Heterologous Expression and Characterization of A Novel Ochratoxin A Degrading Enzyme, N-acyl-L-amino Acid Amidohydrolase, from Alcaligenes faecalis
Abstract
1. Introduction
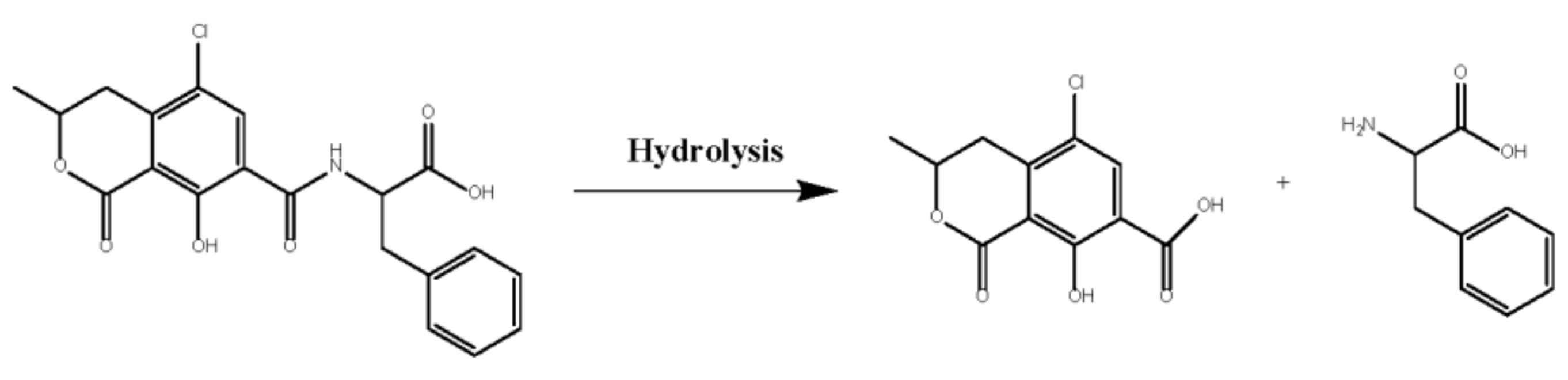
2. Results and Discussion
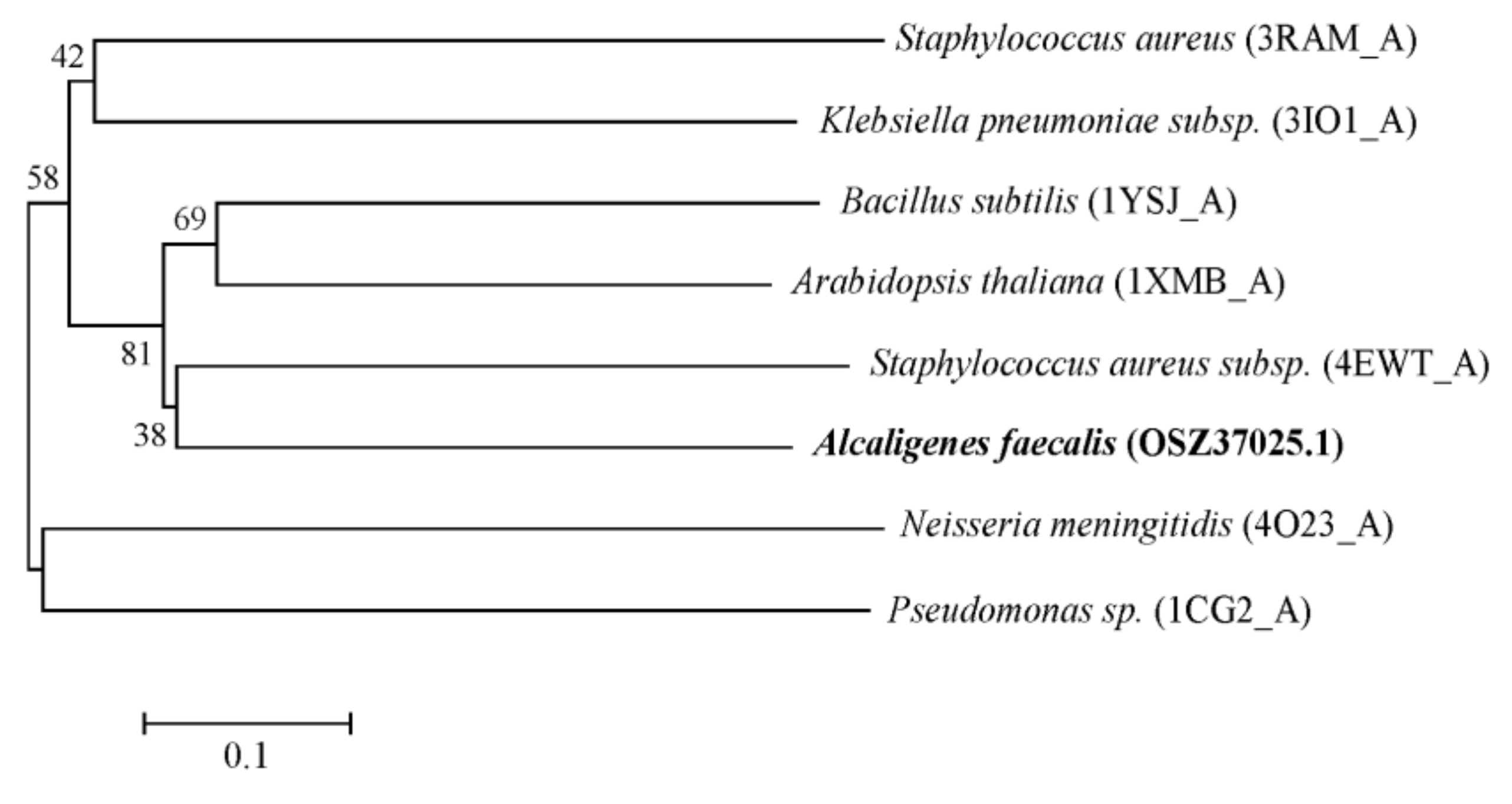
2.1. Cloning and Amino Acid Sequencing of The AfOTase Gene from A. Faecalis DSM 16503
2.2. Expression and Purification of rAfOTase
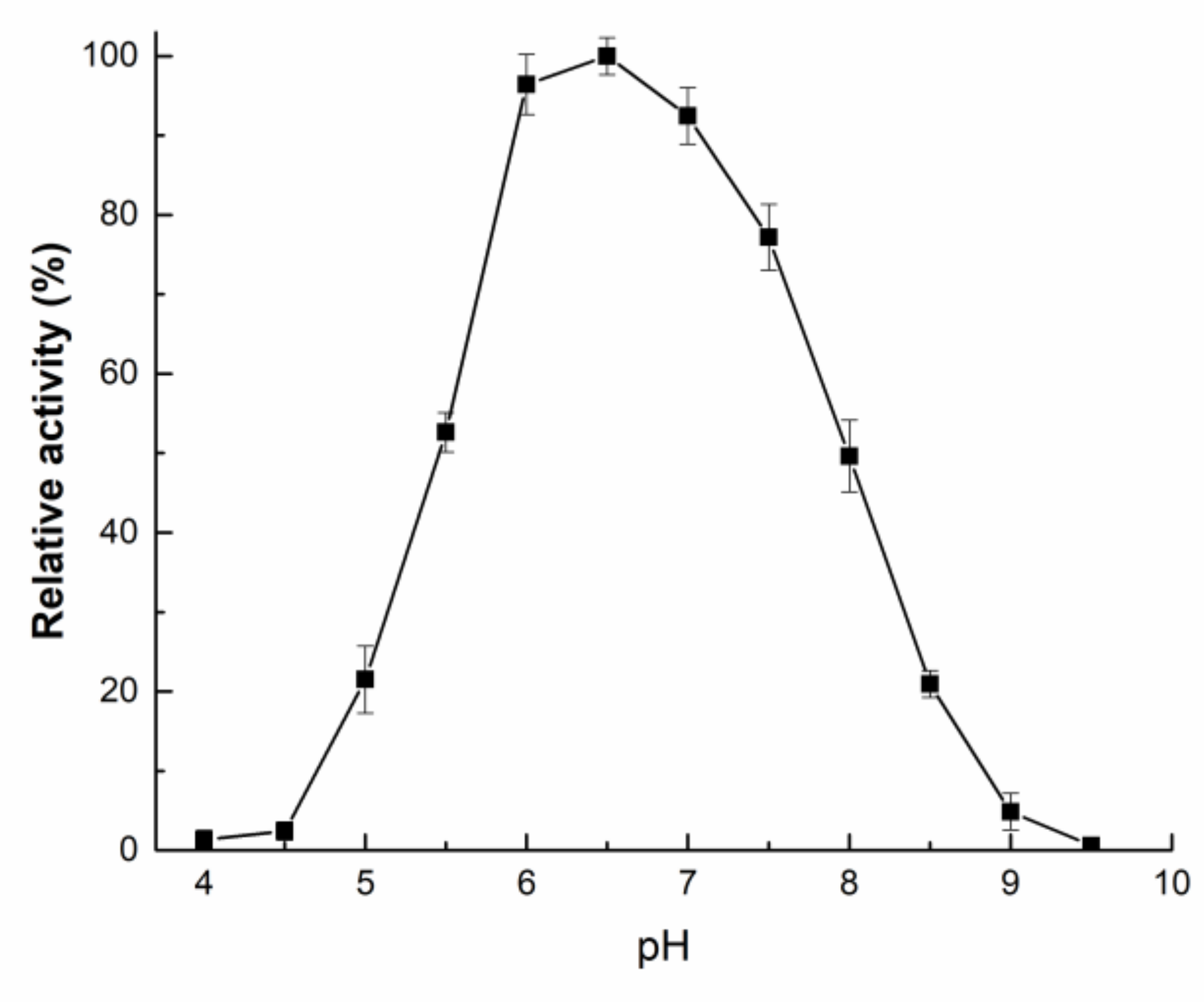
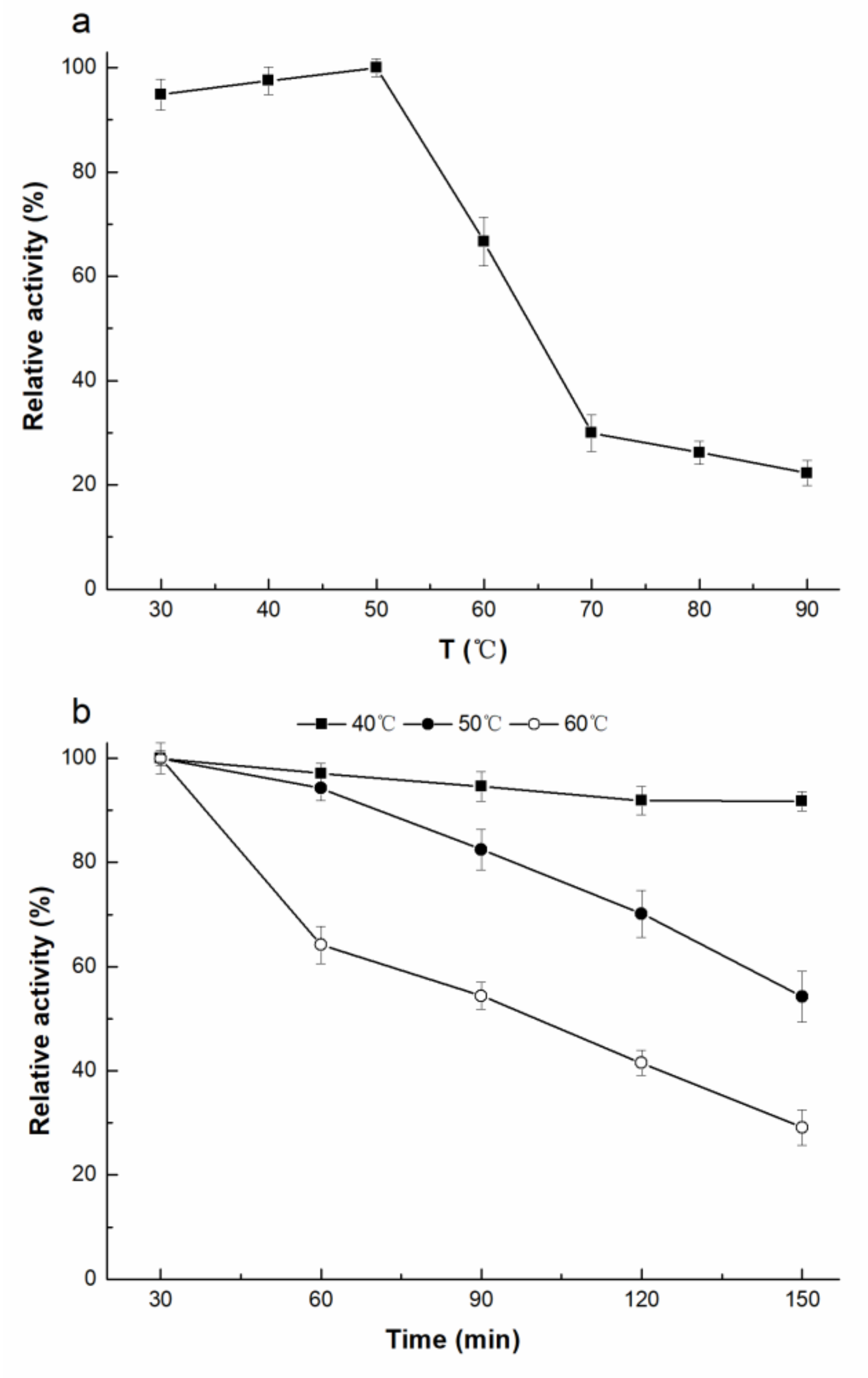
2.3. Enzymatic Properties of rAfOTase
3. Conclusions
4. Materials and Methods
4.1. Strain and Growth Conditions
4.2. DNA Isolation and Sequence Data Analysis
4.3. Cloning of AfOTase Gene
4.4. Expression of AfOTase in E.coli
4.5. Purification of Recombinant AfOTase
4.6. rAfOTase Activity Assays
4.7. Biochemical Characterization of rAfOTase
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bellí, N.; Ramos, A.J.; Coronas, I.; Sanchis, V.; Marín, S. Aspergillus carbonarius growth and ochratoxin A production on a synthetic grape medium in relation to environmental factors. J. Appl. Microbiol. 2005, 98, 839–844. [Google Scholar] [CrossRef]
- André el Khoury, A.; Atoui, A. Ochratoxin A: General overview and actual molecular status. Toxins 2010, 2, 461–493. [Google Scholar] [CrossRef]
- Edite Bezerra da Rocha, M.; Freire, F.d.C.O.; Erlan Feitosa Maia, F.; Izabel Florindo Guedes, M.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control. 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Limay-Rios, V.; Miller, J.D.; Schaafsma, A.W. Occurrence of penicillium verrucosum, ochratoxin A, ochratoxin B and citrinin in on-farm stored winter wheat from the canadian great lakes region. PLoS ONE 2017, 12, e0181239. [Google Scholar] [CrossRef]
- Soto, J.B.; Fernándezfranzón, M.; Ruiz, M.; Juangarcía, A. Presence of ochratoxin A (OTA) mycotoxin in alcoholic drinks from southern european countries: Wine and beer. J. Agr. Food Chem. 2014, 62, 7643–7651. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Manderville, R.A. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Biol. Evol. 2007, 51, 61–99. [Google Scholar]
- Limonciel, A.; Jennings, P. A review of the evidence that ochratoxin A is an nrf2 inhibitor: Implications for nephrotoxicity and renal carcinogenicity. Toxins 2014, 6, 371–379. [Google Scholar] [CrossRef]
- Heussner, A.H.; Bingle, L.E. Comparative ochratoxin toxicity: A review of the available data. Toxins 2015, 7, 4253–4282. [Google Scholar] [CrossRef]
- Buiklimke, T.R.; Wu, F. Ochratoxin A and human health risk: A review of the evidence. Crit. Rev. Food Sci. 2015, 55, 1860–1869. [Google Scholar] [CrossRef]
- Sorrenti, V.; Di Giacomo, C.; Acquaviva, R.; Barbagallo, I.; Bognanno, M.; Galvano, F. Toxicity of ochratoxin A and its modulation by antioxidants: A review. Toxins 2013, 5, 1742–1766. [Google Scholar] [CrossRef]
- Pfohlleszkowicz, A.; Manderville, R.A. An update on direct genotoxicity as a molecular mechanism of ochratoxin A carcinogenicity. J. Chem. Res. in Toxicol. 2012, 25, 252–262. [Google Scholar] [CrossRef]
- NHC. Limits of mycotoxins in foods. In Food Safety National Standard; National Health Commission: Beijing, China, 2017; Volume GB 2716–2017. [Google Scholar]
- Abrunhosa, L.; Paterson, R.R.; Venancio, A. Biodegradation of ochratoxin A for food and feed decontamination. Toxins 2010, 2, 1078–1099. [Google Scholar] [CrossRef]
- Karlovsky, P. Biological detoxification of fungal toxins and its use in plant breeding, feed and food production. Nat. Toxins 1999, 7, 1–23. [Google Scholar] [CrossRef]
- Piotrowska, M. The adsorption of ochratoxin A by lactobacillus species. Toxins 2014, 6, 2826–2839. [Google Scholar] [CrossRef]
- Farbo, M.G.; Urgeghe, P.P.; Fiori, S.; Marceddu, S.; Jaoua, S.; Migheli, Q. Adsorption of ochratoxin A from grape juice by yeast cells immobilised in calcium alginate beads. Int. J. Food. Microbiol. 2016, 217, 29–34. [Google Scholar] [CrossRef]
- Wu, Q.; Dohnal, V.; Huang, L.; Kuca, K.; Wang, X.; Chen, G.; Yuan, Z. Metabolic pathways of ochratoxin A. Curr. Drug Metab. 2011, 12, 1–10. [Google Scholar] [CrossRef]
- Li, S.; Marquardt, R.; Frohlich, A.; Vitti, T.; Crow, G. Pharmacokinetics of ochratoxin A and its metabolites in rats. Toxicol. Appl. Pharm. 1997, 145, 82–90. [Google Scholar] [CrossRef]
- Bruinink, A.; Rasonyi, T.; Sidler, C. Differences in neurotoxic effects of ochratoxin A, ochracin and ochratoxin α in vitro. Nat. Toxins 1998, 6, 173–177. [Google Scholar] [CrossRef]
- Zhang, H.H.; Wang, Y.; Zhao, C.; Wang, J.; Zhang, X.L. Biodegradation of ochratoxin A by alcaligenes faecalis isolated from soil. J. Appl. Microbiol. 2017, 123, 661–668. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J. Evolutionary families of metallopeptidases. Method. Enzymol. 1995, 248, 183–228. [Google Scholar]
- Chen, W.; Li, C.; Zhang, B.; Zhou, Z.; Shen, Y.; Liao, X.; Yang, J.; Wang, Y.; Li, X.; Li, Y.; et al. Advances in biodetoxification of ochratoxin A-A review of the past five decades. Front. Microbiol. 2018, 9, 1386. [Google Scholar] [CrossRef]
- Rodriguez, H.; Reveron, I.; Doria, F.; Costantini, A.; Rivas, B.D.L.; Muňoz, R.; Garcia-Moruno, E. Degradation of ochratoxin A by brevibacterium species. J. Agr. Food Chem. 2011, 59, 10755–10760. [Google Scholar] [CrossRef]
- Pitout, M.J. The hydrolysis of ochratoxin A by some proteolytic enzymes. Biochem. Pharmacol. 1969, 18, 485–491. [Google Scholar] [CrossRef]
- Liuzzi, V.C.; Fanelli, F.; Tristezza, M.; Haidukowski, M.; Picardi, E.; Manzari, C.; Lionetti, C.; Grieco, F.; Logrieco, A.F.; Thon, M.R. Transcriptional analysis of acinetobacter sp. Neg1 capable of degrading ochratoxin A. Front. Microbiol. 2016, 7, 2162. [Google Scholar] [CrossRef]
- Abrunhosa, L.; Santos, L.; Venâncio, A. Degradation of ochratoxin A by proteases and by a crude enzyme of aspergillus niger. Food Biotechnol. 2006, 20, 231–242. [Google Scholar] [CrossRef]
- Stander, M.A.; Bornscheuer, U.T.; Henke, E.; Steyn, P.S. Screening of commercial hydrolases for the degradation of ochratoxin A. J. Agr. Food Chem. 2000, 48, 5736–5739. [Google Scholar] [CrossRef]
- Abrunhosa, L.; Venancio, A. Isolation and purification of an enzyme hydrolyzing ochratoxin A from aspergillus niger. Biotechnol. Lett. 2007, 29, 1909–1914. [Google Scholar] [CrossRef]
- Dobritzsch, D.; Wang, H.; Schneider, G.; Yu, S. Structural and functional characterization of ochratoxinase, a novel mycotoxin-degrading enzyme. Biochem. J. 2014, 462, 441–452. [Google Scholar] [CrossRef]
- Lee, S.B.; Taylor, J.W. PCR protocols: A guide to methods and applications. In Molecular Reproduction and Development; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; Volume 28, pp. 282–287. [Google Scholar]
- Petersen, T.N.; Brunak, S.; Von, H.G.; Nielsen, H. Signalp 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. Mega5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Boil. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhang, Y.; Yin, T.; Wang, J.; Zhang, X. Heterologous Expression and Characterization of A Novel Ochratoxin A Degrading Enzyme, N-acyl-L-amino Acid Amidohydrolase, from Alcaligenes faecalis. Toxins 2019, 11, 518. https://doi.org/10.3390/toxins11090518
Zhang H, Zhang Y, Yin T, Wang J, Zhang X. Heterologous Expression and Characterization of A Novel Ochratoxin A Degrading Enzyme, N-acyl-L-amino Acid Amidohydrolase, from Alcaligenes faecalis. Toxins. 2019; 11(9):518. https://doi.org/10.3390/toxins11090518
Chicago/Turabian StyleZhang, Honghai, Yunpeng Zhang, Tie Yin, Jing Wang, and Xiaolin Zhang. 2019. "Heterologous Expression and Characterization of A Novel Ochratoxin A Degrading Enzyme, N-acyl-L-amino Acid Amidohydrolase, from Alcaligenes faecalis" Toxins 11, no. 9: 518. https://doi.org/10.3390/toxins11090518
APA StyleZhang, H., Zhang, Y., Yin, T., Wang, J., & Zhang, X. (2019). Heterologous Expression and Characterization of A Novel Ochratoxin A Degrading Enzyme, N-acyl-L-amino Acid Amidohydrolase, from Alcaligenes faecalis. Toxins, 11(9), 518. https://doi.org/10.3390/toxins11090518




