Dereplication of Natural Products with Antimicrobial and Anticancer Activity from Brazilian Cyanobacteria
Abstract
:1. Introduction
2. Results
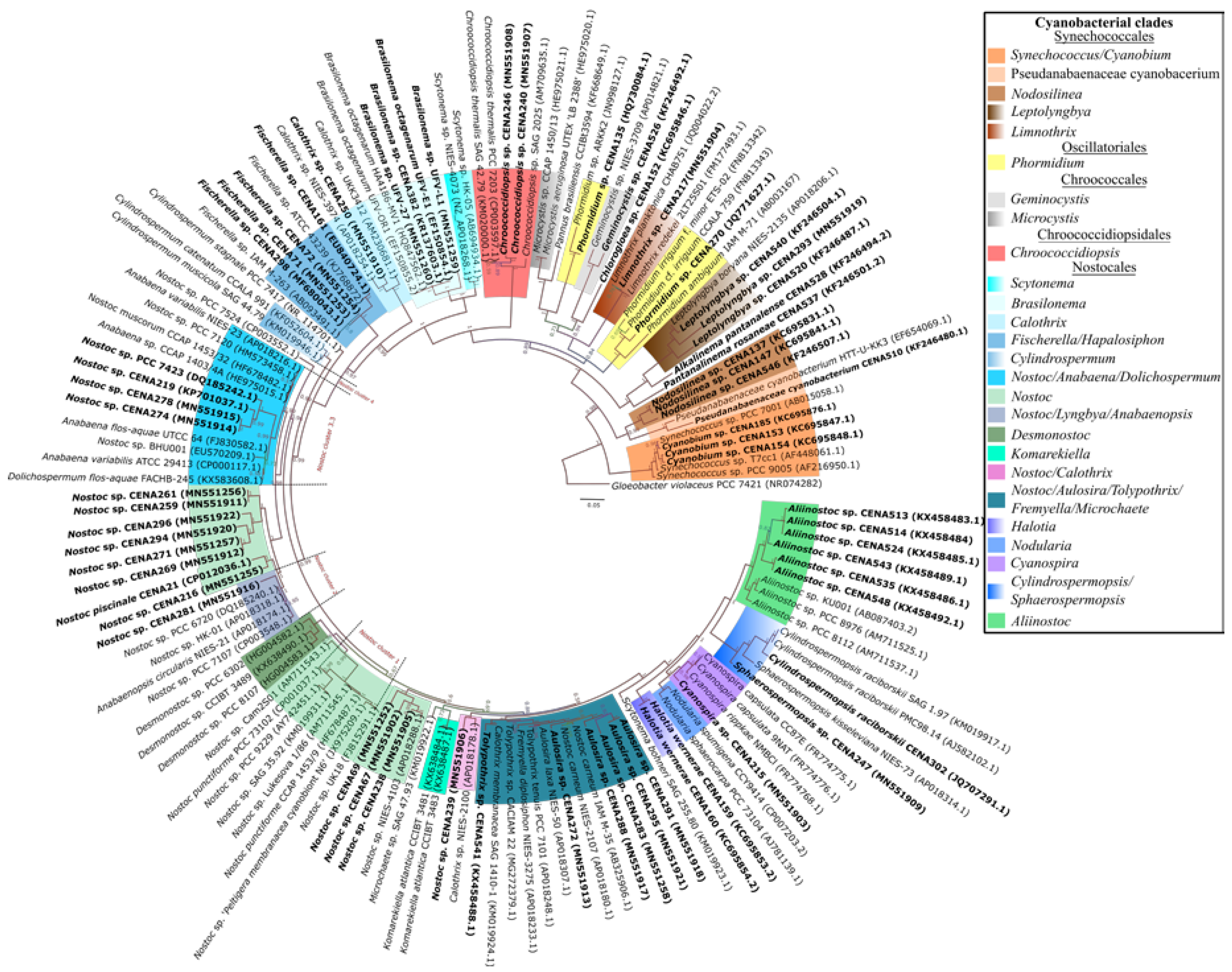
2.1. Cyanobacterial Isolates
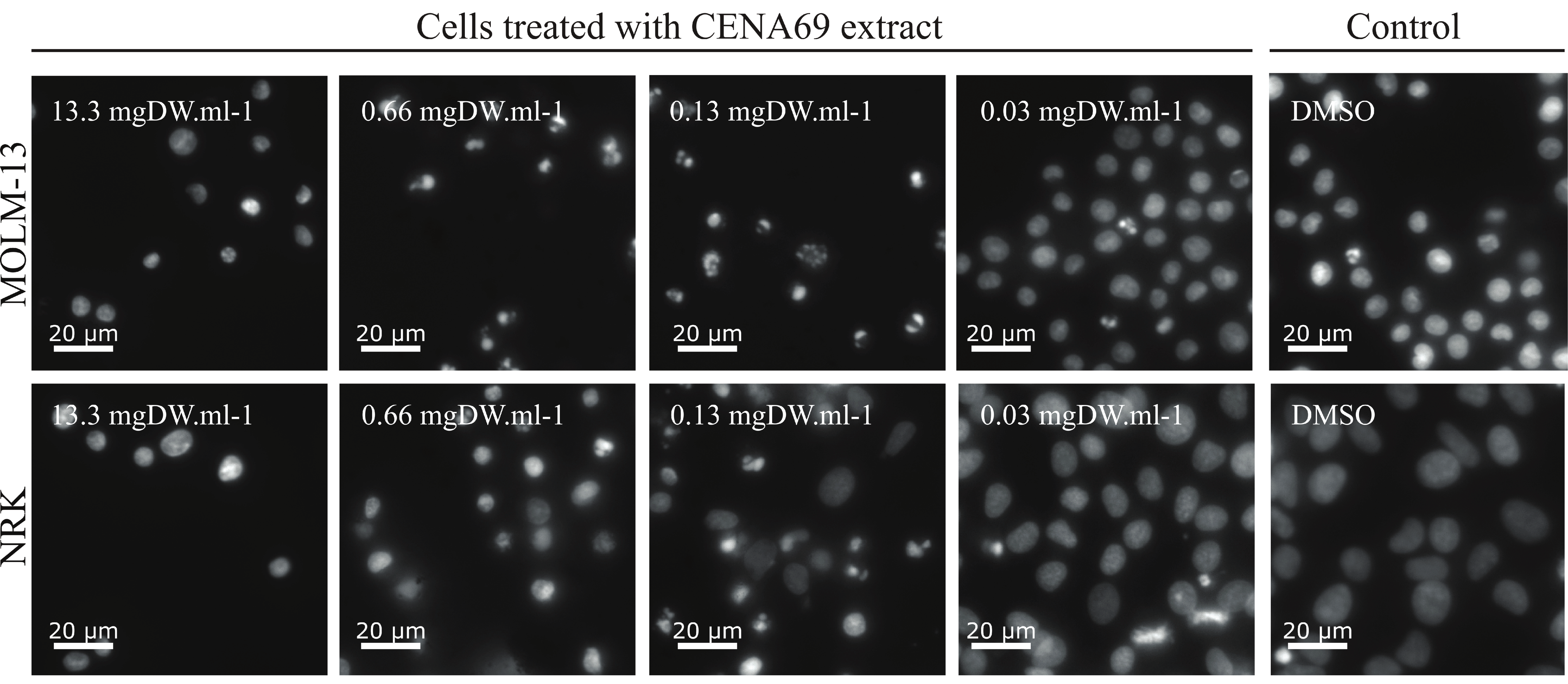
2.2. Bioactivity Screening
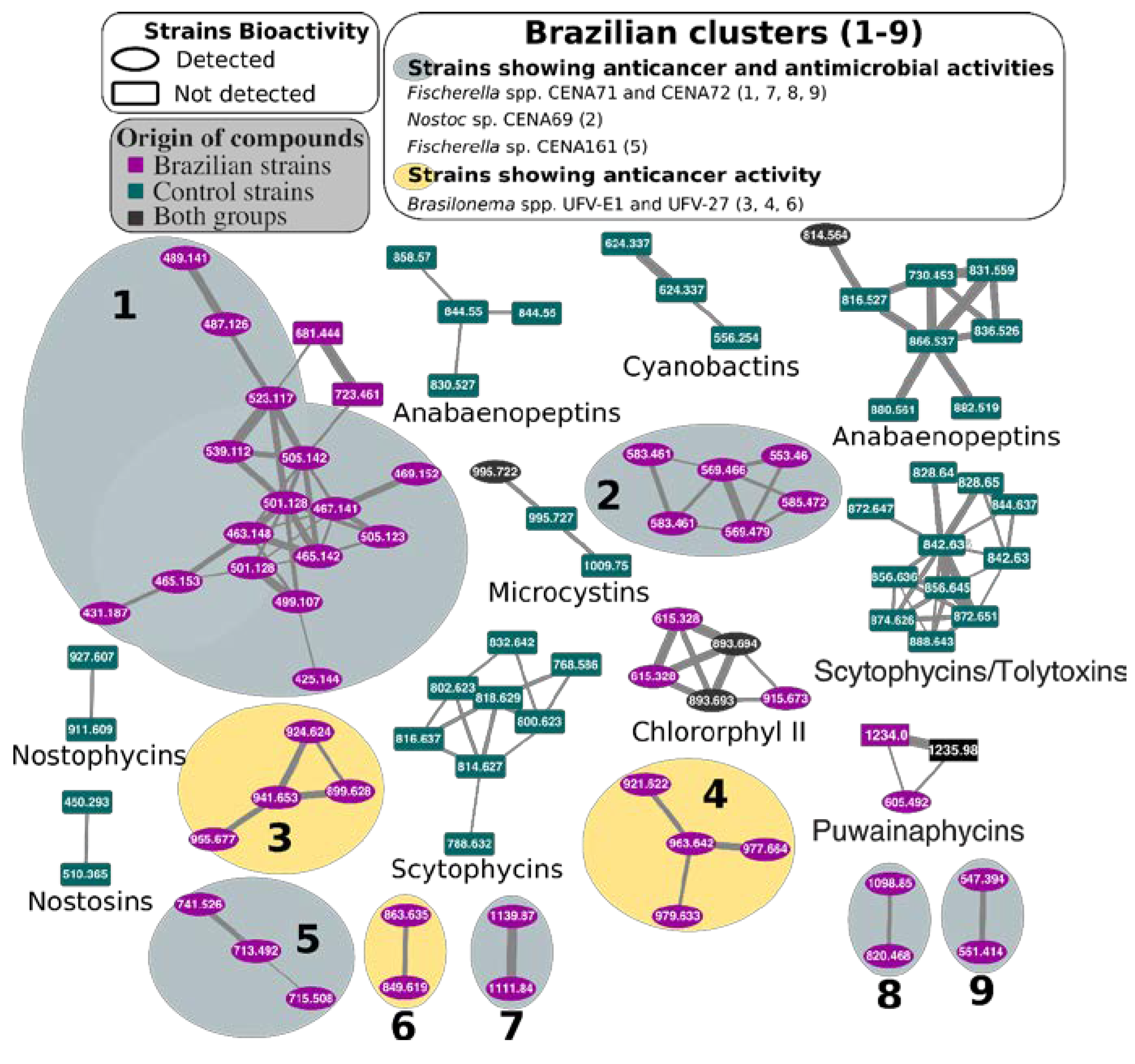
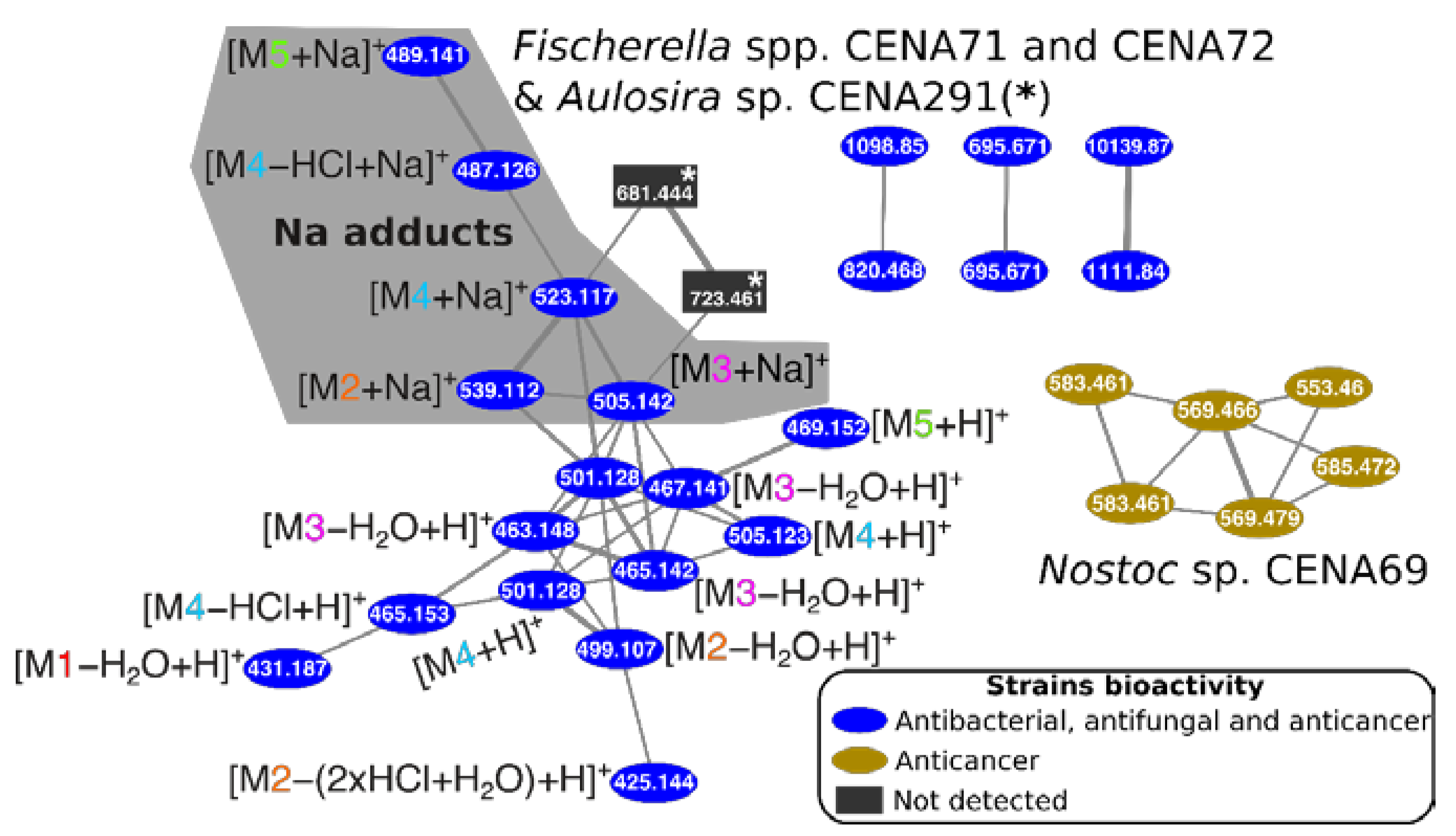
2.3. Natural Products Produced by Brazilian Cyanobacteria
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cyanobacterial Strains
5.2. DNA Extraction and Sequencing, and Phylogenetic Analyses
5.3. Cyanobacterial Extracts
5.4. Cell-Based Assay
5.5. Antifungal and Antibacterial Assays
5.6. Liquid Chromatography-Mass Spectrometry (LC-MS)
5.7. Molecular Network
5.8. Data Availability Statement
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stanier, R.Y.; Bazine, G.C. Phototrophic Prokaryotes: The Cyanobacteria. Annu. Rev. Microbiol. 1977, 31, 225–274. [Google Scholar] [CrossRef]
- Carmichael, W.W. Cyanobacteria secondary metabolites-the cyanotoxins. J. Appl. Microbiol. 1992, 72, 445–459. [Google Scholar] [CrossRef]
- Bohlin, L.; Göransson, U.; Alsmark, C.; Wedén, C.; Backlund, A. Natural products in modern life science. Phytochem. Rev. 2010, 9, 279–301. [Google Scholar] [CrossRef] [Green Version]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welker, M.; Von Döhren, H. Cyanobacterial peptides—Nature’s own combinatorial biosynthesis. FEMS Microbiol. Rev. 2006, 30, 530–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burja, A.M.; Banaigs, B.; Abou-Mansour, E.; Grant Burgess, J.; Wright, P.C. Marine cyanobacteria—A prolific source of natural products. Tetrahedron 2001, 57, 9347–9377. [Google Scholar] [CrossRef]
- Ehrenreich, I.M.; Waterbury, J.B.; Webb, E.A. Distribution and Diversity of Natural Product Genes in Marine and Freshwater Cyanobacterial Cultures and Genomes. Appl. Environ. Microbiol. 2005, 71, 7401–7413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angermayr, S.A.; Hellingwerf, K.J.; Lindblad, P.; Teixeira de Mattos, M.J. Energy biotechnology with cyanobacteria. Curr. Opin. Biotechnol. 2009, 20, 257–263. [Google Scholar] [CrossRef]
- Kehr, J.-C.; Gatte Picchi, D.; Dittmann, E. Natural product biosyntheses in cyanobacteria: A treasure trove of unique enzymes. Beilstein J. Org. Chem. 2011, 7, 1622–1635. [Google Scholar] [CrossRef] [Green Version]
- Marahiel, M.A.; Stachelhaus, T.; Mootz, H.D. Modular peptide synthetases involved in nonribosomal peptide synthesis. Chem. Rev. 1997, 97, 2651–2674. [Google Scholar] [CrossRef]
- Finking, R.; Marahiel, M.A. Biosynthesis of Nonribosomal Peptides. Annu. Rev. Microbiol. 2004, 58, 453–488. [Google Scholar] [CrossRef] [PubMed]
- Sieber, S.A.; Marahiel, M.A. Molecular mechanisms underlying nonribosomal peptide synthesis: Approaches to new antibiotics. Chem. Rev. 2005, 105, 715–738. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Harada, K.; Suzuki, M.; Kondo, F.; Ikai, Y.; Oka, H.; Carmichael, W.W.; Sivonen, K. Occurrence of novel cyclic peptides together with microcystins from toxic cyanobacteria, Anabaena species. In Harmful and Toxic Algal Blooms; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 1996; pp. 559–562. [Google Scholar]
- Moffitt, M.C.; Neilan, B.A. Characterization of the nodularin synthetase gene cluster and proposed theory of the evolution of cyanobacterial hepatotoxins. Appl. Environ. Microbiol. 2004, 70, 6353–6362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouhiainen, L.; Paulin, L.; Suomalainen, S.; Hyytiäinen, H.; Buikema, W.; Haselkorn, R.; Sivonen, K. Genes encoding synthetases of cyclic depsipeptides, anabaenopeptilides, in Anabaena strain 90. Mol. Microbiol. 2000, 37, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Fewer, D.P.; Jokela, J.; Rouhiainen, L.; Wahlsten, M.; Koskenniemi, K.; Stal, L.J.; Sivonen, K. The non-ribosomal assembly and frequent occurrence of the protease inhibitors spumigins in the bloom-forming cyanobacterium Nodularia spumigena. Mol. Microbiol. 2009, 73, 924–937. [Google Scholar] [CrossRef]
- Biondi, N.; Tredici, M.R.; Taton, A.; Wilmotte, A.; Hodgson, D.A.; Losi, D.; Marinelli, F. Cyanobacteria from benthic mats of Antarctic lakes as a source of new bioactivities. J. Appl. Microbiol. 2008, 105, 105–115. [Google Scholar] [CrossRef]
- Kreitlow, S.; Mundt, S.; Lindequist, U. Cyanobacteria—A potential source of new biologically active substances. J. Biotechnol. 1999, 70, 61–63. [Google Scholar] [CrossRef]
- Singh, R.K.; Tiwari, S.P.; Rai, A.K.; Mohapatra, T.M. Cyanobacteria: An emerging source for drug discovery. J. Antibiot. 2011, 64, 401–412. [Google Scholar] [CrossRef] [Green Version]
- Felczykowska, A.; Pawlik, A.; Mazur-Marzec, H.; Toruńska-Sitarz, A.; Narajczyk, M.; Richert, M.; Węgrzyn, G.; Herman-Antosiewicz, A. Selective inhibition of cancer cells’ proliferation by compounds included in extracts from Baltic Sea cyanobacteria. Toxicon 2015, 108, 1–10. [Google Scholar] [CrossRef]
- Humisto, A.; Herfindal, L.; Jokela, J.; Karkman, A.; Bjørnstad, R.; Choudhury, R.R.; Sivonen, K. Cyanobacteria as a source for novel anti-leukemic compounds. Curr. Pharm. Biotechnol. 2016, 17, 78–91. [Google Scholar] [CrossRef]
- Taori, K.; Matthew, S.; Rocca, J.R.; Paul, V.J.; Luesch, H. Lyngbyastatins 5–7, Potent elastase inhibitors from floridian marine cyanobacteria, Lyngbya spp. J. Nat. Prod. 2007, 70, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, W.H.; Proteau, P.J.; Nagle, D.G.; Hamel, E.; Blokhin, A.; Slate, D.L. Structure of Curacin A, a novel antimitotic, antiproliferative and brine shrimp toxic natural product from the marine cyanobacterium Lyngbya majuscula. J. Org. Chem. 1994, 59, 1243–1245. [Google Scholar] [CrossRef]
- Berman, F.W.; Gerwick, W.H.; Murray, T.F. Antillatoxin and kalkitoxin, ichthyotoxins from the tropical cyanobacterium Lyngbya majuscula, induce distinct temporal patterns of NMDA receptor-mediated neurotoxicity. Toxicon 1999, 37, 1645–1648. [Google Scholar] [CrossRef]
- Wu, M.; Okino, T.; Nogle, L.M.; Marquez, B.L.; Williamson, R.T.; Sitachitta, N.; Berman, F.W.; Murray, T.F.; McGough, K.; Jacobs, R.; et al. Structure, synthesis, and biological properties of kalkitoxin, a novel neurotoxin from the marine cyanobacterium Lyngbya majuscula. J. Am. Chem. Soc. 2000, 122, 12041–12042. [Google Scholar] [CrossRef]
- Trimurtulu, G.; Ohtani, I.; Patterson, G.M.L.; Moore, R.E.; Corbett, T.H.; Valeriote, F.A.; Demchik, L. Total structures of cryptophycins, potent antitumor depsipeptides from the blue-green alga Nostoc sp. strain GSV 224. J. Am. Chem. Soc. 1994, 116, 4729–4737. [Google Scholar] [CrossRef]
- Costa, M.; Costa-Rodrigues, J.; Fernandes, M.H.; Barros, P.; Vasconcelos, V.; Martins, R. Marine cyanobacteria compounds with anticancer properties: A review on the implication of apoptosis. Mar. Drugs 2012, 10, 2181–2207. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Herfindal, L.; Jokela, J.; Shishido, T.; Wahlsten, M.; Døskeland, S.; Sivonen, K. Cyanobacteria from terrestrial and marine sources contain apoptogens able to overcome chemoresistance in acute myeloid leukemia cells. Mar. Drugs 2014, 12, 2036–2053. [Google Scholar] [CrossRef]
- Visser, O.; Trama, A.; Maynadié, M.; Stiller, C.; Marcos-Gragera, R.; De Angelis, R.; Mallone, S.; Tereanu, C.; Allemani, C.; Ricardi, U.; et al. Incidence, survival and prevalence of myeloid malignancies in Europe. Eur. J. Cancer 2012, 48, 3257–3266. [Google Scholar] [CrossRef]
- Rowe, J.M. AML in 2016: Where we are now? Best Pract. Res. Clin. Haematol. 2016, 29, 315–319. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [Green Version]
- Shishido, T.; Humisto, A.; Jokela, J.; Liu, L.; Wahlsten, M.; Tamrakar, A.; Fewer, D.; Permi, P.; Andreote, A.; Fiore, M.; et al. Antifungal compounds from cyanobacteria. Mar. Drugs 2015, 13, 2124–2140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leao, T.; Castelão, G.; Korobeynikov, A.; Monroe, E.A.; Podell, S.; Glukhov, E.; Allen, E.E.; Gerwick, W.H.; Gerwick, L. Comparative genomics uncovers the prolific and distinctive metabolic potential of the cyanobacterial genus Moorea. Proc. Natl. Acad. Sci. USA 2017, 114, 3198–3203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaz, M.G.M.V.; Genuario, D.B.; Andreote, A.P.D.; Malone, C.F.S.; Sant’Anna, C.L.; Barbiero, L.; Fiore, M.F. Pantanalinema gen. nov. and Alkalinema gen. nov.: Novel pseudanabaenacean genera (Cyanobacteria) isolated from saline-alkaline lakes. Int. J. Syst. Evol. Microbiol. 2015, 65, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.D.; Branco, L.H.Z. Potamolinea gen. nov. (Oscillatoriales, Cyanobacteria): A phylogenetically and ecologically coherent cyanobacterial genus. Int. J. Syst. Evol. Microbiol. 2016, 66, 3632–3641. [Google Scholar] [CrossRef] [PubMed]
- Genuário, D.B.; de Souza, W.R.; Monteiro, R.T.R.; Sant’Anna, C.L.; Melo, I.S. Amazoninema gen. nov., (Synechococcales, Pseudanabaenaceae) a novel cyanobacteria genus from Brazilian Amazonian rivers. Int. J. Syst. Evol. Microbiol. 2018, 68, 2249–2257. [Google Scholar] [CrossRef] [PubMed]
- Caires, T.A.; de Mattos Lyra, G.; Hentschke, G.S.; de Gusmão Pedrini, A.; Sant’Anna, C.L.; de Castro Nunes, J.M. Neolyngbya gen. nov. (Cyanobacteria, Oscillatoriaceae): A new filamentous benthic marine taxon widely distributed along the Brazilian coast. Mol. Phylogenet. Evol. 2018, 120, 196–211. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Rigonato, J.; Gama, W.A.; Alvarenga, D.O.; Branco, L.H.Z.; Brandini, F.P.; Genuário, D.B.; Fiore, M.F. Aliterella atlantica gen. nov., sp. nov., and Aliterella antarctica sp. nov., novel members of coccoid Cyanobacteria. Int. J. Syst. Evol. Microbiol. 2016, 66, 2853–2861. [Google Scholar] [CrossRef] [Green Version]
- Sanz, M.; Andreote, A.; Fiore, M.; Dörr, F.; Pinto, E. Structural characterization of new peptide variants produced by cyanobacteria from the Brazilian Atlantic coastal forest using liquid chromatography coupled to quadrupole time-of-flight tandem mass spectrometry. Mar. Drugs 2015, 13, 3892–3919. [Google Scholar] [CrossRef]
- Silva, C.S.P.; Genuário, D.B.; Vaz, M.G.M.V.; Fiore, M.F. Phylogeny of culturable cyanobacteria from Brazilian mangroves. Syst. Appl. Microbiol. 2014, 37, 100–112. [Google Scholar] [CrossRef]
- Silva-Stenico, M.E.; Kaneno, R.; Zambuzi, F.A.; Vaz, M.G.M.V.; Alvarenga, D.O.; Fiore, M.F. Natural products from cyanobacteria with antimicrobial and antitumor activity. Curr. Pharm. Biotechnol. 2013, 14, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Jokela, J.; Heinilä, L.M.P.; Shishido, T.K.; Wahlsten, M.; Fewer, D.P.; Fiore, M.F.; Wang, H.; Haapaniemi, E.; Permi, P.; Sivonen, K. Production of high amounts of hepatotoxin nodularin and new protease inhibitors pseudospumigins by the Brazilian benthic Nostoc sp. CENA543. Front. Microbiol. 2017, 8, 1963. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Salinas, R.K.; Pinto, E. Namalides B and C and spumigins k–n from the cultured freshwater cyanobacterium Sphaerospermopsis torques-reginae. J. Nat. Prod. 2017, 80, 2492–2501. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Melnik, A.V.; Koyama, N.; Lu, X.; Schorn, M.; Fang, J.; Aguinaldo, K.; Lincecum, T.L.; Ghequire, M.G.K.; Carrion, V.J.; et al. Indexing the Pseudomonas specialized metabolome enabled the discovery of poaeamide B and the bananamides. Nat. Microbiol. 2016, 2, 16197. [Google Scholar] [CrossRef] [Green Version]
- Watrous, J.; Roach, P.; Alexandrov, T.; Heath, B.S.; Yang, J.Y.; Kersten, R.D.; van der Voort, M.; Pogliano, K.; Gross, H.; Raaijmakers, J.M.; et al. Mass spectral molecular networking of living microbial colonies. Proc. Natl. Acad. Sci. USA 2012, 109, E1743–E1752. [Google Scholar] [CrossRef] [Green Version]
- Silva-Stenico, M.E.; Rigonato, J.; Leal, M.G.; Vaz, M.G.M.V.; Andreote, A.P.D.; Fiore, M.F. Non-ribosomal halogenated protease inhibitors from cyanobacterial isolates as attractive drug targets. Curr. Med. Chem. 2012, 19, 5205–5213. [Google Scholar] [CrossRef]
- Fiore, M.F.; Genuário, D.B.; da Silva, C.S.P.; Shishido, T.K.; Moraes, L.A.B.; Neto, R.C.; Silva-Stenico, M.E. Microcystin production by a freshwater spring cyanobacterium of the genus Fischerella. Toxicon 2009, 53, 754–761. [Google Scholar] [CrossRef]
- Shishido, T.; Kaasalainen, U.; Fewer, D.P.; Rouhiainen, L.; Jokela, J.; Wahlsten, M.; Fiore, M.; Yunes, J.; Rikkinen, J.; Sivonen, K. Convergent evolution of [D-Leucine1] microcystin-LR in taxonomically disparate cyanobacteria. BMC Evol. Biol. 2013, 13, 86. [Google Scholar] [CrossRef] [Green Version]
- Hoff-Risseti, C.; Dörr, F.A.; Schaker, P.D.C.; Pinto, E.; Werner, V.R.; Fiore, M.F. Cylindrospermopsin and saxitoxin synthetase genes in Cylindrospermopsis raciborskii strains from Brazilian freshwater. PLoS ONE 2013, 8, e74238. [Google Scholar] [CrossRef] [Green Version]
- Shishido, T.K.; Jokela, J.; Fewer, D.P.; Wahlsten, M.; Fiore, M.F.; Sivonen, K. Simultaneous production of anabaenopeptins and namalides by the cyanobacterium Nostoc sp. CENA543. ACS Chem. Biol. 2017, 12, 2746–2755. [Google Scholar] [CrossRef]
- Heck, K.; Alvarenga, D.O.; Shishido, T.K.; Varani, A.M.; Dörr, F.A.; Pinto, E.; Rouhiainen, L.; Jokela, J.; Sivonen, K.; Fiore, M.F. Biosynthesis of microcystin hepatotoxins in the cyanobacterial genus Fischerella. Toxicon 2018, 141, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, S.T.; Alvarenga, D.O.; Etchegaray, A.; Fewer, D.P.; Jokela, J.; Varani, A.M.; Sanz, M.; Dörr, F.A.; Pinto, E.; Sivonen, K.; et al. Genetic organization of anabaenopeptin and spumigin biosynthetic gene clusters in the cyanobacterium Sphaerospermopsis torques-reginae ITEP-024. ACS Chem. Biol. 2017, 12, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Costa, N.B.; Kolman, M.A.; Giani, A. Cyanobacteria diversity in alkaline saline lakes in the Brazilian Pantanal wetland: A polyphasic approach. J. Plankton Res. 2016, 38, 1389–1403. [Google Scholar] [CrossRef] [Green Version]
- Genuário, D.B.; Vaz, M.G.M.V.; de Melo, I.S. Phylogenetic insights into the diversity of homocytous cyanobacteria from Amazonian rivers. Mol. Phylogenet. Evol. 2017, 116, 120–135. [Google Scholar] [CrossRef]
- Genuário, D.B.; Vaz, M.G.M.V.; Santos, S.N.; Kavamura, V.N.; Melo, I.S. Cyanobacteria from Brazilian extreme environments. In Microbial Diversity in the Genomic Era; Elsevier: Amsterdam, The Netherlands, 2019; pp. 265–284. ISBN 978-0-12-814849-5. [Google Scholar]
- Jang, I.S.; Neto, E.C.; Guinney, J.; Friend, S.H.; Margolin, A.A. Systematic assessment of analytical methods for drug sensitivity prediction from cancer cell line data. Pac. Symp. Biocomput. 2014, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Selheim, F.; Herfindal, L.; Martins, R.; Vasconcelos, V.; Døskeland, S.O. Neuro-apoptogenic and blood platelet targeting toxins in benthic marine cyanobacteria from the Portuguese coast. Aquat. Toxicol. 2005, 74, 294–306. [Google Scholar] [CrossRef]
- Campos, A.; Vasconcelos, V. Molecular mechanisms of microcystin toxicity in animal cells. Int. J. Mol. 2010, 11, 268–287. [Google Scholar] [CrossRef] [Green Version]
- Fischer, W.J.; Altheimer, S.; Cattori, V.; Meier, P.J.; Dietrich, D.R.; Hagenbuch, B. Organic anion transporting polypeptides expressed in liver and brain mediate uptake of microcystin. Toxicol. Appl. Pharmacol. 2005, 203, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Botha, N.; Gehringer, M.M.; Downing, T.G.; van de Venter, M.; Shephard, E.G. The role of microcystin-LR in the induction of apoptosis and oxidative stress in CaCo2 cells. Toxicon 2004, 43, 85–92. [Google Scholar] [CrossRef]
- Fladmark, K.; Brustugun, O.; Hovland, R.; Bøe, R.; Gjertsen, B.; Zhivotovsky, B.; Døskeland, S. Ultrarapid caspase-3 dependent apoptosis induction by serine/threonine phosphatase inhibitors. Cell Death Differ. 1999, 6, 1099–1108. [Google Scholar] [CrossRef] [Green Version]
- Oftedal, L.; Skjærven, K.H.; Coyne, R.T.; Edvardsen, B.; Rohrlack, T.; Skulberg, O.M.; Døskeland, S.O.; Herfindal, L. The apoptosis-inducing activity towards leukemia and lymphoma cells in a cyanobacterial culture collection is not associated with mouse bioassay toxicity. J. Ind. Microbiol. Biotechnol. 2011, 38, 489–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuhof, T.; Dieckmann, R.; von Hans, D.; Preussel, K.; Seibold, M.; Schmieder, P. Lipopeptides Having Pharmaceutical Activity. WO2006092313A1, 8 September 2006. [Google Scholar]
- Hrouzek, P.; Kuzma, M.; Černý, J.; Novák, P.; Fišer, R.; Šimek, P.; Lukešová, A.; Kopecký, J. The cyanobacterial cyclic lipopeptides puwainaphycins f/g are inducing necrosis via cell membrane permeabilization and subsequent unusual actin relocalization. Chem. Res. Toxicol. 2012, 25, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Welker, M.; Dittmann, E.; von Döhren, H. Cyanobacteria as a source of natural products. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 517, pp. 23–46. ISBN 978-0-12-404634-4. [Google Scholar]
- Zhou, Z.; Tang, X.; Chen, H.; Wang, Y. Comparative studies of saxitoxin (STX)-induced cytotoxicity in Neuro-2a and RTG-2 cell lines: An explanation with respect to changes in ROS. Chemosphere 2018, 192, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Melegari, S.P.; de Carvalho Pinto, C.R.S.; Moukha, S.; Creppy, E.E.; Matias, W.G. Evaluation of cytotoxicity and cell death induced in vitro by saxitoxin in mammalian cells. J Toxicol. Environ. Health Part A 2015, 78, 1189–1200. [Google Scholar] [CrossRef]
- Dodds, W.K.; Gudder, D.A.; Mollenhauer, D. The ecology of Nostoc. J. Appl. Phycol. 1995, 31, 2–18. [Google Scholar] [CrossRef]
- Liaimer, A.; Jensen, J.B.; Dittmann, E. A genetic and chemical perspective on symbiotic recruitment of cyanobacteria of the genus Nostoc into the host plant Blasia pusilla L. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kajiyama, S. Secondary metabolites of cyanobacteria Nostoc sp. Chin. J. Oceanol. Limnol. 1998, 16, 109–117. [Google Scholar] [CrossRef]
- Dittmann, E.; Gugger, M.; Sivonen, K.; Fewer, D.P. Natural product biosynthetic diversity and comparative genomics of the cyanobacteria. Trends Microbiol. 2015, 23, 642–652. [Google Scholar] [CrossRef]
- Oksanen, I.; Jokela, J.; Fewer, D.P.; Wahlsten, M.; Rikkinen, J.; Sivonen, K. Discovery of rare and highly toxic microcystins from lichen-associated cyanobacterium Nostoc sp. Strain IO-102-I. Appl. Environ. Microbiol. 2004, 70, 5756–5763. [Google Scholar] [CrossRef] [Green Version]
- Fujii, K.; Sivonen, K.; Kashiwagi, T.; Hirayama, K.; Harada, K. Nostophycin, a novel cyclic peptide from the toxic cyanobacterium Nostoc sp. 152. J. Org. Chem. 1999, 64, 5777–5782. [Google Scholar] [CrossRef]
- Subbaraju, G.V.; Golakoti, T.; Patterson, G.M.L.; Moore, R.E. Three new cryptophycins from Nostoc sp. GSV 224. J. Nat. Prod. 1997, 60, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Jüttner, F.; Todorova, A.K.; Walch, N.; von Philipsborn, W. Nostocyclamide M: A cyanobacterial cyclic peptide with allelopathic activity from Nostoc 31. Phytochemistry 2001, 57, 613–619. [Google Scholar] [CrossRef]
- Becher, P.G.; Beuchat, J.; Gademann, K.; Jüttner, F. Nostocarboline: Isolation and synthesis of a new cholinesterase inhibitor from Nostoc 78-12A. J. Nat. Prod. 2005, 68, 1793–1795. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Yoshitomi, S.; Dwi, S.; Iwabe, O.; Mahakhant, A.; Polchai, J.; Miyamoto, K. Bioactivities of nostocine a produced by a freshwater cyanobacterium Nostoc spongiaeforme TISTR 8169. J. Biosci. Bioeng. 2003, 95, 512–517. [Google Scholar] [CrossRef]
- Nagatsu, A.; Kajitani, H.; Sakakibara, J. Muscoride A: A new oxazole peptide alkaloid from freshwater cyanobacterium Nostoc muscorum. Tetrahedron Lett. 1995, 36, 4097–4100. [Google Scholar] [CrossRef]
- Gromov, B.V.; Vepritskiy, A.A.; Titova, N.N.; Mamkayeva, K.A.; Alexandrova, O.V. Production of the antibiotic cyanobacterin LU-1 by Nostoc linckia CALU 892 (cyanobacterium). J. Appl. Phycol. 1991, 3, 55–59. [Google Scholar] [CrossRef]
- Jimenez, J.I.; Huber, U.; Moore, R.E.; Patterson, G.M.L. Oxidized welwitindolinones from terrestrial Fischerella spp. J. Nat. Prod. 1999, 62, 569–572. [Google Scholar] [CrossRef]
- Cagide, E.; Becher, P.G.; Louzao, M.C.; Espiña, B.; Vieytes, M.R.; Jüttner, F.; Botana, L.M. Hapalindoles from the cyanobacterium Fischerella: Potential sodium channel modulators. Chem. Res. Toxicol. 2014, 27, 1696–1706. [Google Scholar] [CrossRef]
- Mo, S.; Krunic, A.; Chlipala, G.; Orjala, J. Antimicrobial ambiguine isonitriles from the cyanobacterium Fischerella ambigua. J. Nat. Prod. 2009, 72, 894–899. [Google Scholar] [CrossRef] [Green Version]
- Glaser, B. Prehistorically modified soils of central Amazonia: A model for sustainable agriculture in the twenty-first century. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Pupin, B.; Nahas, E. Microbial populations and activities of mangrove, restinga and Atlantic forest soils from Cardoso Island, Brazil. J. Appl. Microbiol. 2014, 116, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.F.; Moon, D.H.; Tsai, S.M.; Lee, H.; Trevors, J.T. Miniprep DNA isolation from unicellular and filamentous cyanobacteria. J. Microbiol. Methods 2000, 39, 159–169. [Google Scholar] [CrossRef]
- Taton, A.; Grubisic, S.; Brambilla, E.; De Wit, R.; Wilmotte, A. Cyanobacterial diversity in natural and artificial microbial mats of Lake Fryxell (McMurdo Dry Valleys, Antarctica): A morphological and molecular approach. Appl. Environ. Microbiol. 2003, 69, 5157–5169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewing, B.; Green, P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 1998, 8, 186–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewing, B.; Hillier, L.; Wendl, M.C.; Green, P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 1998, 8, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Gordon, D.; Abajian, C.; Green, P. Consed: A graphical tool for sequence finishing. Genome Res. 1998, 8, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef] [Green Version]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Kotai, J. Instructions for Preparation of Modified Nutrient Solution Z8 for Algae; Norwegian Institute for Water Research: Oslo, Norway, 1972. [Google Scholar]
- Matsuo, Y.; MacLeod, R.; Uphoff, C.; Drexler, H.; Nishizaki, C.; Katayama, Y.; Kimura, G.; Fujii, N.; Omoto, E.; Harada, M.; et al. Two acute monocytic leukemia (AML-M5a) cell lines (MOLM-13 and MOLM-14) with interclonal phenotypic heterogeneity showing MLL-AF9 fusion resulting from an occult chromosome insertion, ins(11;9)(q23;p22p23). Leukemia 1997, 11, 1469–1477. [Google Scholar] [CrossRef] [Green Version]
- Oftedal, L.; Selheim, F.; Wahlsten, M.; Sivonen, K.; Døskeland, S.O.; Herfindal, L. Marine Benthic Cyanobacteria contain apoptosis-inducing activity synergizing with daunorubicin to kill leukemia cells, but not cardiomyocytes. Mar. Drugs 2010, 8, 2659–2672. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Cyanobacterial Strain | AUC | EC50 | |||||
|---|---|---|---|---|---|---|---|
| NRK | MOLM-13 | Ratio | |||||
| Total Area | Std. Error | Total Area | Std. Error | NRK | MOLM-13 | ||
| Nostoc sp. CENA69 | 63.01 | 2.902 | 26.61 | 4.412 | 2.4 | 0.098 | 0.054 |
| Fischerella sp. CENA71 * | 106.3 | 9.467 | 45.45 | 4.053 | 2.3 | 1.493 | 0.363 |
| Fischerella sp. CENA72 * | 164.4 | 14.78 | 52.32 | 9.073 | 3.1 | 3.129 | 0.201 |
| Fischerella sp. CENA161 | 134.0 | 6.417 | 69.02 | 7.495 | 1.9 | 0.508 | 0.083 |
| Cyanobium sp. CENA185 | 199.7 | - | 110.7 | − | −§ | >15# | 1.590 |
| Limnothrix sp. CENA217 | 198.6 | 1.033 | 137.7 | 3.028 | −§ | >15# | 1.410 |
| Nostoc sp. CENA296 * | 172.5 | 15.93 | 96.91 | 4.076 | 1.8 | 11.26 | 1.212 |
| Fischerella sp. CENA298 | 124.9 | 8.210 | 98.90 | 12.37 | 1.3 | 2.283 | 1.324 |
| Aliinostoc sp. CENA513 | 129.2 | 6.390 | 53.58 | 5.328 | 2.4 | 0.491 | 0.087 |
| Aliinostoc sp. CENA514 * | 108.2 | 4.706 | 32.96 | 5.631 | 3.3 | 0.842 | 0.140 |
| Aliinostoc sp. CENA524 * | 159.6 | 4.367 | 65.36 | 4.124 | 2.4 | 6.012 | 0.620 |
| Aliinostoc sp. CENA535 | 85.71 | 2.182 | 60.54 | 10.19 | 1.4 | 1.218 | 0.566 |
| Taxon | Strain | Activity | Compounds Detected | Reference | ||
|---|---|---|---|---|---|---|
| F | B | C a | ||||
| Pannus brasiliense | CCIbt3594 | − | − | + | − | This study |
| Nostoc piscinale | CENA21 | − | − | + | − | This study |
| Nostoc sp. | CENA67 | − | − | + * | − | [42] and this study |
| Nostoc sp. | CENA69 | − | − | + * | − | [42] and this study |
| Fischerella sp. | CENA71 | + | + | + | − | This study |
| Fischerella sp. | CENA72 | + | + | + | − | This study |
| Oxynema sp. | CENA135 | − | − | + * | Microcystin, Saxitoxin | [41,42] and this study |
| Nodosilinea sp. | CENA137 | − | − | + | − | this study |
| Nodosilinea sp. | CENA147 | − | − | + | − | this study |
| Chlorogloea sp. | CENA152 | − | − | + | − | this study |
| Cyanobium sp. | CENA153 | − | − | + | Microcystin, Aeruginosin | [41,47] and this study |
| Cyanobium sp. | CENA154 | − | − | + * | Aeruginosin | [42,47] and this study |
| Halotia wernerae | CENA159 | − | − | + | − | This study |
| Halotia wernerae | CENA160 | − | − | + | − | This study |
| Fischerella sp. | CENA161 | + | + | + | Microcystin | [48] and this study |
| Cyanobium sp. | CENA185 | − | − | + | Microcystin | [41] and this study |
| Cyanospira sp. | CENA215 | − | − | + | − | This study |
| Nostoc sp. | CENA216 | − | − | + | − | This study |
| Limnothrix sp. | CENA217 | − | − | + | − | This study |
| Nostoc sp. | CENA219 | − | − | + | Hassallidin | [32] and this study |
| Phormidium sp. | CENA270 | − | − | + | Microcystin | [49] and this study |
| Nostoc sp. | CENA271 | − | − | − | Aeruginosin | [47] and this study |
| Gloeotrichia sp. | CENA272 | − | − | + | − | This study |
| Calothrix sp. | CENA283 | − | − | + | − | This study |
| Nostoc sp. | CENA296 | − | − | + | − | This study |
| Fischerella sp. | CENA298 | + | + | + | − | This study |
| Cylindrospermopsis raciborskii | CENA302 | − | − | + | Saxitoxin | [50] and this study |
| Brasilonema sp. | CENA382 | − | − | + | − | This study |
| Pseudanabaenaceae cyanobacterium | CENA510 | − | − | + | − | This study |
| Aliinostoc sp. | CENA513 | + | + | + | − | This study |
| Aliinostoc sp. | CENA514 | + | − | + | − | This study |
| Aliinostoc sp. | CENA524 | − | + | + | − | This study |
| Geminocystis sp. | CENA526 | − | − | + | − | This study |
| Aliinostoc sp. | CENA535 | + | − | + | Puwainaphycin | This study |
| Tolypothrix sp. | CENA541 | − | − | + | − | This study |
| Aliinostoc sp. | CENA543 | − | − | + | Nodularin, Anabaenopeptin, Pseudoaeruginosin | [11,43] and this study |
| Aliinostoc sp. | CENA548 | + | − | + | Puwainaphycin | This study |
| Brasilonema octagenarum | UFV-E1 | − | − | + | − | This study |
| Brasilonema sp. | UFV-L1 | − | − | + | − | This study |
| Brasilonema sp. | UFV-27 | − | − | + | − | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shishido, T.K.; Popin, R.V.; Jokela, J.; Wahlsten, M.; Fiore, M.F.; Fewer, D.P.; Herfindal, L.; Sivonen, K. Dereplication of Natural Products with Antimicrobial and Anticancer Activity from Brazilian Cyanobacteria. Toxins 2020, 12, 12. https://doi.org/10.3390/toxins12010012
Shishido TK, Popin RV, Jokela J, Wahlsten M, Fiore MF, Fewer DP, Herfindal L, Sivonen K. Dereplication of Natural Products with Antimicrobial and Anticancer Activity from Brazilian Cyanobacteria. Toxins. 2020; 12(1):12. https://doi.org/10.3390/toxins12010012
Chicago/Turabian StyleShishido, Tania Keiko, Rafael Vicentini Popin, Jouni Jokela, Matti Wahlsten, Marli Fatima Fiore, David P. Fewer, Lars Herfindal, and Kaarina Sivonen. 2020. "Dereplication of Natural Products with Antimicrobial and Anticancer Activity from Brazilian Cyanobacteria" Toxins 12, no. 1: 12. https://doi.org/10.3390/toxins12010012
APA StyleShishido, T. K., Popin, R. V., Jokela, J., Wahlsten, M., Fiore, M. F., Fewer, D. P., Herfindal, L., & Sivonen, K. (2020). Dereplication of Natural Products with Antimicrobial and Anticancer Activity from Brazilian Cyanobacteria. Toxins, 12(1), 12. https://doi.org/10.3390/toxins12010012





