, A.L.; Verma, A.; Kohli, G.S.; Murray, S. Development of a quantitative PCR assay for the detection and enumeration of a potentially ciguatoxin-producing dinoflagellate, Gambierdiscus lapillus (Gonyaulacales, Dinophyceae). PLoS ONE 2019, 14, e0224664. [Google Scholar] [CrossRef] [PubMed]
- Litaker, R.W.; Tester, P.A.; Vandersea, M.W. Species-specific PCR assays for Gambierdiscus excentricus and Gambierdiscus silvae (Gonyaulacales, Dinophyceae). J. Phycol. 2019, 53, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Jeong, H.J.; Yoo, Y.D. Gambierdiscus jejuensis sp. nov., an epiphytic dinoflagellate from the waters of Jeju Island, Korea, effect of temperature on the growth, and its global distribution. Harmful Algae 2018, 80, 149–157. [Google Scholar] [CrossRef]
- Nishimura, T.; Hariganeya, N.; Tawong, W.; Sakanari, H.; Yamaguchi, H.; Adachi, M. Quantitative PCR assay for detection and enumeration of ciguatera-causing dinoflagellate Gambierdiscus spp. (Gonyaulacales) in coastal areas of Japan. Harmful Algae 2016, 52, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Sato, S.; Tawong, W.; Sakanari, H.; Uehara, K.; Shah, M.M.R.; Suda, S.; Yasumoto, T.; Taira, Y.; Yamaguchi, H. Genetic diversity and distribution of the ciguatera-causing dinoflagellate Gambierdiscus spp. (Dinophyceae) in coastal areas of Japan. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Kohli, G.S.; Uwe, J.; Figueroa, R.I.; Rhodes, L.L.; Harwood, D.T.; Groth, M.; Bolch, C.J.S.; Murray, S.A. Polyketide synthesis genes associated with toxin production in two species of Gambierdiscus (Dinophyceae). BMC Genom. 2015, 16, 410–420. [Google Scholar] [CrossRef]
- Kohli, G.S.; Campbell, K.; Uwe, J.; Smith, K.F.; Fraga, S.; Rhodes, L.L.; Murray, S.A. Role of modular polyketide synthases in the production of polyether ladder compounds in ciguatoxin-producing Gambierdiscus polynesiensis and G. excentricus (Dinophyceae). J. Euk. Microbiol. 2017, 64, 691–706. [Google Scholar] [CrossRef]
- Verma, A.; Barua, A.; Ruvindy, R.; Savela, H.; Ajani, P.A.; Murray, S.A. The genetic basis of toxin biosynthesis in dinoflagellates. Microorganisms 2019, 7, 222. [Google Scholar] [CrossRef]
- Yogi, K.; Oshiro, N.; Inafuku, Y.; Hirama, N.; Yasumoto, T. Detailed LC-MS/MS analysis of ciguatoxins revealing distinct regional and species characteristics in fish and causative alga from the Pacific. Anal. Chem. 2011, 83, 8886–8891. [Google Scholar] [CrossRef]
- Roeder, K.; Erler, K.; Kibler, S.; Tester, P.; Van The, H.; Nguyen-Ngoc, L.; Gerdts, G.; Luckas, B. Characteristic profiles of Ciguatera toxins in different strains of Gambierdiscus spp. Toxicon 2010, 56, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Ikehara, T.; Kuniyoshi, K.; Oshiro, N.; Yasumoto, T. Biooxidation of ciguatoxins leads to species-specific toxin profiles. Toxins 2017, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Naoki, H.; Iwashita, T.; Matsunaga, S.; Sasaki, M.; Yokoyama, T. Structure of maitotoxin. J. Am. Chem. Soc. 1993, 115, 2060–2062. [Google Scholar] [CrossRef]
- Selwood, A.; Rhodes, L.; Smith, K.; Harwood, D.T. Development of two novel UPLC-MS/MS methods for the analysis of maitotoxin from micro-algae cultures. In Marine and Freshwater Harmful Algae. Proceeding 16th International Conference on Harmful Algae, Wellington, New Zealand, 27–31 October 2014; MacKenzie, A.L., Ed.; Cawthron Institute: Nelson, New Zealand, 2014; pp. 66–69. [Google Scholar]
- Murray, J.S.; Selwood, A.I.; Harwood, D.T.; van Ginkel, R.; Puddick, J.; Rhodes, L.L.; Rise, F.; Wilkins, A.L. 44-Methylgambierone, a new gambierone analogue isolated from Gambierdiscus australes. Tetrahedron Lett. 2019, 60, 621–625. [Google Scholar] [CrossRef]
- Fraga, S.; Rodríguez, F.; Riobó, P.; Bravo, I. Gambierdiscus balechii sp. nov. (Dinophyceae), a new benthic toxic dinoflagellate from the Celebes Sea (SW Pacific Ocean). Harmful Algae 2016, 58, 93–105. [Google Scholar] [CrossRef]
- Rodríguez, I.; Genta-Jouve, G.; Alfonso, C.; Calabro, K.; Alonso, E.; Sanchez, J.A.; Alfonso, A.; Thomas, O.P.; Botana, L.M. Gambierone, a ladder-shaped polyether from the dinoflagellate Gambierdiscus belizeanus. Org. Lett. 2015, 17, 2392–2395. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Inserra, M.; Vetter, I.; Holland, W.C.; Hardison, D.R.; Tester, P.A.; Litaker, R.W. Rapid extraction and identification of Maitotoxin and Ciguatoxin-Like toxins from Caribbean and Pacific Gambierdiscus using a new functional bioassay. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Boente-Juncal, A.; Álvarez, M.; Antelo, A.; Rodríguez, I.; Calabro, K.; Vale, C.; Thomas, O.P.; Botana, L.M. Structure elucidation and biological evaluation of Maitotoxin-3, a homologue of gambierone, from Gambierdiscus belizeanus. Toxins 2019, 11, 79. [Google Scholar] [CrossRef]
- Tawong, W.; Yoshimatsu, T.; Yamaguchi, H.; Adachi, M. Temperature and salinity effects and toxicity of Gambierdiscus caribaeus (Dinophyceae) from Thailand. Phycologia 2016, 55, 274–278. [Google Scholar] [CrossRef]
- Kretzschmar, A.L.; Larsson, M.; Hoppenrath, M.; Doblin, M.; Murray, S. Characterisation of two toxic Gambierdiscus spp. (Gonyaulacales, Dinophyceae) from the Great Barrier Reef (Australia): G. lewisii sp. nov. and G. holmesii sp. nov. Protist 2019. Accepted. [Google Scholar] [CrossRef]
- Nishimura, T.; Sato, S.; Tawong, W.; Sakanari, H.; Yamaguchi, H.; Adachi, M. Morphology of Gambierdiscus scabrosus sp. nov. (Gonyaulacales): A new epiphytic toxic dinoflagellate from coastal areas of Japan. J. Phycol. 2014, 50, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.J.; Lewis, R.J.; Gillespie, N.C. Toxicity of Australian and French Polynesian strains of Gambierdiscus toxicus (Dinophyceae) grown in culture: Characterisation of a new type of maitotoxin. Toxicon 1990, 28, 1159–1172. [Google Scholar] [CrossRef]
- Nagai, H.; Murata, M.; Torigoe, K.; Satake, M.; Yasumoto, T. Gambieric acids, new potent antifungal substances with unprecedented polyether structures from a marine dinoflagellate Gambierdiscus toxicus. J. Org. Chem. 1992, 57, 5448–5453. [Google Scholar] [CrossRef]
- Satake, M.; Murata, M.; Yasumoto, T. Structure of CTX3C, a ciguatoxin congener isolated from cultured Gambierdiscus toxicus. Tetrahedron. Lett. 1993, 34, 1975–1978. [Google Scholar] [CrossRef]
- Watanabe, R.; Uchida, H.; Suzuki, T.; Matsushima, R.; Nagae, M.; Toyohara, Y.; Satake, M.; Oshima, Y.; Inoue, A.; Yasumoto, T. Gambieroxide, a novel epoxy polyether compound from the dinoflagellate Gambierdiscus toxicus GTP2 strain. Tetrahedron 2013, 69. [Google Scholar] [CrossRef]
- Leung, P.T.Y.; Yan, M.; Lam, V.; Yiu, S.K.F.; Chen, C.-Y.; Murray, J.S.; Harwood, D.T.; Rhodes, L.L.; Lam, P.K.S.; Wai, T.-C. Phylogeny, morphology and toxicity of benthic dinoflagellates of the genus Fukuyoa (Goniodomataceae, Dinophyceae) from a subtropical reef ecosystem in the South China Sea. Harmful Algae 2018, 74, 78–97. [Google Scholar] [CrossRef]
- Holmes, M.J. Gambierdiscus yasumotoi sp. nov. (Dinophyceae), a toxic benthic dinoflagellate from Southeastern Asia. J. Phycol. 1998, 34, 661–668. [Google Scholar] [CrossRef]
- Manger, R.L.; Leja, L.S.; Lee, S.Y.; Hungerford, J.M.; Wekell, M.M. Tetrazolium-based cell bioassay for neurotoxins active on voltage-sensitive sodium channels: Semiautomated assay for saxitoxins, brevetoxins, and ciguatoxins. Anal. Biochem. 1993, 214, 190–194. [Google Scholar] [CrossRef]
- Hardison, D.R.; Holland, W.C.; McCall, J.R.; Bourdelais, A.J.; Baden, D.G.; Darius, H.T.; Chinain, M.; Tester, P.A.; Shea, D.; Flores Quintana, H.A.; et al. Fluorescent Receptor Binding Assay for Detecting Ciguatoxins in Fish. PLoS ONE 2016, 11, e0153348. [Google Scholar] [CrossRef]
- Holland, W.C.; Litaker, R.W.; Tomas, C.R.; Kibler, S.R.; Place, A.R.; Davenport, E.D.; Tester, P.A. Differences in the toxicity of six Gambierdiscus (Dinophyceae) species measured using an in vitro human erythrocyte lysis assay. Toxicon 2013, 65, 15–33. [Google Scholar] [CrossRef]
- Martin-Yken, H.; Gironde, C.; Derick, S.; Darius, H.T.; Furger, C.; Laurent, D.; Chinain, M. Ciguatoxins activate the Calcineurin signalling pathway in yeasts: Potential for development of an alternative detection tool? Environ. Res. 2018, 162, 144–151. [Google Scholar] [CrossRef]
- Holmes, M.J.; Lewis, R.J. Purification and characterisation of large and small maitotoxins from cultured Gambierdiscus toxicus. Natural Toxins 1994, 2, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.A.; Monigliano, P.; Heimann, K.; Blair, D. Molecular phylogenetics and morphology of Gambierdiscus yasumotoi from tropical eastern Australia. Harmful Algae 2014, 39, 242–252. [Google Scholar] [CrossRef]
- Chinain, M.; Faust, M.A.; Pauillac, S. Morphology and molecular analyses of three toxic species of Gambierdiscus (Dinophycae): G. pacificus sp. nov., G. australes sp. nov., and G. polynesiensis sp. nov. J. Phycol. 1999, 35, 1282–1296. [Google Scholar] [CrossRef]
- Munday, R. Toxicology of Seafood Toxins: A Critical Review. In Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection, 3rd ed.; Botana, L.M., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 197–290. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain. Scientific opinion on marine biotoxins in shellfish—Emerging toxins: Ciguatoxin group. EFSA J. 2010, 8, 1627. [Google Scholar] [CrossRef]
- Dickey, R.W.; Plakas, S.M. Ciguatera: A public health perspective. Toxicon 2010, 56, 123–136. [Google Scholar] [CrossRef]
- Yasumoto, T.; Igarashi, T.; Legrand, A.-M.; Cruchet, P.; Chinain, M.; Fujita, T.; Naoki, H. Structural elucidation of ciguatoxin congeners by fast-atom bombardment tandem mass spectroscopy. J. Am. Chem. Soc. 2000, 122, 4988–4989. [Google Scholar] [CrossRef]
- Clausing, R.J.; Losen, B.; Oberhaensli, F.R.; Darius, H.T.; Sibat, M.; Hess, P.; Swarzenski, P.W.; Chinain, M.; Dechraoui Bottein, M.Y. Experimental evidence of dietary ciguatoxin accumulation in an herbivorous coral reef fish. Aquat. Toxicol. 2018, 200, 257–265. [Google Scholar] [CrossRef]
- Kohli, G.S.; Gimenez Papiol, G.; Rhodes, L.; Harwood, T.; Selwood, A.; Jerrett, A.; Murray, S.A.; Neilan, B.A. A feeding study to probe the uptake of Maitotoxin by snapper (Pagrus auratus). Harmful Algae 2014, 37, 125–132. [Google Scholar] [CrossRef]
- Neves, R.A.F.; Pardal, M.A.; Nascimento, S.M.; Oliveira, P.J.; Rodrigues, E.T. A contribution for establishing a specific, simple, fast and sensitive cell-based assay for ciguatera toxin screening. In Proceedings of the 18th International Conference on Harmful Algae, Nantes, France, 21–26 October 2018. in press. [Google Scholar]
- Tsumuraya, T.; Sato, T.; Oshiro, N.; Hirama, M.; Fujii, I. Highly sensitive and practical fluorescent sandwich ELISA for ciguatoxins. In Proceedings of the 18th International Conference on Harmful Algae, Nantes, France, 21–26 October 2018. in press. [Google Scholar]
- Hales, S.; Weinstein, P.; Woodward, A. Ciguatera (fish poisoning), El Niño, and Pacific sea surface temperatures. Ecosyst. Health 1999, 5, 20–25. [Google Scholar] [CrossRef]
- Llewellyn, L.E. Revisiting the association between sea surface temperature and the epidemiology of fish poisoning in the South Pacific: Reassessing the link between ciguatera and climate change. Toxicon 2010, 56, 691–697. [Google Scholar] [CrossRef]
- Sutton, P.J.H.; Bowen, M. Ocean temperature change around New Zealand over the last 36 years. N. Z. J. Mar. Freshw. Res. 2019. [Google Scholar] [CrossRef]
- Climate Change Projections for New Zealand. Available online: https://www.mfe.govt.nz/sites/default/files/media/Climate%20Change/Climate-change-projections-2nd-edition-final.pdf (accessed on 10 January 2020).
- Law, C.S.; Bell, J.J.; Bostock, H.C.; Cornwall, C.E.; Cummings, V.J.; Currie, K.; Davy, S.K.; Gammon, M.; Hepburn, C.D.; Hurd, C.L.; et al. Ocean acidification in New Zealand waters: Trends and impacts. N. Z. J. Mar. Freshw. Res. 2018, 52, 155–195. [Google Scholar] [CrossRef]
- Wang, B.; Yao, M.; Zhou, L. Growth and toxin production of Gambierdiscus spp. can be regulated by quorum-sensing bacteria. Toxins 2018, 10, 257. [Google Scholar] [CrossRef] [PubMed]
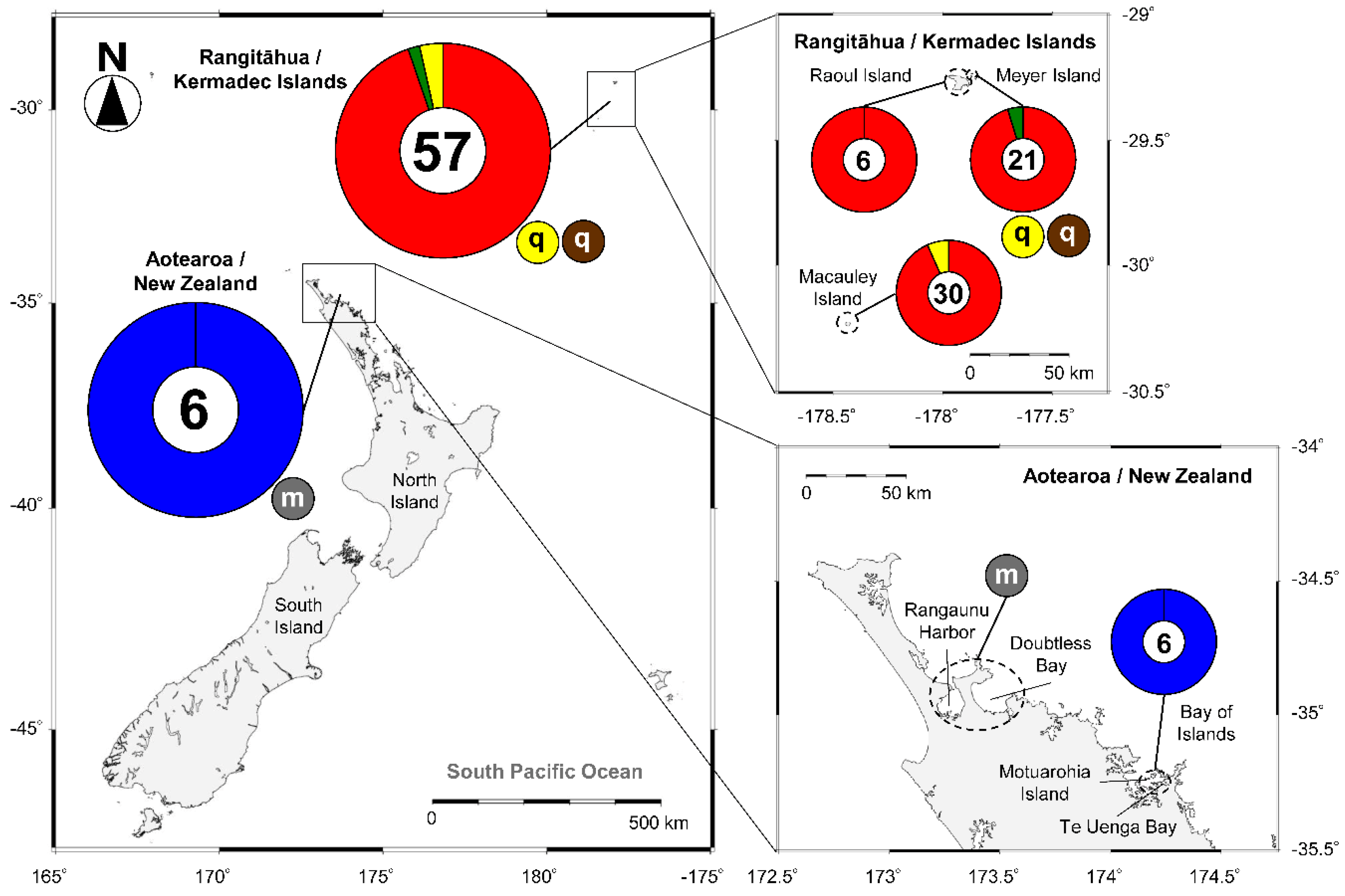
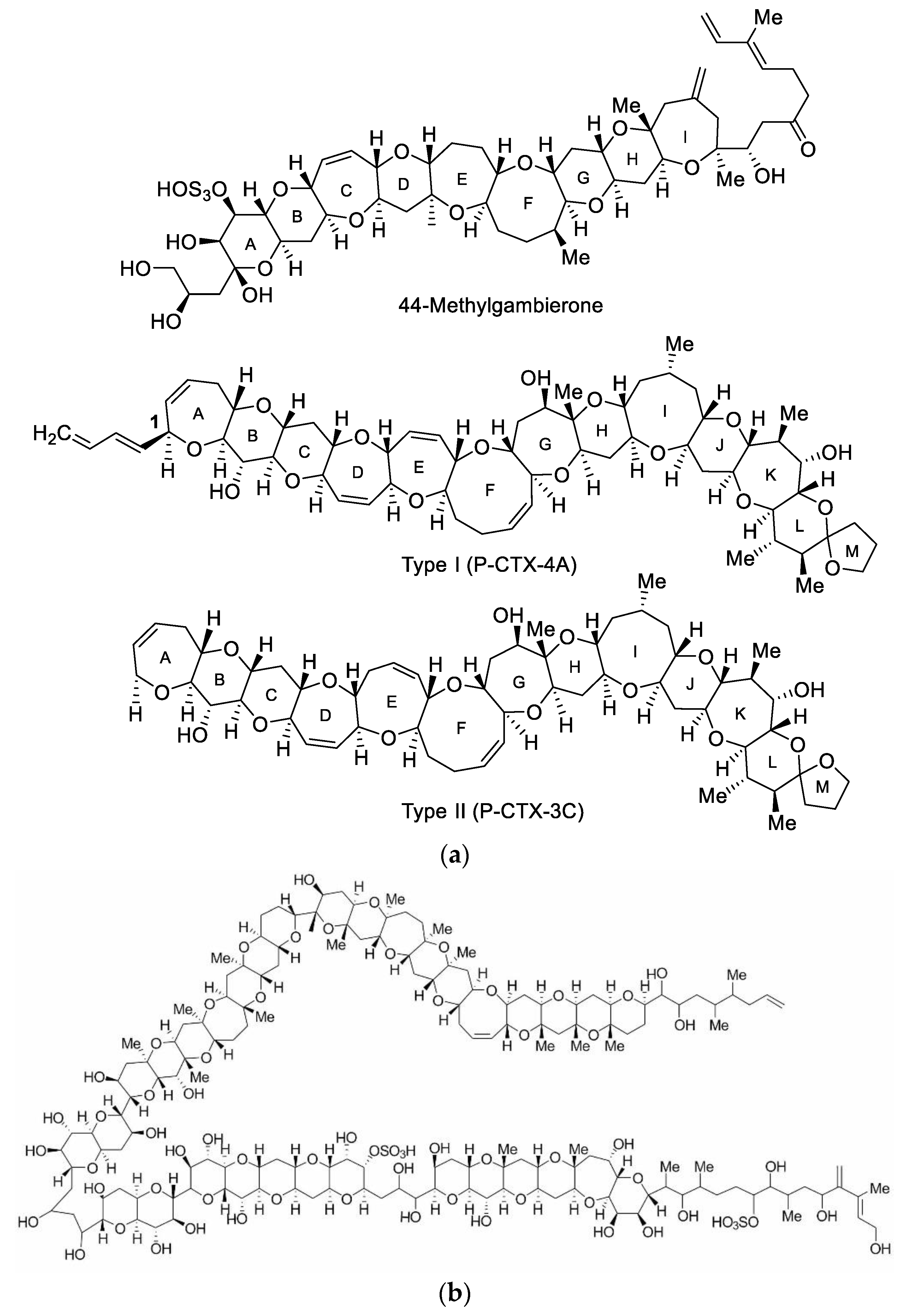
| Species | Sampling Site | GenBank Code | Toxins (pg/cell) | CICCM Code | Reference |
|---|---|---|---|---|---|
| G. australes | North Meyer Is. 29°14.485′ S, 172°52.717′ W | KY069059 | MTX-1 (5.9); 44-MG | CAWD244 | Rhodes et al. 2017 [37] |
| North Meyer Is. 29°14.485′ S, 172°52.717′ W | MN709498 | MTX-1 (8.9); 44-MG | CAWD246 | Munday et al. 2017 [38] | |
| Macauley Is. 30°14′ S, 178°26′ W | MF109033 | MTX-1 (36); 44-MG | CAWD255 | Rhodes et al. 2017 [12,37] | |
| Macauley Is. 30°14′ S, 178°26′ W | MF109034 | MTX-1 (31); 44-MG | CAWD256 | Rhodes et al. 2017 [12] | |
| G. honu | North Meyer Is. 29°14.485′ S, 172°52.717′ W | KY062662 | 44-MG | CAWD242 | Rhodes et al. 2017 [39] |
| G. polynesiensis | Macauley Is. 30°14′ S, 178°26′ W | MF109032 | Traces CTX-3C; iso peaks CTX-3B/C and 4A/B; 44-MG | CAWD254 | Unpublished data |
| Macauley Is. 30°14′ S, 178°26′ W | NE | Neg. CTXs, MTX, 44-MG | CAWD259 | Rhodes et al. 2017 [12] | |
| G. cf. toxicus * | Northland | NS | NT | Chang 1996 [18] | |
| F. paulensis | Te Uenga Bay, Northland 35°25.58′ S, 174°24.17′ E | MN305995 | 44-MG | CAWD306 | Unpublished data |
| Species | Toxins | Toxicity | Reference |
|---|---|---|---|
| G. australes | MTX-1, 44-MG | High toxicity by i.p. using the MBA, less by oral administration | Munday et al. 2017 [38] |
| G. balechii | 44-MG | CTX- and MTX-like toxicity using the MBA and the N2a cytotoxicity assay | Fraga et al., 2016 [74]; Pisapia et al., 2017 [23] |
| G. belizeanus | Gambierone, 44-MG | CTX-like toxicity using the N2a cytotoxicity assay; CTX- and MTX-like activity using the Ca2+ flux assay; CTX-like activity using the RBA | Chinain et al. 2010 [19]; Rodriguez et al. 2015 [75]; Lewis et al. 2016 [76]; Litaker et al. 2017 [22]; Boente-Juncal et al. 2019 [77] |
| G. caribaeus | MTX-2, 44-MG | CTX- and MTX-like toxicity using the N2a cytotoxicity assay; CTX- and MTX-like activity using the MBA | Tawong et al. 2016 [78]; Litaker et al. 2017 [22]; Pisapia et al. 2017 [23] |
| G. carolinianus | 44-MG | Extremely low CTX- and MTX-like toxicity using the N2a cytotoxicity and Ca2+ flux assays | Tester et al. 2014 [51]; Lewis et al. 2016 [77]; Litaker et al. 2017 [22]; Pisapia et al. 2017 [23] |
| G. carpenteri | 44-MG* | Low toxicity by i.p. using the MBA, less by oral administration; CTX- and MTX-like toxicity using the N2a cytotoxicity assay; MTX-like activity using the Ca2+ flux assay | Munday et al. 2017 [38]; Litaker et al. 2017 [22]; Pisapia et al. 2017 [23]; Larsson et al. 2018 [26] |
| G. cheloniae | 44-MG | High toxicity by i.p. using the MBA, less by oral administration | Smith et al. 2016 [44]; Munday et al. 2017 [38] |
| G. excentricus | MTX-2, MTX-4, 44-MG | High CTX- and MTX-like toxicity using the N2a cytotoxicity and Ca2+ flux assays | Fraga et al. 2011 [21]; Pisapia et al. 2017 [23] |
| G. holmesii | 44-MG | CTX- and MTX-like activity using the Ca2+ flux assay | Larsson et al. 2018 [26]; Kretzschmar et al. 2019 [79] |
| G. honu | 44-MG | High toxicity by i.p. using the MBA, less by oral administration | Munday et al. 2017 [38]; Rhodes et al. 2017 [39] |
| G. jejuensis | Unknown | Unknown (non-toxic by i.p. using MBA) | Nishimura et al. 2014 [64] |
| G. lapillus | 44-MG | CTX- and MTX-like activity using the Ca2+ flux assay | Kretzschmar et al. 2017 [60]; Larsson et al. 2018 [26] |
| G. lewisii | 44-MG | CTX- and MTX-like activity using the Ca2+ flux assay | Larsson et al. 2018 26]; Kretzschmar et al. 2019 [79] |
| G. pacificus | 44-MG, MTX-2 | High toxicity by i.p. using the MBA, less by oral administration, CTX- and MTX-like activity using the N2a cytotoxicity assay | Munday et al. 2017 [38]; Pisapia et al. 2017 [23] |
| G. polynesiensis | P-CTX-3B *, P-CTX-3C *, P-CTX-4A *, P-CTX-4B *, P-CTX-3B/C isomers *, P-CTX-4A/B isomers *, 44-MG | Highly toxic by both i.p. and oral administration using the MBA | Rhodes et al. 2014 [33]; Munday et al. 2017 [38]; Rhodes et al. 2017 [12]; Chinain et al. 2010 [19] |
| G. scabrosus | 44-MG | CTX- and MTX-like toxicity using the MBA and N2a cytotoxicity assay | Nishimura et al. 2014 [64,80]; Pisapia et al. 2017 [23] |
| G. silvae | 44-MG | CTX- and MTX-like toxicity using the N2a cytotoxicity assay | Litaker et al. 2017 [22]; Pisapia et al. 2017 [23] |
| G. toxicus† | P-CTX-3C, P-CTX-4A/B, 44-MG, Gambieric acids A, B, C, and D, Gambieroxide, Gambierol, MTX-1, MTX-2 | CTX- and MTX-like toxicity using the N2a cytotoxicity and RBA assays; MTX-like activity using the MBA | Holmes et al. 1990 [81]; Nagai et al. 1992 [82]; Satake et al. 1993 [83]; Watanabe et al. 2013 [84]; Pisapia et al. 2017 [23] |
| F. paulensis | 44-MG | Low toxicity by i.p. using the MBA, extremely low by gavage | Rhodes et al. 2014 [32]; Munday et al. 2017 [38] |
| F. ruetzleri | 44-MG * | CTX-like toxicity using the N2a cytotoxicity, Ca2+ flux and brine shrimp assays | Tester et al. 2014 [51]; Litaker et al. 2017 [22]; Leung et al. 2018 [85] |
| F. yasumotoi | Unknown | MTX-like activity using the MBA | Holmes 1998 [86] |
| Fukuyoa sp. HK Type 1 | 44-MG | Activity using the brine shrimp bioassay | Leung et al. 2018 [85] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhodes, L.L.; Smith, K.F.; Murray, J.S.; Nishimura, T.; Finch, S.C. Ciguatera Fish Poisoning: The Risk from an Aotearoa/New Zealand Perspective. Toxins 2020, 12, 50. https://doi.org/10.3390/toxins12010050
Rhodes LL, Smith KF, Murray JS, Nishimura T, Finch SC. Ciguatera Fish Poisoning: The Risk from an Aotearoa/New Zealand Perspective. Toxins. 2020; 12(1):50. https://doi.org/10.3390/toxins12010050
Chicago/Turabian StyleRhodes, Lesley L., Kirsty F. Smith, J. Sam Murray, Tomohiro Nishimura, and Sarah C. Finch. 2020. "Ciguatera Fish Poisoning: The Risk from an Aotearoa/New Zealand Perspective" Toxins 12, no. 1: 50. https://doi.org/10.3390/toxins12010050
APA StyleRhodes, L. L., Smith, K. F., Murray, J. S., Nishimura, T., & Finch, S. C. (2020). Ciguatera Fish Poisoning: The Risk from an Aotearoa/New Zealand Perspective. Toxins, 12(1), 50. https://doi.org/10.3390/toxins12010050





