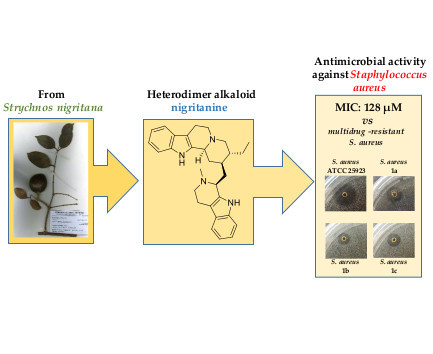
Nigritanine as a New Potential Antimicrobial Alkaloid for the Treatment of Staphylococcus aureus-Induced Infections
Abstract
1. Introduction
2. Results and Discussion
2.1. Alkaloids Collection
2.2. Antimicrobial Activity
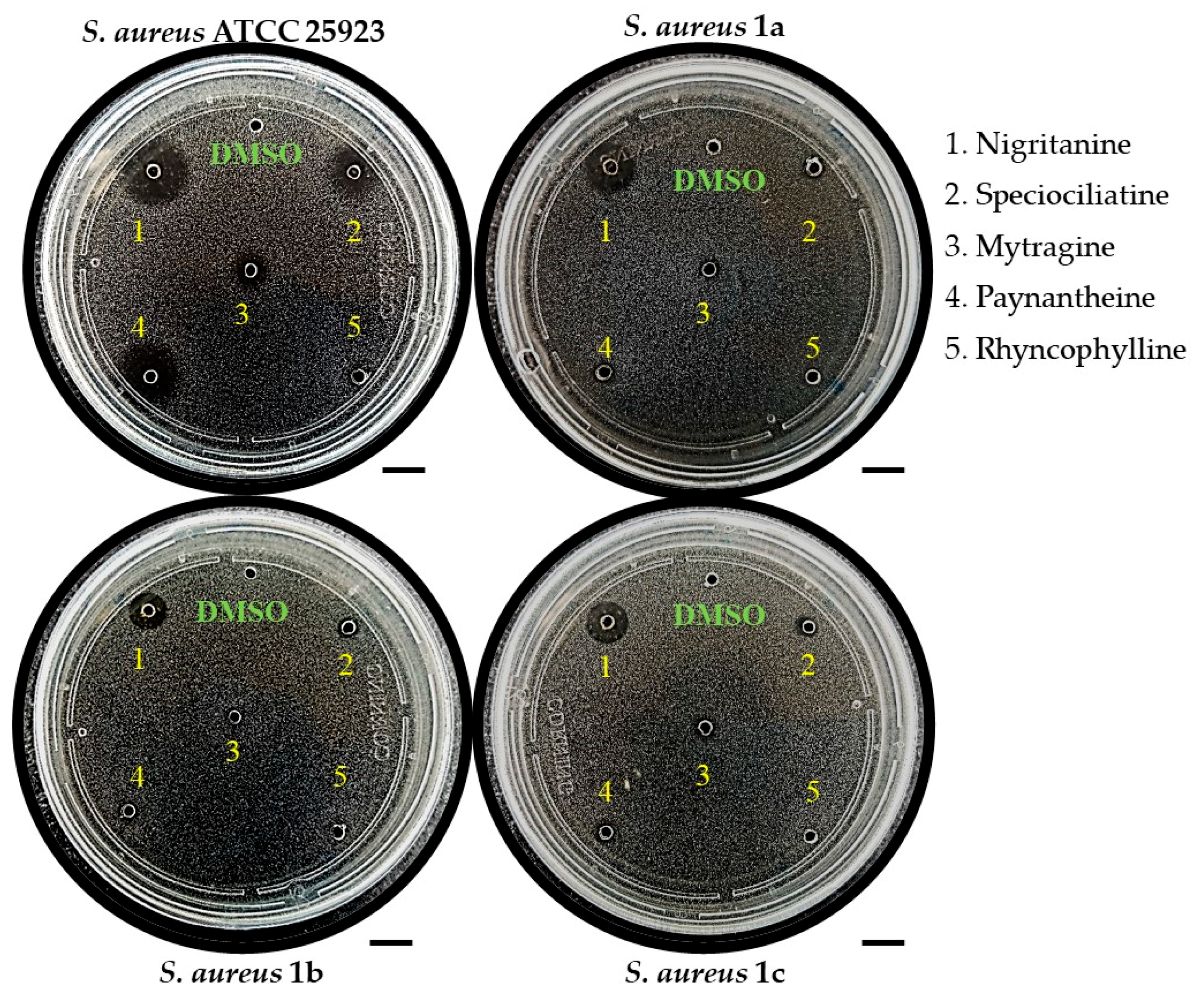
2.2.1. Inhibition Zone Assay
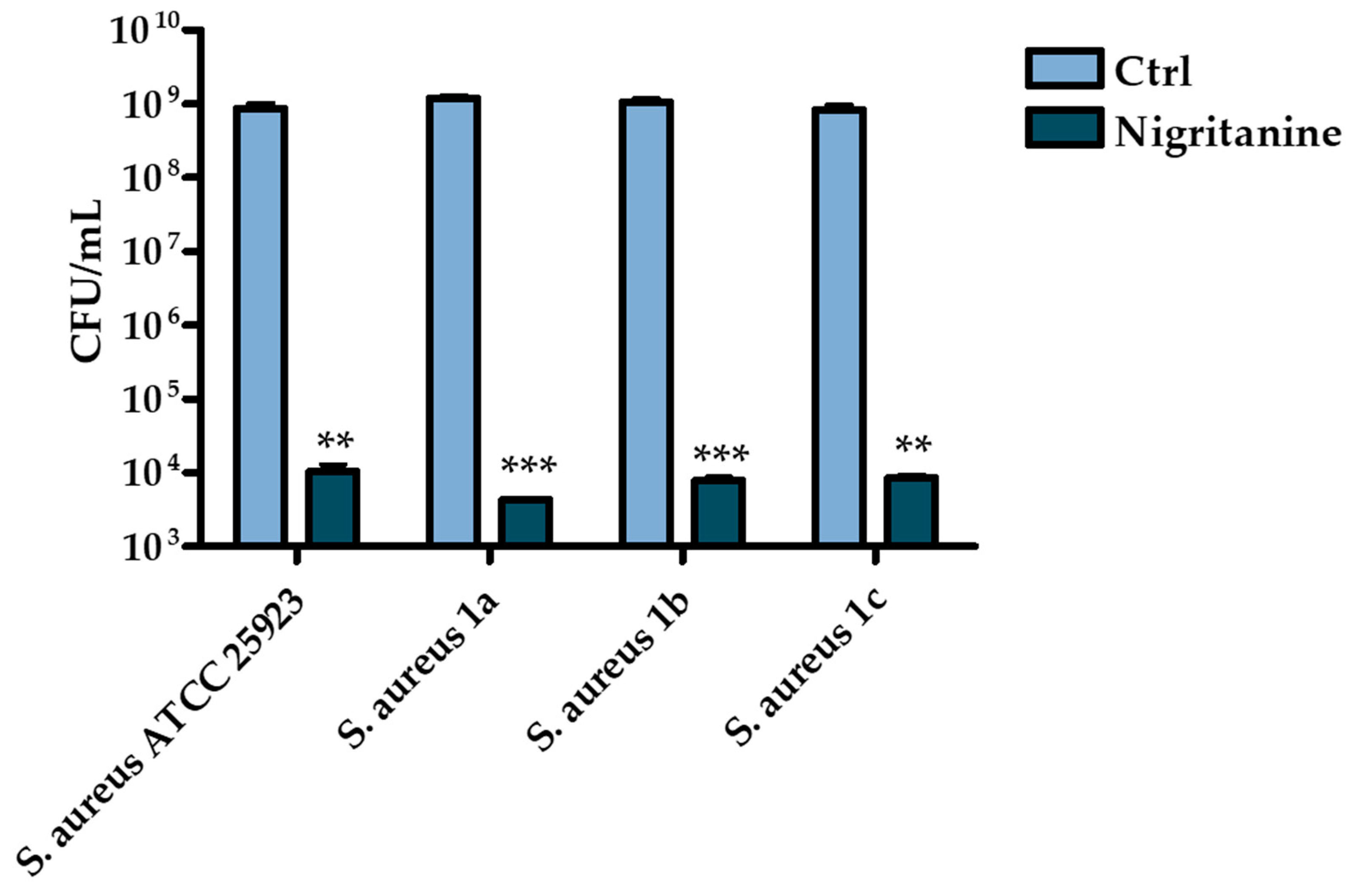
2.2.2. Determination of the Minimum Inhibitory Concentration
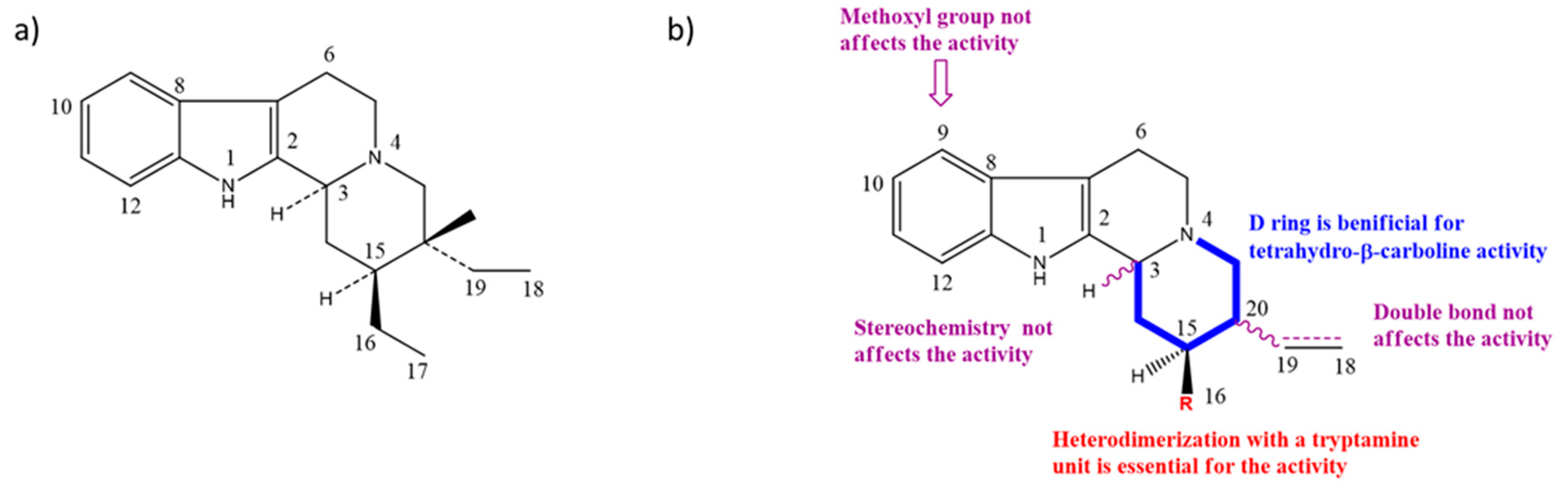
2.3. Structure–Activity Relationships (SARs) of Nigritanine for Its Antibacterial Activity
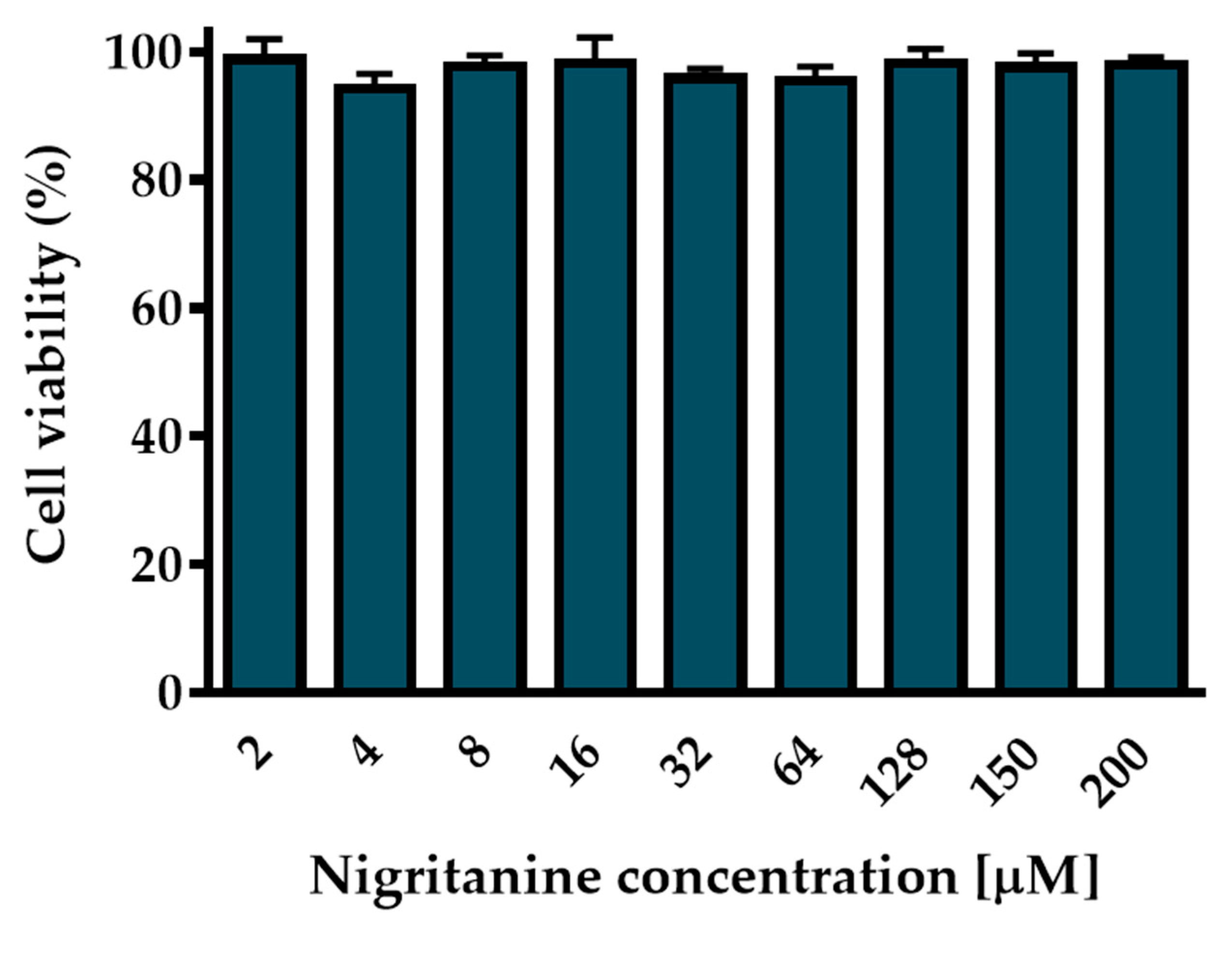
2.4. Cytotoxicity
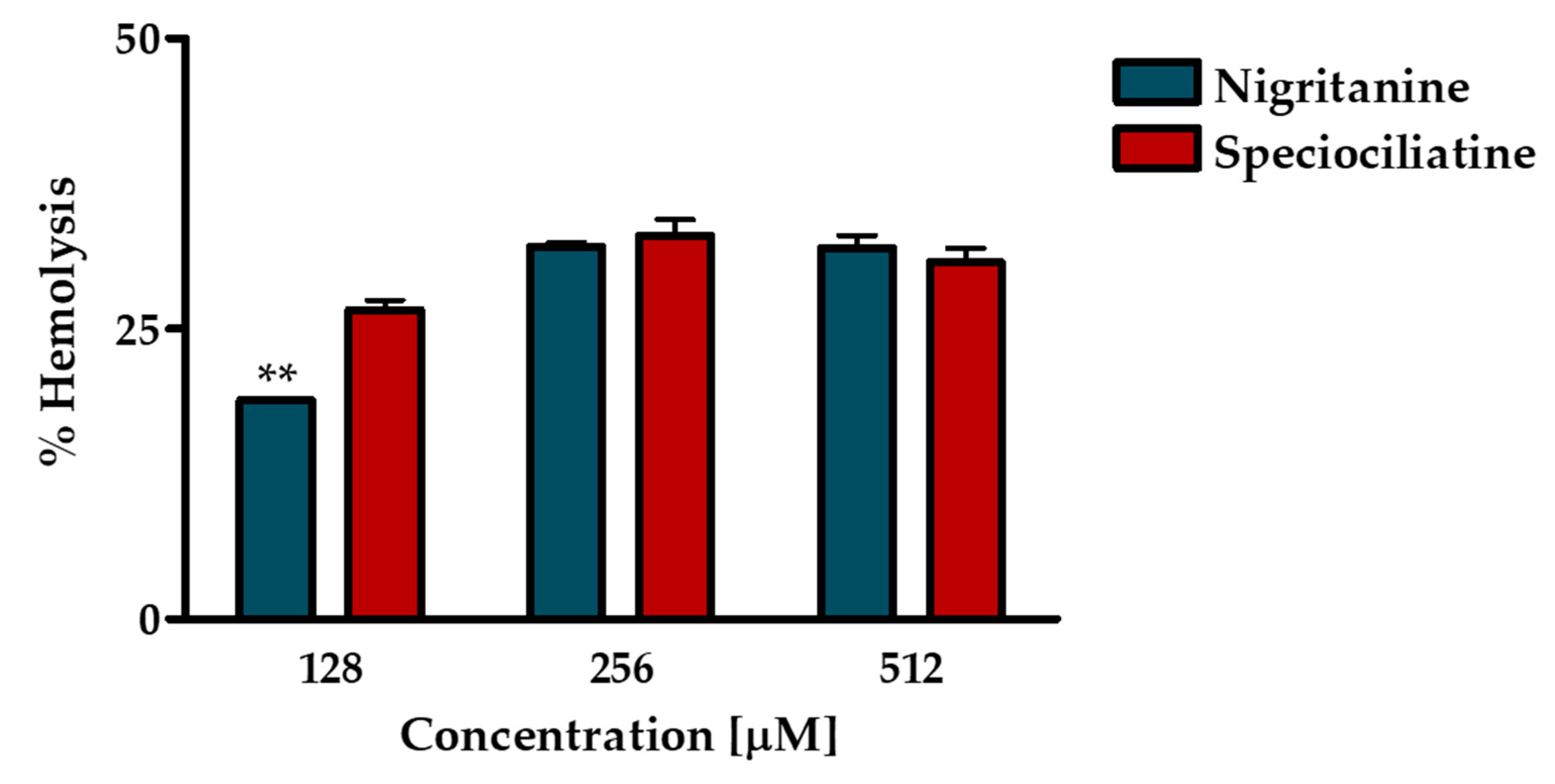
2.4.1. Hemolytic Assay
2.4.2. Cytotoxic Effect on HaCaT Cells
3. Conclusions
4. Material and Methods
4.1. Chemistry
4.2. Microorganisms and Cell Line
4.3. Antimicrobial Assays
4.4. Cytotoxicity Assays
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Jha, R.K.; Mishra, S.K.; Tiwari, B.R.; Asaad, A.M. Recent advances in Staphylococcus aureus infection: Focus on vaccine development. Infect. Drug Resist. 2019, 12, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Gajdacs, M. The continuing threat of methicillin-resistant Staphylococcus aureus. Antibiotics 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Mermel, L.A.; Cartony, J.M.; Covington, P.; Maxey, G.; Morse, D. Methicillin-resistant Staphylococcus aureus colonization at different body sites: A prospective, quantitative analysis. J. Clin. Microbiol. 2011, 49, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Keihanian, F.; Saeidinia, A.; Abbasi, K. Epidemiology of antibiotic resistance of blood culture in educational hospitals in Rasht, North of Iran. Infect. Drug Resist. 2018, 11, 1723–1728. [Google Scholar] [CrossRef]
- Chung, P.Y. Novel targets of pentacyclic triterpenoids in Staphylococcus aureus: A systematic review. Phytomedicine Int. J. Phytother. Phytopharm. 2019. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, A.; D’Acquarica, I. The market of chiral drugs: Chiral switches versus de novo enantiomerically pure compounds. J. Pharm. Biomed. Anal. 2018, 147, 323–340. [Google Scholar] [CrossRef]
- Debnath, B.; Singh, W.S.; Das, M.; Goswami, S.; Singh, M.K.; Maiti, D.; Manna, K. Role of plant alkaloids on human health: A review of biological activities. Mater. Today Chem. 2018, 9, 56–72. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef]
- Ingallina, C.; D’Acquarica, I.; Delle Monache, G.; Ghirga, F.; Quaglio, D.; Ghirga, P.; Berardozzi, S.; Markovic, V.; Botta, B. The pictet-spengler reaction still on stage. Curr. Pharm. Des. 2016, 22, 1808–1850. [Google Scholar] [CrossRef]
- Ghirga, F.; Bonamore, A.; Calisti, L.; D’Acquarica, I.; Mori, M.; Botta, B.; Boffi, A.; Macone, A. Green routes for the production of enantiopure benzylisoquinoline alkaloids. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Amirkia, V.; Heinrich, M. Alkaloids as drug leads—A predictive structural and biodiversity-based analysis. Phytochem. Lett. 2014, 10. [Google Scholar] [CrossRef]
- Infante, P.; Alfonsi, R.; Ingallina, C.; Quaglio, D.; Ghirga, F.; D’Acquarica, I.; Bernardi, F.; Di Magno, L.; Canettieri, G.; Screpanti, I.; et al. Inhibition of hedgehog-dependent tumors and cancer stem cells by a newly identified naturally occurring chemotype. Cell Death Dis. 2016, 7, E2376. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Tottone, L.; Quaglio, D.; Zhdanovskaya, N.; Ingallina, C.; Fusto, M.; Ghirga, F.; Peruzzi, G.; Crestoni, M.E.; Simeoni, F.; et al. Identification of a novel chalcone derivative that inhibits Notch signaling in T-cell acute lymphoblastic leukemia. Sci. Rep. 2017, 7, 2213. [Google Scholar] [CrossRef] [PubMed]
- Infante, P.; Mori, M.; Alfonsi, R.; Ghirga, F.; Aiello, F.; Toscano, S.; Ingallina, C.; Siler, M.; Cucchi, D.; Po, A.; et al. Gli1/DNA interaction is a druggable target for Hedgehog-dependent tumors. EMBO. J. 2015, 34, 200–217. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Srivastava, M.; Misra, A.; Pandey, G.; Rawat, A. A review on biological and chemical diversity in Berberis (Berberidaceae). Excli J. 2015, 14, 247–267. [Google Scholar] [CrossRef]
- Meyer, A.; Imming, P. Benzylisoquinoline alkaloids from the Papaveraceae: The heritage of Johannes Gadamer (1867–1928). J. Nat. Prod. 2011, 74, 2482–2487. [Google Scholar] [CrossRef]
- Urzúa, A.P.A. Alkaloids from the bark of Peumus boldus. Fitoterapia 1983, 54, 175–177. [Google Scholar]
- Imaki, N.M.Y.; Shimpuku, T.; Shirasaka, T. A Process for Preparing Cotarnine; Technical Report No. 4,963,684; U.S. Patent and Trademark Office: Washington, DC, USA, 1986.
- Colombo, M.L.; Bosisio, E. Pharmacological activities of Chelidonium majus L. (Papaveraceae). Pharmacol. Res. 1996, 33, 127–134. [Google Scholar] [CrossRef]
- Akinboye, E.; Bakare, O. Biological activities of emetine. Open Nat. Prod. J. 2011, 411, 8–15. [Google Scholar] [CrossRef]
- Lapa, G.P.; Sheichenko, O.; Serezhechkin, A.G.N.; Tolkachev, O. HPLC determination of glaucine in yellow horn poppy grass (Glaucium flavum Crantz). Pharm. Chem. J. 2004, 38, 441–442. [Google Scholar] [CrossRef]
- Brown, P.N.; Roman, M.C. Determination of hydrastine and berberine in goldenseal raw materials, extracts, and dietary supplements by high-performance liquid chromatography with UV: Collaborative study. J. AOAC Int. 2008, 91, 694–701. [Google Scholar] [PubMed]
- Ramanathan, V.S.; Chandra, P. Recovery, separation and purification of narcotine and papaverine from Indian opium. Bull. Narc. 1981, 33, 55–64. [Google Scholar]
- Lee, M.R. Curare: The South American arrow poison. J. R. Coll. Physicians Edinb. 2005, 35, 83–92. [Google Scholar] [PubMed]
- Martins, D.; Nunez, C.V. Secondary metabolites from Rubiaceae species. Molecules 2015, 20, 13422–13495. [Google Scholar] [CrossRef] [PubMed]
- Dellemonache, F.; Dellemonache, G.; Souza, M.A.D.; Cavalcanti, M.D.; Chiappeta, A. Isopentenylindole derivatives and other components of Esenbeckia leiocarpa. Gazz. Chim. Ital. 1989, 119, 435–439. [Google Scholar]
- Guimaraes, H.A.; Braz-Filho, R.; Vieira, I.J.C. H-1 and C-13-NMR data of the simplest plumeran indole alkaloids isolated from Aspidosperma species. Molecules 2012, 17, 3025–3043. [Google Scholar] [CrossRef]
- Galeffi, C.; Ciasca-Rendina, M.A.; Miranda Delle Monache, E.; Villar Del Fresno, A.; Marini Bettòlo, G.B. Gradient method for the counter-current separation of alkaloids using a heavy organic phase. J. Chromatogr. 1969, 45, 407–414. [Google Scholar] [CrossRef]
- Zhao, B.; Moochhala, S.M.; Tham, S.Y. Biologically active components of Physostigma venenosum. J. Chromatogr. B 2004, 812, 183–192. [Google Scholar] [CrossRef]
- Ohiri, F.C.; Verpoorte, R.; Baerheim Svendsen, A. The African Strychnos species and their alkaloids: A review. J. Ethnopharmacol. 1983, 9, 167–223. [Google Scholar] [CrossRef]
- Mohammad-Zadeh, L.F.; Moses, L.; Gwaltney-Brant, S.M. Serotonin: A review. J. Vet. Pharm. 2008, 31, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Kousar, S.; Noreen Anjuma, S.; Jaleel, F.; Khana, J.; Naseema, S. Biomedical significance of tryptamine: A review. J. Pharmacovigil. 2017, 5. [Google Scholar] [CrossRef]
- Song, K.M.; Park, S.W.; Hong, W.H.; Lee, H.; Kwak, S.S.; Liu, J.R. Isolation of vindoline from Catharanthus roseus by supercritical fluid extraction. Biotechnol. Prog. 1992, 8, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Noldin, V.F.; de Oliveira Martins, D.T.; Marcello, C.M.; da Silva Lima, J.C.; Delle Monache, F.; Cechinel Filho, V. Phytochemical and antiulcerogenic properties of rhizomes from Simaba ferruginea St. Hill. (Simaroubaceae). Zeitschrift für Naturforschung C 2005, 60, 701–706. [Google Scholar] [CrossRef]
- Kumar, A.S.; Nagarajan, R. Synthesis of alpha-carbolines via Pd-catalyzed amidation and Vilsmeier-Haack reaction of 3-acetyl-2-chloroindoles. Org. Lett. 2011, 13, 1398–1401. [Google Scholar] [CrossRef]
- Claudia, F.; Amaral, A.; Ramos, A.D.S.; Ferreira, J.; Santos, A.D.S.; Cruz, J.D.S.; De Luna, A.V.M.; Nery, V.V.C.; de Lima, I.C.; Chaves, M.H.d.C.; et al. LC-HRMS for the Identification of β-carboline and canthinone alkaloids isolated from natural sources. Mass Spectrometry 2017, 187. [Google Scholar] [CrossRef]
- Alper, K.R. Ibogaine: A review. Alkaloids. Chem. Biol. 2001, 56, 1–38. [Google Scholar]
- Takayama, H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem. Pharm. Bull. 2004, 52, 916–928. [Google Scholar] [CrossRef]
- Leon, F.; Habib, E.; Adkins, J.E.; Furr, E.B.; McCurdy, C.R.; Cutler, S.J. Phytochemical characterization of the leaves of Mitragyna speciosa grown in USA. Nat. Prod. Commun. 2009, 4, 907–910. [Google Scholar] [CrossRef]
- Cinosi, E.; Martinotti, G.; Simonato, P.; Singh, D.; Demetrovics, Z.; Roman-Urrestarazu, A.; Bersani, F.S.; Vicknasingam, B.; Piazzon, G.; Li, J.H.; et al. Following the roots of kratom (Mitragyna speciosa): The evolution of an enhancer from a traditional use to increase work and productivity in Southeast Asia to a recreational psychoactive drug in western countries. Biomed. Res. Int. 2015, 968786. [Google Scholar] [CrossRef]
- Evans, W.C. Pharmacopoeial and Related Drugs of Biological Origin, 16th ed.; Saunders—Elsevier: Amsterdam, The Netherlands, 2009; pp. 353–416. [Google Scholar]
- Dai, J.K.; Dan, W.J.; Schneider, U.; Wang, J.R. β-Carboline alkaloid monomers and dimers: Occurrence, structural diversity, and biological activities. Eur. J. Med. Chem. 2018, 157, 622–656. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.K.; Dan, W.J.; Ren, S.Y.; Shang, C.G.; Wang, J.R. Design, synthesis and biological evaluations of quaternization harman analogues as potential antibacterial agents. Eur. J. Med. Chem. 2018, 160, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Nenaah, G. Antibacterial and antifungal activities of (β)-carboline alkaloids of Peganum harmala (L) seeds and their combination effects. Fitoterapia 2010, 81, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Reza, V.R.; Hadjiakhoondi, A. Cytotoxicity and antimicrobial activity of harman alkaloids. J. Pharmacol. Toxicol. 2007, 2, 677–680. [Google Scholar] [CrossRef][Green Version]
- Parthasarathy, S.; Bin Azizi, J.; Ramanathan, S.; Ismail, S.; Sasidharan, S.; Said, M.I.; Mansor, S.M. Evaluation of antioxidant and antibacterial activities of aqueous, methanolic and alkaloid extracts from Mitragyna speciosa (Rubiaceae family) leaves. Molecules 2009, 14, 3964–3974. [Google Scholar] [CrossRef]
- Benziane Maatalah, M.; Kambuche Bouzidi, N.; Bellahouel, S.; Merah, B.; Fortas, Z.; Soulimani, R.; Saidi, S.; Derdour, A. Antimicrobial activity of the alkaloids and saponin extracts of Anabasis articulate. J. Biotechnol. Pharm. Res. 2012, 3, 54. [Google Scholar]
- Ozcelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef]
- Gurrapu, S.; Estari, M. In vitro antibacterial activity of alkaloids isolated from leaves of Eclipta alba against human pathogenic bacteria. Pharmacogn. J. 2017, 9, 573–577. [Google Scholar] [CrossRef]
- Karou, D.; Savadogo, A.; Canini, A.; Yameogo, S.; Montesano, C.; Simpore, J.; Colizzi, V.; Traore, A.S. Antibacterial activity of alkaloids from Sida acuta. Afr. J. Biotechnol. 2006, 5, 195–200. [Google Scholar]
- Slobodnikova, L.; Kost’alova, D.; Labudova, D.; Kotulova, D.; Kettman, V. Antimicrobial activity of Mahonia aquifolium crude extract and its major isolated alkaloids. Phytother. Res. 2004, 18, 674–676. [Google Scholar] [CrossRef]
- Su, Y.F.; Li, S.K.; Li, N.; Chen, L.L.; Zhang, J.W.; Wang, J.R. Seven alkaloids and their antibacterial activity from Hypecoum erectum L. J. Med. Plants Res. 2011, 5, 5428–5432. [Google Scholar]
- Manosalva, L.; Mutis, A.; Urzua, A.; Fajardo, V.; Quiroz, A. Antibacterial activity of alkaloid fractions from Berberis microphylla G. Forst and study of synergism with ampicillin and cephalothin. Molecules 2016, 21, 76. [Google Scholar] [CrossRef] [PubMed]
- Tegos, G.; Stermitz, F.R.; Lomovskaya, O.; Lewis, K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob. Agents Chemother. 2002, 46, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
- Locher, H.H.; Ritz, D.; Pfaff, P.; Gaertner, M.; Knezevic, A.; Sabato, D.; Schroeder, S.; Barbaras, D.; Gademann, K. Dimers of nostocarboline with potent antibacterial activity. Chemotherapy 2010, 56, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Soong, G.; Paulino, F.; Wachtel, S.; Parker, D.; Wickersham, M.; Zhang, D.; Brown, A.; Lauren, C.; Dowd, M.; West, E.; et al. Methicillin-resistant Staphylococcus aureus adaptation to human keratinocytes. mBio 2015, 6, e00289-15. [Google Scholar] [CrossRef] [PubMed]
- Malikova, J.; Zdarilova, A.; Hlobilkova, A.; Ulrichova, J. The effect of chelerythrine on cell growth, apoptosis, and cell cycle in human normal and cancer cells in comparison with sanguinarine. Cell Biol. Toxicol. 2006, 22, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.S.; Li, M.; Gao, F.; Peng, J.Y.; Xiao, H.B.; Dai, L.X.; Lin, S.R.; Zhang, R.; Jin, L.Y. Arecoline suppresses HaCaT cell proliferation through cell cycle regulatory molecules. Oncol. Rep. 2013, 29, 2438–2444. [Google Scholar] [CrossRef] [PubMed]
- Delle Monache, F.C.; Rossi, E.; Cartoni, C.; Carpi, A.; Marini Bettolo, G.B. The alkaloids of Strychnos castelneana. Strychnos Alkaloids 1970, 33, 279–283. [Google Scholar]
- Martin, G.E. Configuration and total assignment of the 1H-and 13C-NMR spectra of the alkaloid holstiine. J. Nat. Prod. 1990, 53, 793–800. [Google Scholar]
- Tavernier, D.; Anteunis, M.; Tits, M.; Angenot, L. The 1H NMR spectra of the strychnos alkaloids retuline isoretuline, and their N-deacetyl compounds. Bull. Des Sociétés Chim. Belg. 2010, 87, 595–607. [Google Scholar] [CrossRef]
- Cao, M.; Muganga, R.; Nistor, I.; Tits, M.; Angenot, L.; Frederich, M. LC–SPE–NMR–MS analysis of Strychnos usambarensis fruits from Rwanda. Phytochem. Lett. 2012, 5, 170–173. [Google Scholar] [CrossRef]
- Nicoletti, M.; Oguakwa, J.U.; Messana, I. On the alkaloids of two African Strychnos: Strychnos nigritana bak and Strychnos barteri Sol. carbon-13 NMR spectroscopy of nigritanins. Fitoterapia 1980, 87, 595–607. [Google Scholar]
- Islas-Rodriguez, A.E.; Marcellini, L.; Orioni, B.; Barra, D.; Stella, L.; Mangoni, M.L. Esculentin 1-21: A linear antimicrobial peptide from frog skin with inhibitory effect on bovine mastitis-causing bacteria. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2009, 15, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; Fiocco, D.; Mignogna, G.; Barra, D.; Simmaco, M. Functional characterisation of the 1–18 fragment of esculentin-1b, an antimicrobial peptide from Rana esculenta. Peptides 2003, 24, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Falciani, C.; Lozzi, L.; Pollini, S.; Luca, V.; Carnicelli, V.; Brunetti, J.; Lelli, B.; Bindi, S.; Scali, S.; Di Giulio, A.; et al. Isomerization of an antimicrobial peptide broadens antimicrobial spectrum to gram-positive bacterial pathogens. PLoS ONE 2012, 7, e46259. [Google Scholar] [CrossRef] [PubMed]
- Buommino, E.; Carotenuto, A.; Antignano, I.; Bellavita, R.; Casciaro, B.; Loffredo, M.R.; Merlino, F.; Novellino, E.; Mangoni, M.L.; Nocera, F.P.; et al. The outcomes of decorated prolines in the discovery of antimicrobial peptides from Temporin-L. ChemMedChem 2019, 14, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Merlino, F.; Carotenuto, A.; Casciaro, B.; Martora, F.; Loffredo, M.R.; Di Grazia, A.; Yousif, A.M.; Brancaccio, D.; Palomba, L.; Novellino, E.; et al. Glycine-replaced derivatives of [Pro(3),DLeu(9)]TL, a temporin L analogue: Evaluation of antimicrobial, cytotoxic and hemolytic activities. Eur. J. Med. Chem. 2017, 139, 750–761. [Google Scholar] [CrossRef]
- Grieco, P.; Carotenuto, A.; Auriemma, L.; Saviello, M.R.; Campiglia, P.; Gomez-Monterrey, I.M.; Marcellini, L.; Luca, V.; Barra, D.; Novellino, E.; et al. The effect of d-amino acid substitution on the selectivity of temporin L towards target cells: Identification of a potent anti-Candida peptide. Biochimica et Biophysica Acta 2013, 1828, 652–660. [Google Scholar] [CrossRef]
- Cappiello, F.; Di Grazia, A.; Segev-Zarko, L.A.; Scali, S.; Ferrera, L.; Galietta, L.; Pini, A.; Shai, Y.; Di, Y.P.; Mangoni, M.L. Esculentin-1a-derived peptides promote clearance of Pseudomonas aeruginosa internalized in bronchial cells of cystic fibrosis patients and lung cell migration: Biochemical properties and a plausible mode of action. Antimicrob. Agents Chemother. 2016, 60, 7252–7262. [Google Scholar] [CrossRef]
- Di Grazia, A.; Luca, V.; Segev-Zarko, L.A.; Shai, Y.; Mangoni, M.L. Temporins A and B stimulate migration of HaCaT keratinocytes and kill intracellular Staphylococcus aureus. Antimicrob. Agents Chemother. 2014, 58, 2520–2527. [Google Scholar] [CrossRef]
| Mol. | Common Name | Chemical Structure | M.W. | Molecular Formula | Source | Ref. |
|---|---|---|---|---|---|---|
 Isoquinoline Alkaloids | ||||||
| 1 | Dihydroberberine·HCl | 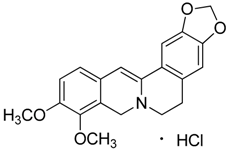 | 337.37 373.83 (+ HCl) | C20H19NO4·HCl | Berberis species: Berberis aristata, Berberis lyceum, Berberis petiolaris, Berberis tinctoria (Berberidaceae family) | [16] |
| 2 | Bulbocapnine·HCl |  | 325.36 361.82 (+ HCl) | C19H19NO4·HCl | Species: Corydalis cava (Papaveraceae family) | [17] |
| 3 | Boldine | 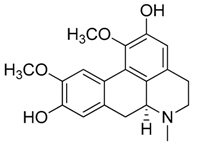 | 327.37 | C19H21NO4 | Species: Peumus boldus (Monimiaceae family) | [18] |
| 4 | Cotarmine·HCl | 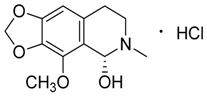 | 237.25 273.71 | C12H15NO4·HCl | Synthetic | [19] |
| 5 | Chelidonine | 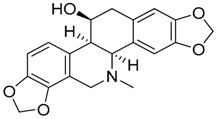 | 353.37 | C20H19NO5 | Species: Chelidonium majus L. (Papaveraceae family) | [20] |
| 6 | Emetine·HCl | 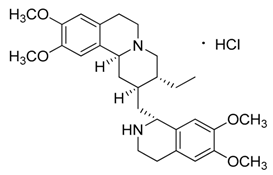 | 480.64 517.10 (+HCl) | C29H40N2O4 | Species: Psychotria ipecacuanha Stokes (Rubiaceae family) | [21] |
| 7 | (S)-Glaucine | 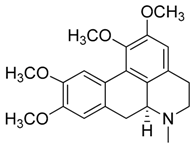 | 355.43 | C21H25NO4 | Species: Glaucium luteum L. (Papaveraceae family) | [22] |
| 8 | Hydrastine | 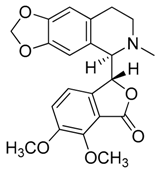 | 383.39 | C21H21NO6 | Species: Hydrastis canadensis L. (Ranunculaceae family) | [23] |
| 9 | Noscapine (Narcotine) |  | 413.42 | C22H23NO7 | Species: Papaver somniferum (Papaveraceae family) | [24] |
| 10 | Papaverine |  | 339.39 | C20H21NO4 | Species: P. somniferum (Papaveraceae family) | [24] |
| 11 | Tubocurarine Chloride·HCl |  | 609.73 681.65 (+Cl-+HCl) | C37H41N2O6·HCl + Cl- | Species: Liana Chondrodendron (Menispermaceae family) | [25] |
 Quinoline Alkaloids | ||||||
| 12 | Cinchonine |  | 294.39 | C19H22N2O | Species: Cinchona ledgeriana, Remijia peruviana (Rubiaceae family) | [26] |
| 13 | Kokusaginine | 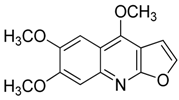 | 259.26 | C14H13NO4 | Species: Esenbeckia leiocarpa (Rutaceae family) | [27] |
| 14 | Maculine | 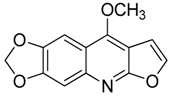 | 243.21 | C13H9NO4 | Species: E. leiocarpa (Rutaceae family) | [27] |
| 15 | 4-methoxy-2-(1-ethylpropyl)-quinoline | 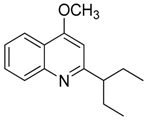 | 229.32 | C15H19NO | Species: E. leiocarpa (Rutaceae family) | [27] |
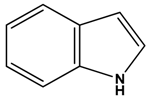 Indole Alkaloids | ||||||
| 16 | Aspidospermine |  | 354.49 | C22H30N2O2 | Aspidosperma species: Aspidosperma album, Aspidosperma australe, Aspidosperma exalatum, Aspidosperma peroba, Aspidosperma polyneuron, Aspidosperma pyricollum, Aspidosperma pyrifolium, Aspidosperma quebracho-blanco, Aspidosperma quirandy, Aspidosperma sessiflorum, Aspidosperma rhombeosignatum (Apocynaceae family) | [28] |
| 17 | Brucine |  | 394.47 | C23H26N2O4 | Species: Strychnos nux-vomica (Apocynaceae family) | [29] |
| 18 | Diaboline |  | 353.41 | C21H23NO4 | Species: Strychnos castelneana (Loganiaceae family) | [27] |
| 19 | Physostigmine (Eserine) |  | 275.35 | C15H21N3O2 | Physostigma venenosum (Fabaceae family) | [30] |
| 20 | Holstiine |  | 382.45 | C22H26N2O4 | Species: Strychnos henningsii Gilg (Loganiaceae family) | [31] |
| 21 | Pseudobrucine |  | 410.46 | C23H26N2O5 | Species: S. nux-vomica (Loganiaceae family) | [29] |
| 22 | Retuline |  | 338.44 | C21H26N2O2 | Strychnos species: Strychnos camptoneura, S. henningsii (Loganiaceae family) | [31] |
| 23 | Serotonin |  | 176.22 | C10H12N2O | Species: Laphophora williamsii (Cactaceae family) | [32] |
| 24 | Triptamine·HCl |  | 160.22 196.68 (+HCl) | C10H12N2 HCl | Acacia species (Fabacee family) | [33] |
| 25 | Vomicine·HC1 |  | 380.44 416.90 (+HCl) | C22H24N2O4 HCl | Strychnos icaja (Loganiaceae family) | [27,31] |
| 26 | Vindoline |  | 456.53 | C25H32N2O6 | Catharanthus roseus (Apocynaceae family) | [34] |
 Carboline Alkaloids (Indole Subclass) | ||||||
| 27 | Akagerine | 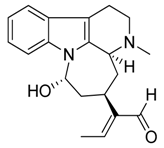 | 324.42 | C20H24N2O2 | Strychnos species: Strychnos barteri Solered, S. camptoneurine, Strychnos nigritana Bak (Loganiaceae family) | [31] |
| 28 | Canthin-6-one |  | 220.23 | C14H8N2O | Species: Simaba ferruginea (Simaroubaceae family) | [35] |
| 29 | α-Carboline |  | 168.19 | C11H8N2 | Synthetic | [36] |
| 30 | Harmane |  | 182.22 | C12H10N2 | Species: Chimarrhis turbinata, Ophiorrhiza communis, Ophiorrhiza liukiuensis, Ophiorrhiza tomentosa, Psychotria barbiflora (Rubiaceae family) | [26] |
| 31 | Norharmane |  | 168.19 | C11H8N2 | Species: Hygrophorus eburneus (Tricholomataceae family) | [37] |
| 32 | Harmine | 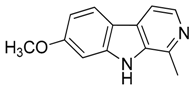 | 212.25 | C13H12N2O | Species: Banisteriopsis caapi (Malpighiaceae family), Grewia bicolor (Malvaceae family), Passiflora edulis f. flavicarpa O. Deg., Passiflora incarnata L. (Passifloraceae family), Tribulus terrestris L., Peganum harmala L. (Zygophyllaceae family) | [37] |
| 33 | Ibogaine | 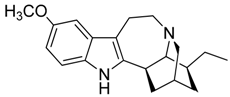 | 310.43 | C20H26N2O | Species: Tabernanthe iboga (Apocynaceae family) | [38] |
| 34 | Mitragynine | 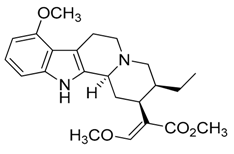 | 398.50 | C23H30N2O4 | Species: Mitragyna speciosa (Rubiaceae family) | [39] |
| 35 | Nigritanine | 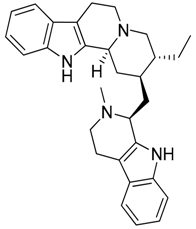 | 452.63 | C30H36N4 | Strychnos species: Strychnos borteri, S. nigritana Bak. (Loganiaceae family) | [31] |
| 36 | Paynantheine |  | 396.48 | C23H28N2O4 | Species: M. speciosa (Rubiaceae family) | [40] |
| 37 | Rhynchophylline |  | 384.47 | C22H28N2O4 | Species: M. speciosa, Uncaria rhynchophylla (Rubiaceae family) | [40] |
| 38 | Speciociliatine | 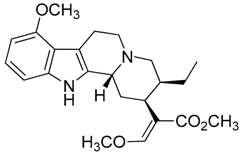 | 398.50 | C23H30N2O4 | Species: M. speciosa (Rubiaceae family) | [41] |
| 39 | Yohimbine·HCl |  | 354.44 390.90 (+HCl) | C21H26N2O3 HCl | Apocynaceae species: Aspidosperma discolor A. DC., Aspidosperma excelsum Benth, Aspidosperma eburneum F. Allem, Aspidosperma marcgravianum Woodson, Aspidosperma oblongum A. DC. | [37] |
| Inhibition Zone Assay | Diameter of Inhibition Zone (mm) 1 | |
|---|---|---|
| Compound | Gram-Positive Staphylococcus aureus ATCC 25923 | Gram-Negative Escherichia coli ATCC 25922 |
| Dihydroberberine·HCl (1) | 7.800 | n.a. |
| (S)-Glaucine (7) | 7.600 | n.a. |
| Canthin-6-one (28) | 6.100 | n.a. |
| Harmane (30) | 4.360 | 8.640 |
| Harmine (32) | n.a. | 6.250 |
| Mytragine (34) | 5.420 | n.a. |
| Nigritanine (35) | 10.39 | n.a. |
| Paynantheine (36) | 8.440 | n.a. |
| Speciociliatine (38) | 8.240 | n.a. |
| Inhibition Zone Assay | Diameter of Inhibition Zone (mm) 1 | |||
|---|---|---|---|---|
| Compound | S. aureus ATCC 25923 | S. aureus 1a | S. aureus 1b | S. aureus 1c |
| Mytragine (34) | 5.420 | 4.000 | n.a. | n.a. |
| Nigritanine (35) | 10.39 | 11.20 | 8.440 | 9.100 |
| Paynantheine (36) | 8.440 | 3.800 | n.a. | n.a. |
| Speciociliatine (38) | 8.240 | 4.520 | 4.340 | 4.580 |
| Strains | Nigritanine | Speciociliatine | Mytragine | Paynantheine | Rhyncophylline |
|---|---|---|---|---|---|
| S. aureus ATCC 25923 | 128 μM | > 256 μM | > 256 μM | > 256 μM | > 256 μM |
| S. aureus 1a | 128 μM | > 256 μM | > 256 μM | > 256 μM | > 256 μM |
| S. aureus 1b | 128 μM | > 256 μM | > 256 μM | > 256 μM | > 256 μM |
| S. aureus 1c | 128 μM | > 256 μM | > 256 μM | > 256 μM | > 256 μM |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casciaro, B.; Calcaterra, A.; Cappiello, F.; Mori, M.; Loffredo, M.R.; Ghirga, F.; Mangoni, M.L.; Botta, B.; Quaglio, D. Nigritanine as a New Potential Antimicrobial Alkaloid for the Treatment of Staphylococcus aureus-Induced Infections. Toxins 2019, 11, 511. https://doi.org/10.3390/toxins11090511
Casciaro B, Calcaterra A, Cappiello F, Mori M, Loffredo MR, Ghirga F, Mangoni ML, Botta B, Quaglio D. Nigritanine as a New Potential Antimicrobial Alkaloid for the Treatment of Staphylococcus aureus-Induced Infections. Toxins. 2019; 11(9):511. https://doi.org/10.3390/toxins11090511
Chicago/Turabian StyleCasciaro, Bruno, Andrea Calcaterra, Floriana Cappiello, Mattia Mori, Maria Rosa Loffredo, Francesca Ghirga, Maria Luisa Mangoni, Bruno Botta, and Deborah Quaglio. 2019. "Nigritanine as a New Potential Antimicrobial Alkaloid for the Treatment of Staphylococcus aureus-Induced Infections" Toxins 11, no. 9: 511. https://doi.org/10.3390/toxins11090511
APA StyleCasciaro, B., Calcaterra, A., Cappiello, F., Mori, M., Loffredo, M. R., Ghirga, F., Mangoni, M. L., Botta, B., & Quaglio, D. (2019). Nigritanine as a New Potential Antimicrobial Alkaloid for the Treatment of Staphylococcus aureus-Induced Infections. Toxins, 11(9), 511. https://doi.org/10.3390/toxins11090511