Potential Application of Lactic Acid Bacteria to Reduce Aflatoxin B1 and Fumonisin B1 Occurrence on Corn Kernels and Corn Ears
Abstract
1. Introduction
2. Results and Discussion
2.1. In Vitro Antifungal Activity of L. plantarum spp.
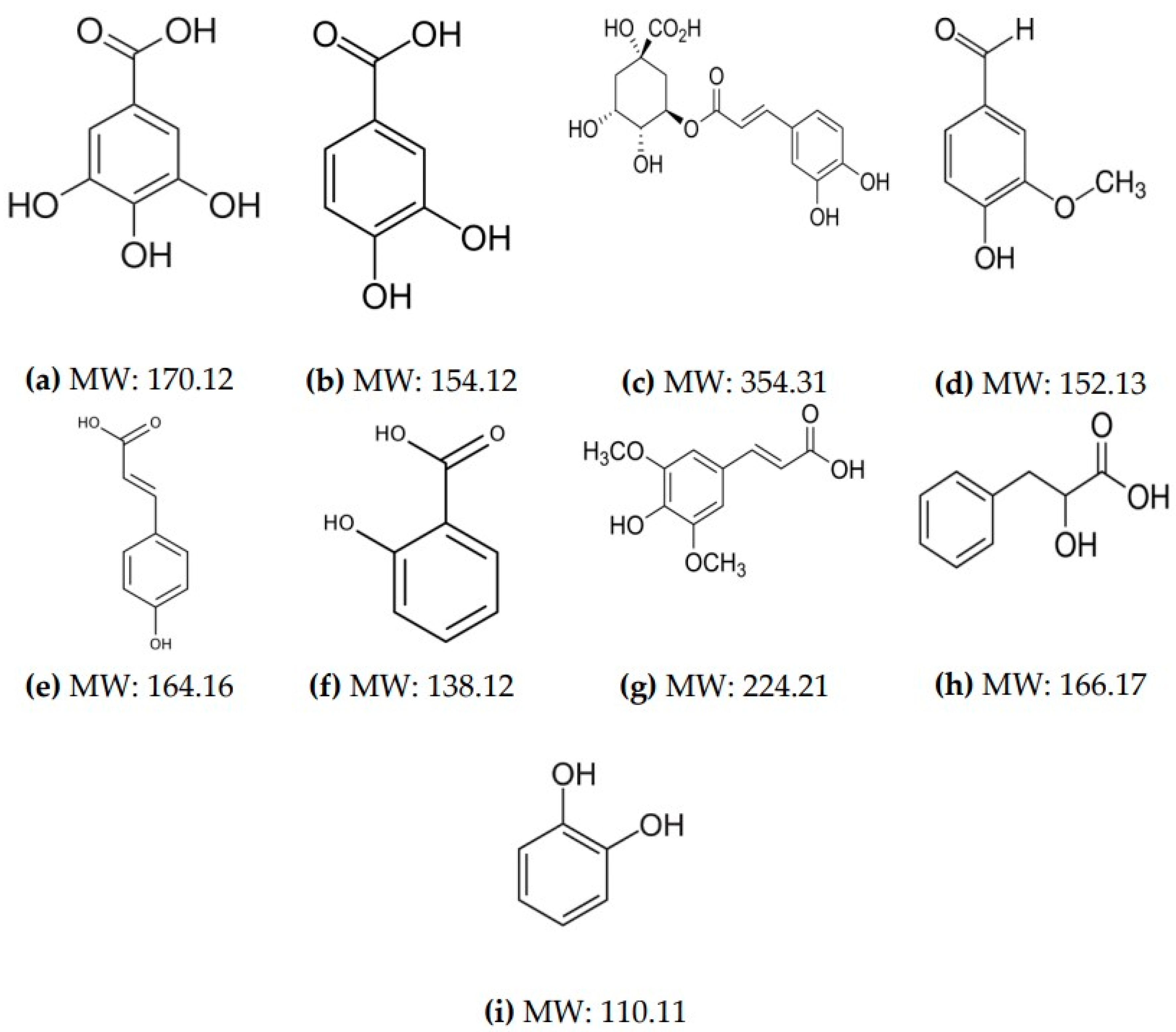
2.2. Phenolic Acids Produced by L. plantarum spp.
2.3. L. plantarum CECT 749 CFS Characteristics and Composition
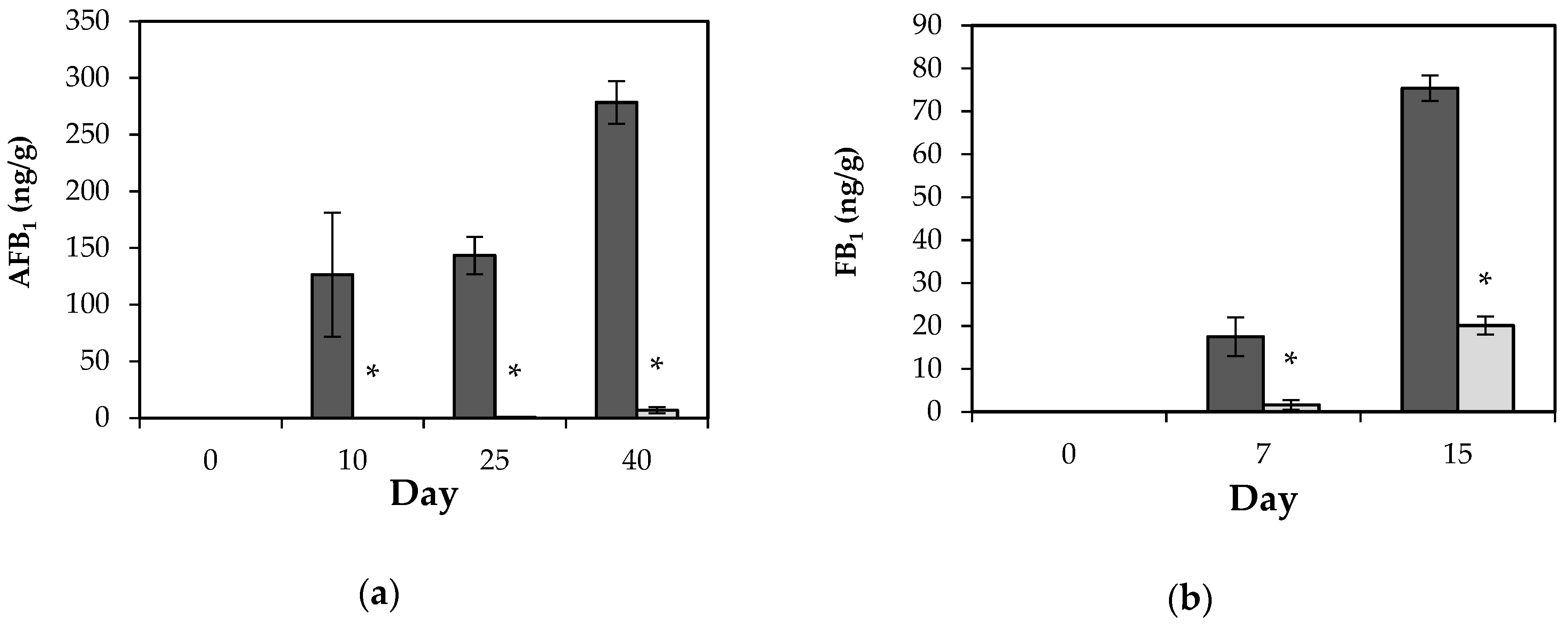
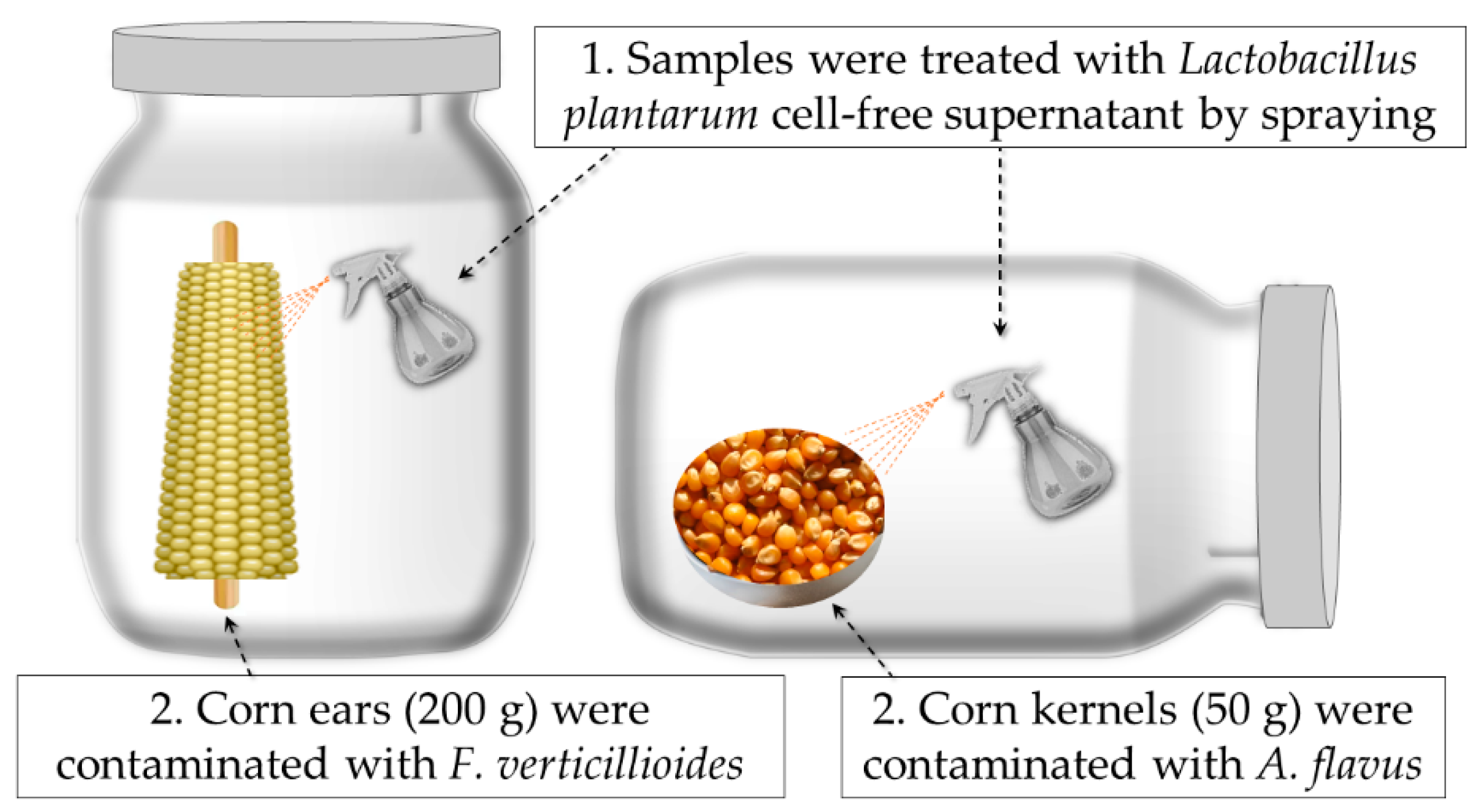
2.4. Biopreservation and Shelf-Life Improvement by L. plantarum CECT 749 CFS
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Microorganisms and Culture Conditions
4.3. LAB Growth and Preparation of Cell-Free Cupernatant
4.4. Qualitative Antifungal Activity in Solid Medium
4.5. Quantitative Antifungal Activity in 96-Well Microplates
4.6. Extraction and Identification of Phenolic Acids by LC-ESI-qTOF-MS
4.7. Biopreservation of Corn Kernels and Corn Ears
4.8. Determination of Mycotoxins by LC-MS/MS Spectrometry
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alshannaq, A.; Yu, J.H. Occurrence, toxicity and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef]
- Fleurat-Lessard, F. Integrated management of the risks of stored grain spoilage by seedborne fungi and contamination by storage mould mycotoxins—An update. J. Stored Prod. Res. 2017, 71, 22–40. [Google Scholar] [CrossRef]
- Gonçalves, B.L.; Coppa, C.F.S.C.; de Neeff, D.V.; Corassin, C.H.; Oliveira, C.A.F. Mycotoxins in fruits and fruit-based products: Occurrence and methods for decontamination. Toxin Rev. 2019, 38, 263–272. [Google Scholar] [CrossRef]
- Mditshwa, A.; Magwaza, L.S.; Tesfay, S.Z.; Mbili, N. Postharvest quality and composition of organically and conventionally produced fruits: A review. Sci. Hortic. 2017, 216, 148–159. [Google Scholar] [CrossRef]
- Azaiez, I.; Font, G.; Mañes, J.; Fernández-Franzón, M. Survey of mycotoxins in dates and dried fruits from Tunisian and Spanish markets. Food Control 2015, 51, 340–346. [Google Scholar] [CrossRef]
- Pereira, C.S.; Cunha, S.C.; Fernandes, J.O. Prevalent mycotoxins in animal feed: Occurrence and analytical methods. Toxins 2019, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Pizzolato Montanha, F.; Anater, A.; Burchard, J.F.; Luciano, F.B.; Meca, G.; Manyes, L.; Pimpão, C.T. Mycotoxins in dry-cured meats: A review. Food Chem. Toxicol. 2018, 111, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Filtenborg, O.; Frisvad, J.C.; Thrane, U. Moulds in food spoilage. Int. J. Food Microbiol. 1996, 33, 85–102. [Google Scholar] [CrossRef]
- Torrijos, R.; Nazareth, T.M.; Pérez, J.; Mañes, J.; Meca, G. Development of a bioactive sauce based on oriental mustard flour with antifungal properties for PITA bread shelf life improvement. Molecules 2019, 24, 1019. [Google Scholar] [CrossRef]
- Giorni, P.; Bertuzzi, T.; Battilani, P. Impact of fungi co-occurrence on mycotoxin contamination in maize during the growing season. Front. Microbiol. 2019, 10, 1265. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Alonso, V.A.; Pereyra, C.M.; Keller, L.A.M.; Dalcero, A.M.; Rosa, C.A.R.; Chiacchiera, S.M.; Cavaglieri, L.R. Fungi and mycotoxins in silage: An overview. J. Appl. Microbiol. 2013, 115, 637–643. [Google Scholar] [CrossRef]
- Reddy, K.R.N.; Salleh, B.; Saad, B.; Abbas, H.K.; Abel, C.A.; Shier, W.T. An overview of mycotoxin contamination in foods and its implications for human health. Toxin Rev. 2010, 29, 3–26. [Google Scholar] [CrossRef]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Degraeve, S.; Madege, R.R.; Audenaert, K.; Kamala, A.; Ortiz, J.; Kimanya, M.; Tiisekwa, B.; De Meulenaer, B.; Haesaert, G. Impact of local pre-harvest management practices in maize on the occurrence of Fusarium species and associated mycotoxins in two agro-ecosystems in Tanzania. Food Control 2015, 59, 225–233. [Google Scholar] [CrossRef]
- Jackson, L.S.; Katta, S.K.; Fingerhut, D.D.; De Vries, J.W.; Bullerman, L.B. Effects of Baking and Frying on the Fumonisin B1 Content of Corn-Based Foods. J. Agric. Food Chem. 1997, 45, 4800–4805. [Google Scholar] [CrossRef]
- Magan, N.; Medina, A. Integrating gene expression, ecology and mycotoxin production by Fusarium and Aspergillus species in relation to interacting environmental factors. World Mycotoxin J. 2016, 9, 673–684. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N. Thermophilic fungi to dominate aflatoxigenic/mycotoxigenic fungi on food under global warming. Int. J. Environ. Res. Public Health 2017, 14, 199. [Google Scholar] [CrossRef]
- Kabak, B.; Dobson, A.D.W.; Var, I. Strategies to prevent mycotoxin contamination of food and animal feed: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2019, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Thanushree, M.P.; Sailendri, D.; Yoha, K.S.; Moses, J.A.; Anandharamakrishnan, C. Mycotoxin contamination in food: An exposition on spices. Trends Food Sci. Technol. 2019, 93, 69–80. [Google Scholar] [CrossRef]
- Stockmann-Juvala, H.; Savolainen, K. A review of the toxic effects and mechanisms of action of fumonisin B 1. Hum. Exp. Toxicol. 2008, 27, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Human Health and their Management Strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Available online: https://monographs.iarc.fr/wp-content/uploads/2018/06/mono82.pdf (accessed on 31 December 2019).
- Stanojević-Nikolić, S.; Dimić, G.; Mojović, L.; Pejin, J.; Djukić-Vuković, A.; Kocić-Tanackov, S. Antimicrobial Activity of Lactic Acid Against Pathogen and Spoilage Microorganisms. J. Food Process. Preserv. 2016, 40, 990–998. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Saladino, F.; Luz, C.; Manyes, L.; Fernández-Franzón, M.; Meca, G. In vitro antifungal activity of lactic acid bacteria against mycotoxigenic fungi and their application in loaf bread shelf life improvement. Food Control 2016, 67, 273–277. [Google Scholar] [CrossRef]
- Niku-Paavola, M.L.; Laitila, A.; Mattila-Sandholm, T.; Haikara, A. New types of antimicrobial compounds produced by Lactobacillus plantarum. J. Appl. Microbiol. 1999, 86, 29–35. [Google Scholar] [CrossRef]
- Ndagano, D.; Lamoureux, T.; Dortu, C.; Vandermoten, S.; Thonart, P. Antifungal Activity of 2 Lactic Acid Bacteria of the Weissella Genus Isolated from Food. J. Food Sci. 2011, 76, M305–M311. [Google Scholar] [CrossRef]
- Broberg, A.; Jacobsson, K.; Ström, K.; Schnürer, J. Metabolite profiles of lactic acid bacteria in grass silage. Appl. Environ. Microbiol. 2007, 73, 5547–5552. [Google Scholar] [CrossRef]
- Schnürer, J.; Magnusson, J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci. Technol. 2005, 16, 70–78. [Google Scholar] [CrossRef]
- Corrêa, J.A.F.; Evangelista, A.G.; de Melo Nazareth, T.; Luciano, F.B. Fundamentals on the molecular mechanism of action of antimicrobial peptides. Materialia 2019, 8, 100494. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Yan, B.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Lactic Acid Bacteria as Antifungal and Anti-Mycotoxigenic Agents: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436. [Google Scholar] [CrossRef]
- Ghanbari, M.; Jami, M.; Domig, K.J.; Kneifel, W. Seafood biopreservation by lactic acid bacteria—A review. LWT Food Sci. Technol. 2013, 54, 315–324. [Google Scholar] [CrossRef]
- Sanders, M.E.; Akkermans, L.M.A.; Haller, D.; Hammerman, C.; Heimbach, J.; Hörmannsperger, G.; Huys, G.; Levy, D.D.; Lutgendorff, F.; Mack, D.; et al. Safety assessment of probiotics for human use. Gut Microbes 2010, 1, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Rodríguez, H.; Landete, J.M.; de las Rivas, B.; Muñoz, R. Metabolism of food phenolic acids by Lactobacillus plantarum CECT 748T. Food Chem. 2008, 107, 1393–1398. [Google Scholar] [CrossRef]
- Brosnan, B.; Coffey, A.; Arendt, E.K.; Furey, A. Rapid identification, by use of the LTQ Orbitrap hybrid FT mass spectrometer, of antifungal compounds produced by lactic acid bacteria. Anal. Bioanal. Chem. 2012, 403, 2983–2995. [Google Scholar] [CrossRef]
- Sánchez-Maldonado, A.F.; Schieber, A.; Gänzle, M.G. Structure-function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef]
- Cray, J.A.; Stevenson, A.; Ball, P.; Bankar, S.B.; Eleutherio, E.C.A.; Ezeji, T.C.; Singhal, R.S.; Thevelein, J.M.; Timson, D.J.; Hallsworth, J.E. Chaotropicity: A key factor in product tolerance of biofuel-producing microorganisms. Curr. Opin. Biotechnol. 2015, 33, 228–259. [Google Scholar] [CrossRef]
- Le Lay, C.; Coton, E.; Le Blay, G.; Chobert, J.M.; Haertlé, T.; Choiset, Y.; Van Long, N.N.; Meslet-Cladière, L.; Mounier, J. Identification and quantification of antifungal compounds produced by lactic acid bacteria and propionibacteria. Int. J. Food Microbiol. 2016, 239, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Souza, M.M.; Badiale-Furlong, E. Antioxidant and antifungal activity of phenolic compounds and their relation with aflatoxin B1 occurrence in soybeans (Glycine max L.). J. Sci. Food Agric. 2019. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cortes, T.; Pérez España, V.H.; López Pérez, P.A.; Rodríguez-Jimenes, G.D.C.; Robles-Olvera, V.J.; Aparicio Burgos, J.E.; Cuervo-Parra, J.A. Antifungal activity of vanilla juice and vanillin against Alternaria alternate. CyTA J. Food 2019, 17, 375–383. [Google Scholar] [CrossRef]
- Luz, C.; Izzo, L.; Ritieni, A.; Mañes, J.; Meca, G. Antifungal and antimycotoxigenic activity of hydrolyzed goat whey on Penicillium spp: An application as biopreservation agent in pita bread. LWT 2020, 118, 108717. [Google Scholar] [CrossRef]
- Gerez, C.L.; Torino, M.I.; Rollán, G.; Font de Valdez, G. Prevention of bread mould spoilage by using lactic acid bacteria with antifungal properties. Food Control 2009, 20, 144–148. [Google Scholar] [CrossRef]
- Willits, C.O. Methods for Determination of Moisture-Oven Drying. Anal. Chem. 1951, 23, 1058–1062. [Google Scholar] [CrossRef]
- Reiners, W.A.; Reiners, N.M. Comparison of Oxygen-Bomb Combustion with Standard Ignition Techniques for Determining Total Ash. Ecology 1972, 53, 132–136. [Google Scholar] [CrossRef]
- Miloski, K.; Wallace, K.; Fenger, A.; Schneider, E.; Bendinskas, K. Comparison of Biochemical and Chemical Digestion and Detection Methods for Carbohydrates. Am. J. Undergrad. Res. 2008, 7, 7–18. [Google Scholar] [CrossRef]
- Nuryana, I.; Andriani, A.; Lisdiyanti, P. Analysis of organic acids produced by lactic acid bacteria. IOP Conf. Ser. Earth Environ. Sci. 2019, 251, 012054. [Google Scholar] [CrossRef]
- Reddy, K.R.N.; Reddy, C.S.; Muralidharan, K. Potential of botanicals and biocontrol agents on growth and aflatoxin production by Aspergillus flavus infecting rice grains. Food Control 2009, 20, 173–178. [Google Scholar] [CrossRef]
- Ahlberg, S.H.; Joutsjoki, V.; Korhonen, H.J. Potential of lactic acid bacteria in aflatoxin risk mitigation. Int. J. Food Microbiol. 2015, 207, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Haskard, C.A.; El-Nezami, H.S.; Kankaanpää, P.E.; Salminen, S.; Ahokas, J.T. Surface Binding of Aflatoxin B 1 by Lactic Acid Bacteria. Appl. Environ. Microbiol. 2001, 67, 3086–3091. [Google Scholar] [CrossRef] [PubMed]
- Niderkorn, V.; Boudra, H.; Morgavi, D.P. Binding of Fusarium mycotoxins by fermentative bacteria in vitro. J. Appl. Microbiol. 2006, 101, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Juodeikiene, G.; Bartkiene, E.; Cernauskas, D.; Cizeikiene, D.; Zadeike, D.; Lele, V.; Bartkevics, V. Antifungal activity of lactic acid bacteria and their application for Fusarium mycotoxin reduction in malting wheat grains. LWT Food Sci. Technol. 2018, 89, 307–314. [Google Scholar] [CrossRef]
- Damayanti, E.; Indriati, R.; Sembiring, L.; Julendra, H. Antifungal Activities of Lactic Acid Bacteria against Aspergillus flavus, A. parasiticus and enicillium citrinum as Mycotoxin Producing Fungi. In Proceedings of the 16th AAAP Animal Science Congress, Yogyakarta, Indonesia, 10–14 November 2014; Volume 3, pp. 1742–1745. [Google Scholar]
- Russo, P.; Arena, M.P.; Fiocco, D.; Capozzi, V.; Drider, D.; Spano, G. Lactobacillus plantarum with broad antifungal activity: A promising approach to increase safety and shelf-life of cereal-based products. Int. J. Food Microbiol. 2017, 247, 48–54. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 401/2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur Union 2006, 70, 12–34. [Google Scholar]
- Dohlman, E. Mycotoxin Hazards and Regulations. Available online: https://www.ers.usda.gov/webdocs/publications/41603/15640_aer828h_1_.pdf?v=42055 (accessed on 31 December 2019).
- Nazareth, T.M.; Corrêa, J.A.F.; Pinto, A.C.S.M.; Palma, J.B.; Meca, G.; Bordin, K.; Luciano, F.B. Evaluation of gaseous allyl isothiocyanate against the growth of mycotoxigenic fungi and mycotoxin production in corn stored for 6 months. J. Sci. Food Agric. 2018, 98, 5235–5241. [Google Scholar] [CrossRef]
- Suleiman, R.A.; Rosentrater, K.A.; Bern, C.J. Effects of Deterioration Parameters on Storage of Maize: A Review. J. Nat. Sci. Res. 2013, 3, 147–165. [Google Scholar]
- Varsha, K.K.; Priya, S.; Devendra, L.; Nampoothiri, K.M. Control of spoilage fungi by protective lactic acid bacteria displaying probiotic properties. Appl. Biochem. Biotechnol. 2014, 172, 3402–3413. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of fi Lamentous Fungi; Approved Standard CLSI Document M38-A2; Clinical and Laboratory Standards Institute, WayneCLS: Wayne, PA, USA, 2008. [Google Scholar]
- Brosnan, B.; Coffey, A.; Arendt, E.K.; Furey, A. The QuEChERS approach in a novel application for the identification of antifungal compounds produced by lactic acid bacteria cultures. Talanta 2014, 129, 364–373. [Google Scholar] [CrossRef]
- Denardi-Souza, T.; Luz, C.; Mañes, J.; Badiale-Furlong, E.; Meca, G. Antifungal effect of phenolic extract of fermented rice bran with Rhizopus oryzae and its potential use in loaf bread shelf life extension. J. Sci. Food Agric. 2018, 98, 5011–5018. [Google Scholar] [CrossRef] [PubMed]
- Nazareth, T.D.M.; Quiles, J.M.; Torrijos, R.; Luciano, F.B.; Mañes, J.; Meca, G. Antifungal and antimycotoxigenic activity of allyl isothiocyanate on barley under different storage conditions. LWT 2019, 112, 108237. [Google Scholar] [CrossRef]
- Quiles, J.M.; Nazareth, T.d.M.; Luz, C.; Luciano, F.B.; Mañes, J.; Meca, G. Development of an antifungal and antimycotoxigenic device containing allyl isothiocyanate for silo fumigation. Toxins 2019, 11, 137. [Google Scholar] [CrossRef] [PubMed]
| Fungal Strain | L. plantarum | ||||||
|---|---|---|---|---|---|---|---|
| CECT 220 | CECT 221 | CECT 223 | CECT 224 | CECT 748 | CECT 749 | CECT 750 | |
| F. graminearum ITEM 126 | + | + | + | + | + | + + + | + |
| F. graminearum ITEM 6415 | + | + | + | + | + | + | + |
| F. cerealis CECT 20488 | + | + | + | + | + | + | + |
| F. cerealis CECT 20489 | + | + | + + | + | + | + | + |
| F. verticillioides CECT 20926 | + | + | + | + | + + | + + + | + |
| F. verticillioides CECT 2152 | + + | + | + + | + | + + | + + + | + + |
| F. verticillioides CECT 2982 | + + | + + | + + | + | + + | + + + | + |
| F. mesoamericanum CECT 20490 | + | + | + | + | + | + | + |
| F. poae CECT 20165 | + | + | + | + | + | + | + |
| A. flavus ITEM 8111 | + | + | + | + | + | + + | + |
| A. parasiticus CECT 2681 | − | − | − | − | + | + | − |
| A. niger CECT 2088 | − | + | − | − | + | + | + |
| Fungal Strain | L. plantarum | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CECT 220 | CECT 221 | CECT 223 | CECT 224 | CECT 748 | CECT 749 | CECT 750 | ||||||||
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
| F. graminearum ITEM 126 | 8 | 8 | 8 | 16 | 8 | 16 | 8 | 16 | 8 | 8 | 8 | 16 | 8 | 16 |
| F. graminearum ITEM 6415 | 8 | 16 | 8 | 13 | 8 | 13 | 8 | 13 | 4 | 16 | 4 | 16 | 4 | 16 |
| F. cerealis CECT 20488 | 8 | 16 | 8 | 31 | 8 | 16 | 8 | 16 | 8 | 8 | 8 | 16 | 8 | 16 |
| F. cerealis CECT 20489 | 8 | 16 | 16 | 16 | 16 | 31 | 4 | 16 | 4 | 16 | 4 | 16 | 4 | 31 |
| F. verticillioides CECT 20926 | 16 | 31 | 16 | 31 | 16 | 31 | 16 | 31 | 8 | 16 | 8 | 31 | 16 | 31 |
| F. verticillioides CECT 2152 | 4 | 31 | 4 | 31 | 4 | 31 | 4 | 31 | 4 | 31 | 4 | 31 | 4 | 31 |
| F. verticillioides CECT 2982 | 16 | 31 | 16 | 31 | 16 | 31 | 16 | 31 | 16 | 31 | 16 | 31 | 16 | 31 |
| F. mesoamericanum CECT 20490 | 8 | 16 | 8 | 31 | 8 | 16 | 8 | 16 | 8 | 16 | 8 | 16 | 8 | 16 |
| F. poae CECT 20165 | 16 | 31 | 8 | 16 | 8 | 16 | 8 | 16 | 8 | 16 | 8 | 16 | 8 | 16 |
| A. flavus ITEM 8111 | 250 | nd 1 | 250 | nd 1 | 250 | nd 1 | 250 | nd 1 | 125 | 250 | 62 | 125 | 250 | nd 1 |
| A. Parasiticus CECT 2681 | nd 1 | nd 1 | nd 1 | nd 1 | nd 1 | nd 1 | nd 1 | nd 1 | 250 | nd 1 | 125 | 250 | 250 | nd 1 |
| A. niger CECT 2088 | nd 1 | nd 1 | 250 | nd 1 | nd 1 | nd 1 | nd 1 | nd 1 | 250 | nd 1 | 250 | nd 1 | 250 | nd 1 |
| Compound | Molecular Formula | L. plantarum | ||||||
|---|---|---|---|---|---|---|---|---|
| CECT 220 | CECT 221 | CECT 223 | CECT 224 | CECT 748 | CECT 749 | CECT 750 | ||
| Gallic acid | C7H6O5 | 0.7 ± 0.2 | nd 1 | nd 1 | nd 1 | nd 1 | nd 1 | nd 1 |
| Protocatechuic | C7H6O4 | nd 1 | nd 1 | 0.4 ± 0.1 | nd 1 | nd 1 | nd 1 | nd 1 |
| Chlorogenic acid | C16H18O9 | 0.6 ± 0.1 | nd 1 | nd 1 | nd 1 | 0.5 ± 0.1 | nd 1 | nd 1 |
| Vanillin | C8H8O3 | nd 1 | 0.3 ± 0.1 | nd 1 | nd 1 | nd 1 | 0.5 ± 0.2 | nd 1 |
| p-coumaric acid | C9H8O3 | nd 1 | 0.3 ± 0.1 | nd 1 | 0.6 ± 0.1 | nd 1 | nd 1 | nd 1 |
| Salicylic acid | C7H6O3 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.5 ± 0.2 | 0.3 ± 0.1 | 0.6 ± 0.2 | 0.5 ± 0.1 | 0.6 ± 0.1 |
| Sinapic acid | C11H12O5 | 0.7 ± 0.1 | nd 1 | nd 1 | nd 1 | 1.0 ± 0.2 | nd 1 | 0.4 ± 0.1 |
| Phenyllactic acid | C9H10O3 | 1.0 ± 0.2 | 1.1 ± 0.1 | 0.9 ± 0.1 | 1.4 ± 0.2 | 3.9 ± 0.1 | 5.3 ± 0.3 | 2.8 ± 0.2 |
| 1,2-Dihydroxybenzene | C6H6O2 | nd 1 | 0.5 ± 0.1 | nd 1 | 0.6 ± 0.2 | 1.1 ± 0.2 | nd 1 | 0.9 ± 0.2 |
| Parameters | L. plantarum CECT 749 CFS (%) | MRS (%) |
|---|---|---|
| Moisture [47] | 94.5 ± 2.10 | 94.3 ± 2.40 |
| Proteins [46] | 0.3 ± 0.07 | 2.5 ± 0.30 |
| Ash [48] | 0.2 ± 0.06 | 0.2 ± 0.01 |
| Carbohydrates [49] | 3.2 ± 0.31 | 2.7 ± 0.5 |
| pH | 3.3 ± 0.21 | 6.2 ± 0.22 |
| Lactic acid [50] | 1.5 ± 0.50 | nd 1 |
| Samples | A. flavus ITEM 8111 | F. verticillioides CECT 2982 | ||||||
|---|---|---|---|---|---|---|---|---|
| Days | ||||||||
| 0 | 7 | 15 | 40 | 0 | 5 | 7 | 15 | |
| Control | − | + | + | + | − | + | + | + |
| CFS | − | − | + | + | − | − | + | + |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazareth, T.d.M.; Luz, C.; Torrijos, R.; Quiles, J.M.; Luciano, F.B.; Mañes, J.; Meca, G. Potential Application of Lactic Acid Bacteria to Reduce Aflatoxin B1 and Fumonisin B1 Occurrence on Corn Kernels and Corn Ears. Toxins 2020, 12, 21. https://doi.org/10.3390/toxins12010021
Nazareth TdM, Luz C, Torrijos R, Quiles JM, Luciano FB, Mañes J, Meca G. Potential Application of Lactic Acid Bacteria to Reduce Aflatoxin B1 and Fumonisin B1 Occurrence on Corn Kernels and Corn Ears. Toxins. 2020; 12(1):21. https://doi.org/10.3390/toxins12010021
Chicago/Turabian StyleNazareth, Tiago de Melo, Carlos Luz, Raquel Torrijos, Juan Manuel Quiles, Fernando Bittencourt Luciano, Jordi Mañes, and Giuseppe Meca. 2020. "Potential Application of Lactic Acid Bacteria to Reduce Aflatoxin B1 and Fumonisin B1 Occurrence on Corn Kernels and Corn Ears" Toxins 12, no. 1: 21. https://doi.org/10.3390/toxins12010021
APA StyleNazareth, T. d. M., Luz, C., Torrijos, R., Quiles, J. M., Luciano, F. B., Mañes, J., & Meca, G. (2020). Potential Application of Lactic Acid Bacteria to Reduce Aflatoxin B1 and Fumonisin B1 Occurrence on Corn Kernels and Corn Ears. Toxins, 12(1), 21. https://doi.org/10.3390/toxins12010021






