Botulinum Neurotoxin A Intravesical Injections in Interstitial Cystitis/Bladder Painful Syndrome: A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Results
2.1. Outcomes
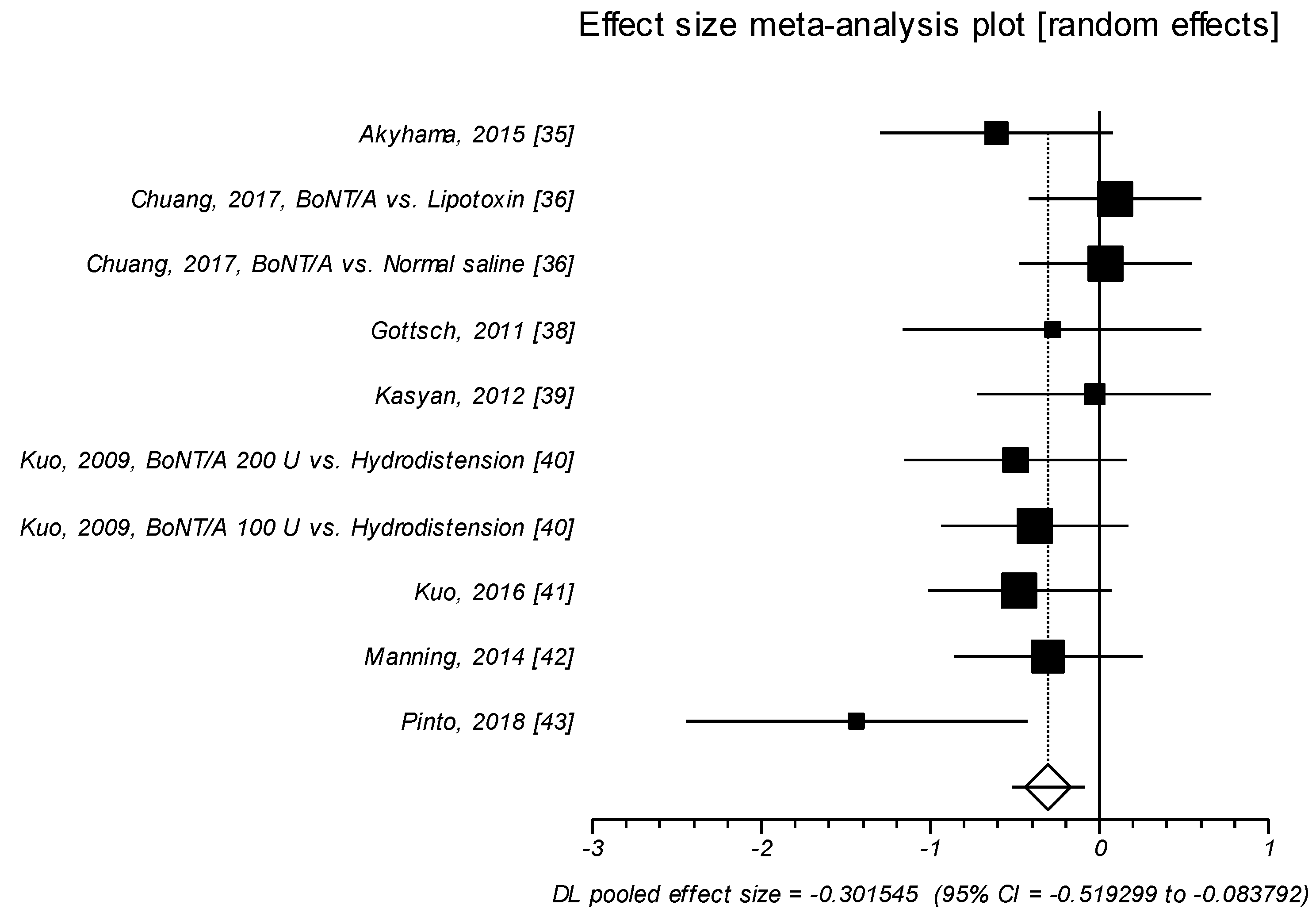
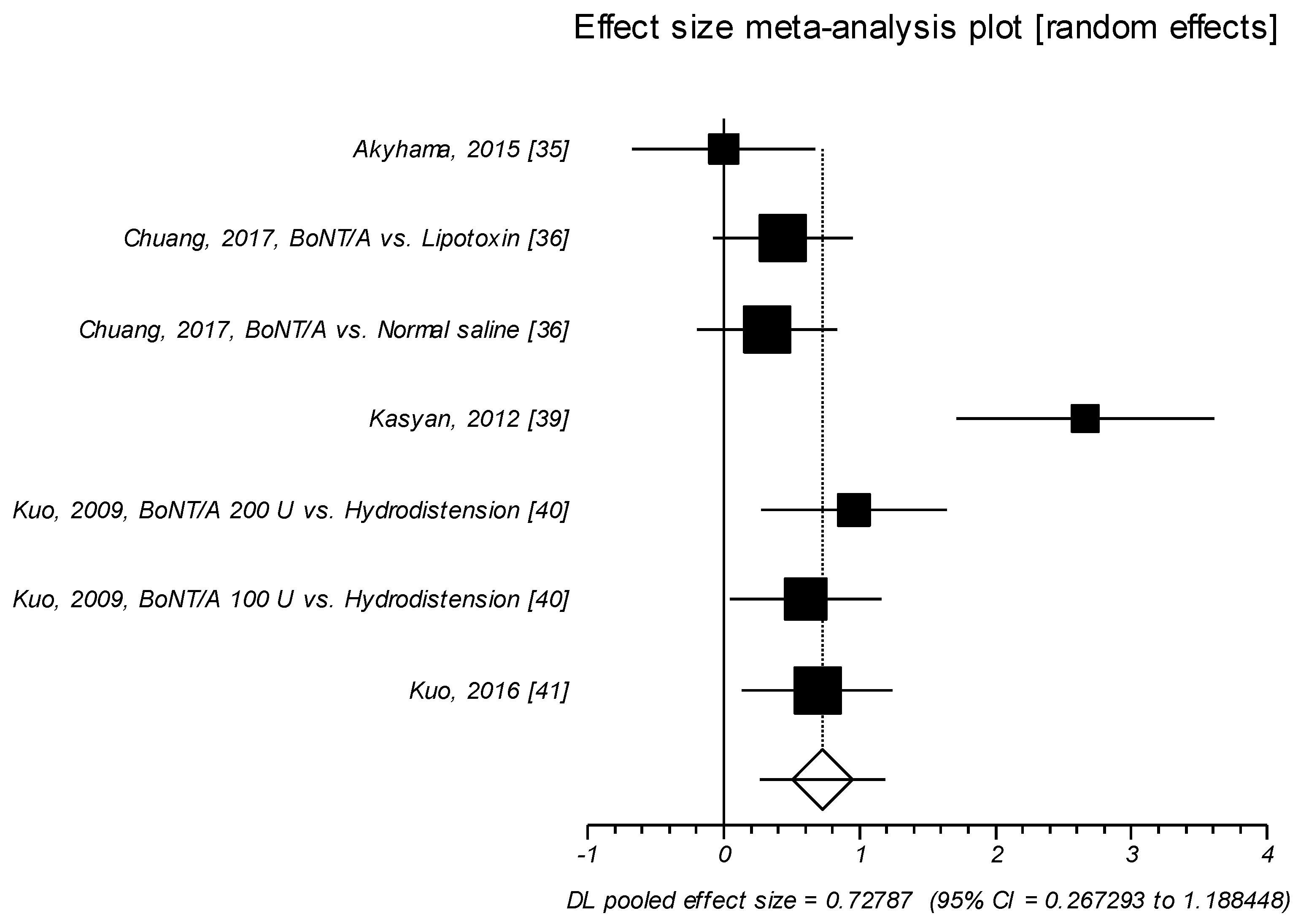
2.1.1. Effect Size of Standardized Mean Difference on Interstitial Cystitis Symptom Index
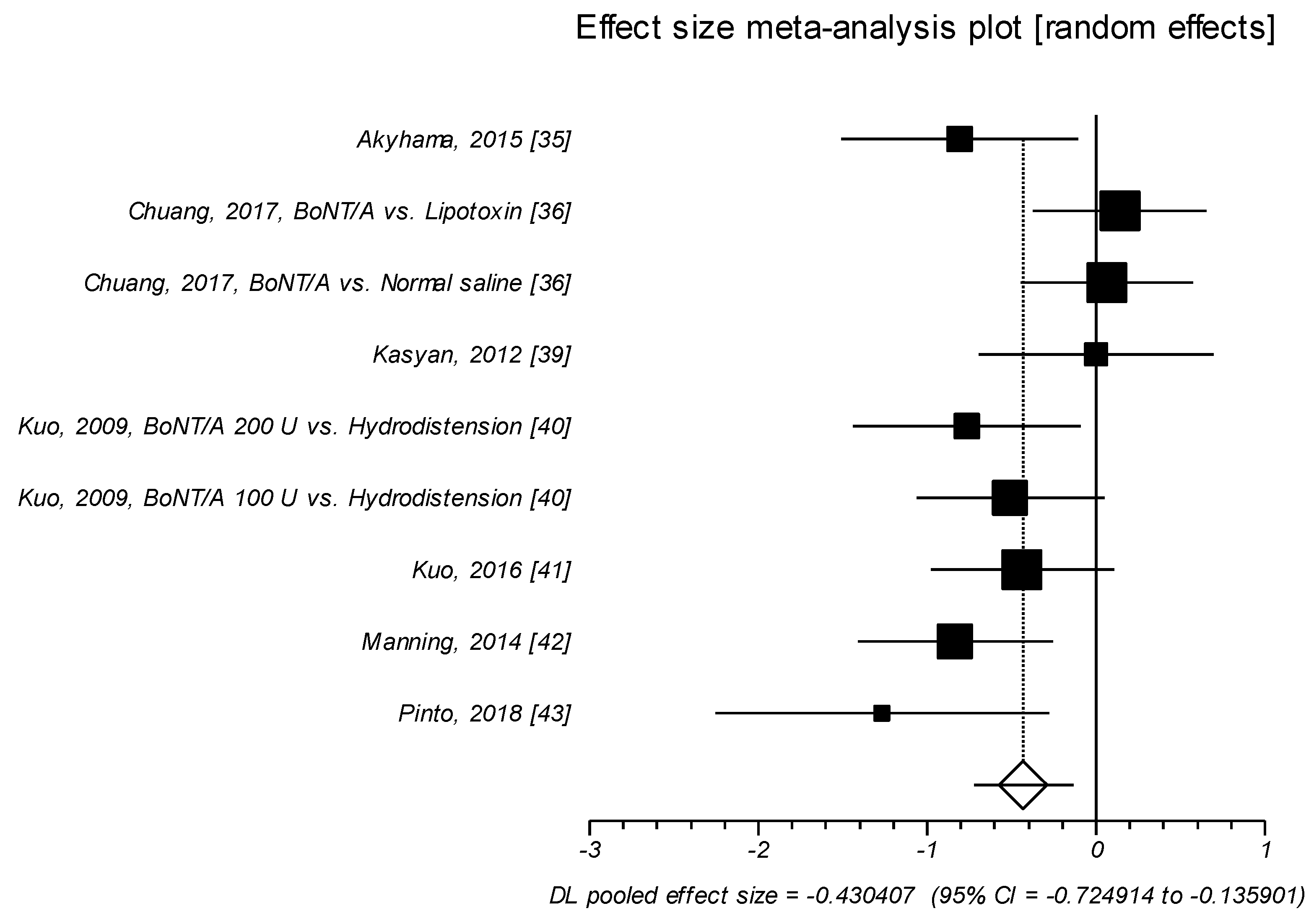
2.1.2. Effect Size of Standardized Mean Difference on Interstitial Cystitis Problem Index
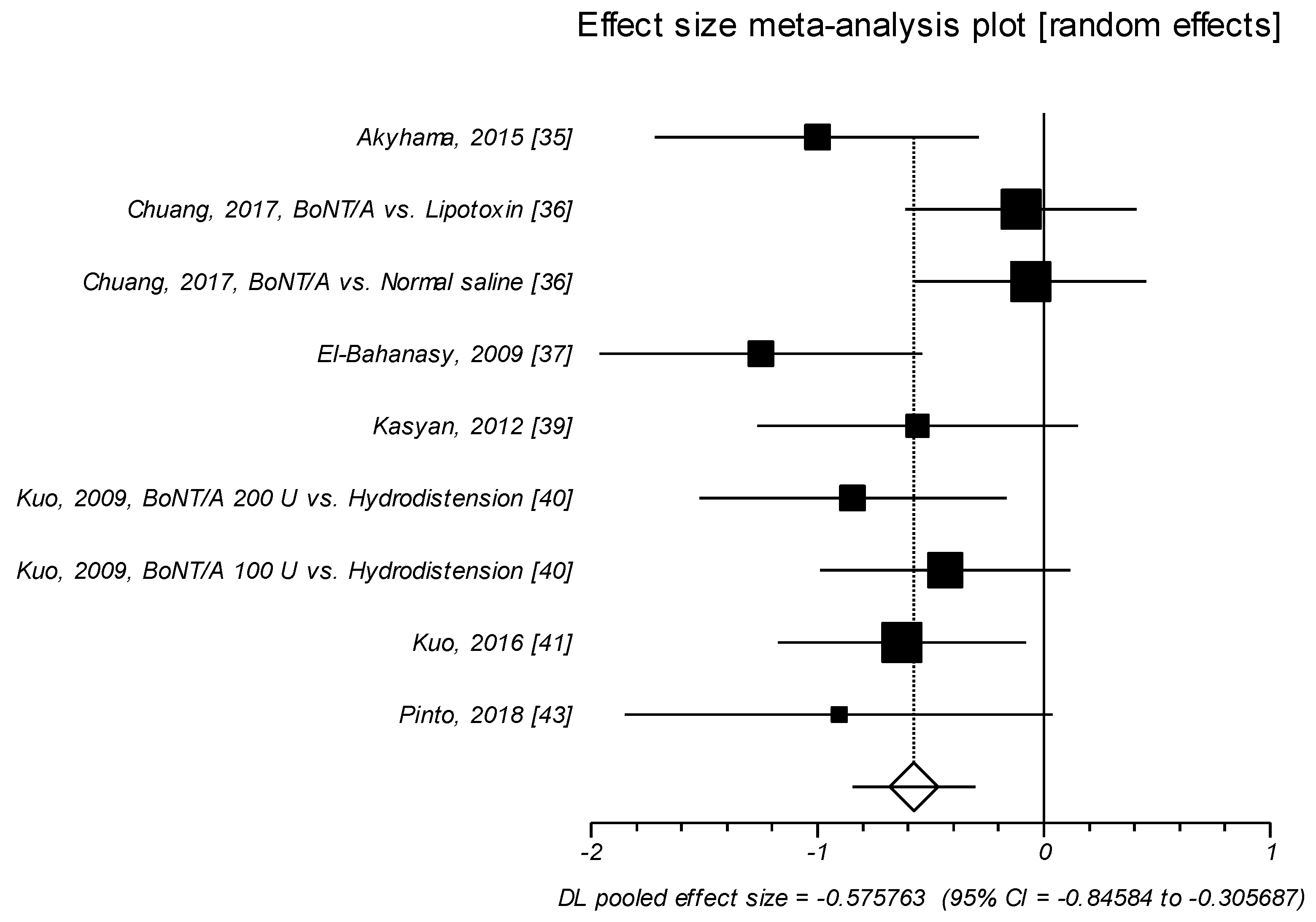
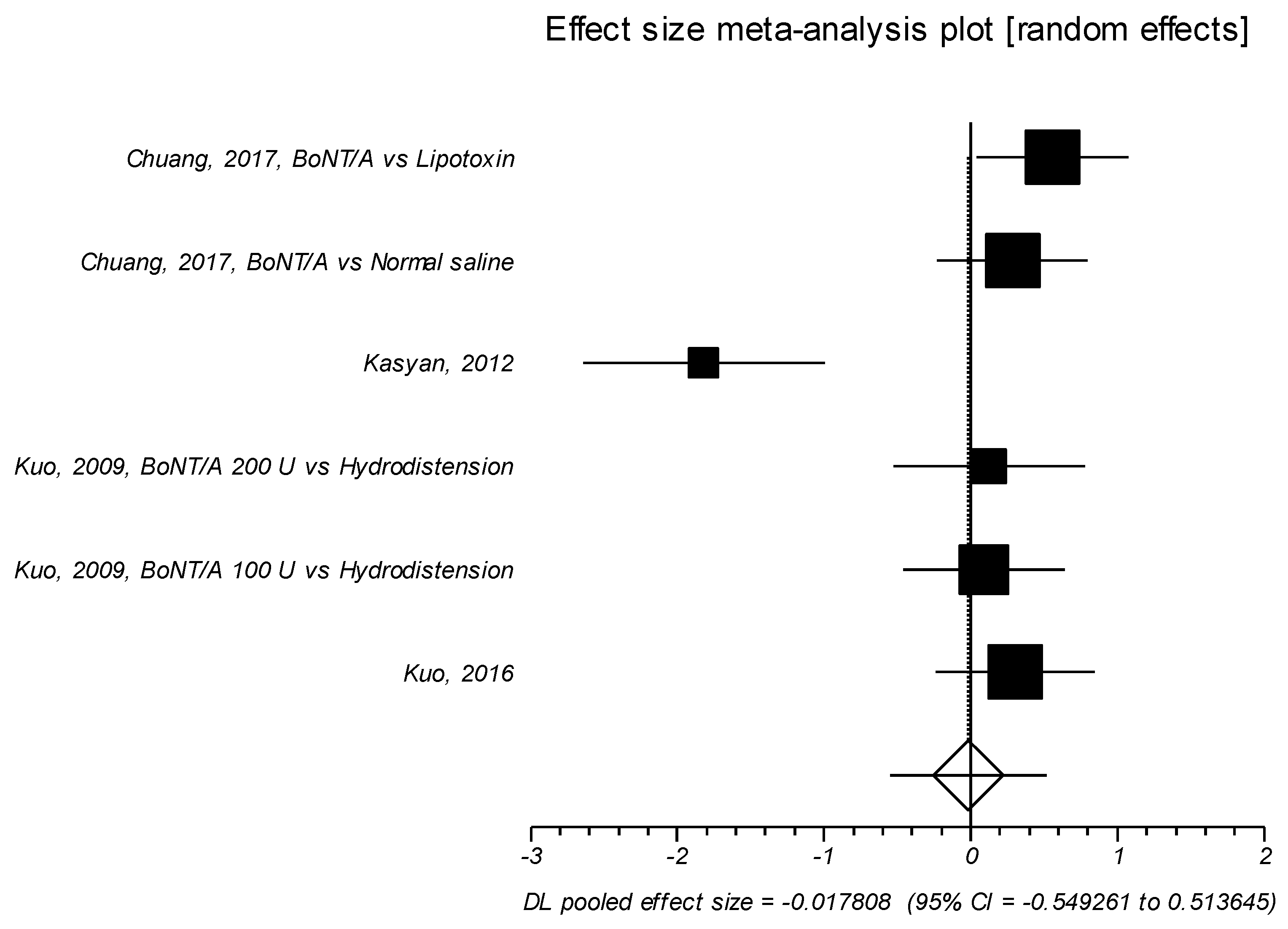
2.1.3. Effect Size of Standardized Mean Difference on Visual Analog Scale or Likert Scale
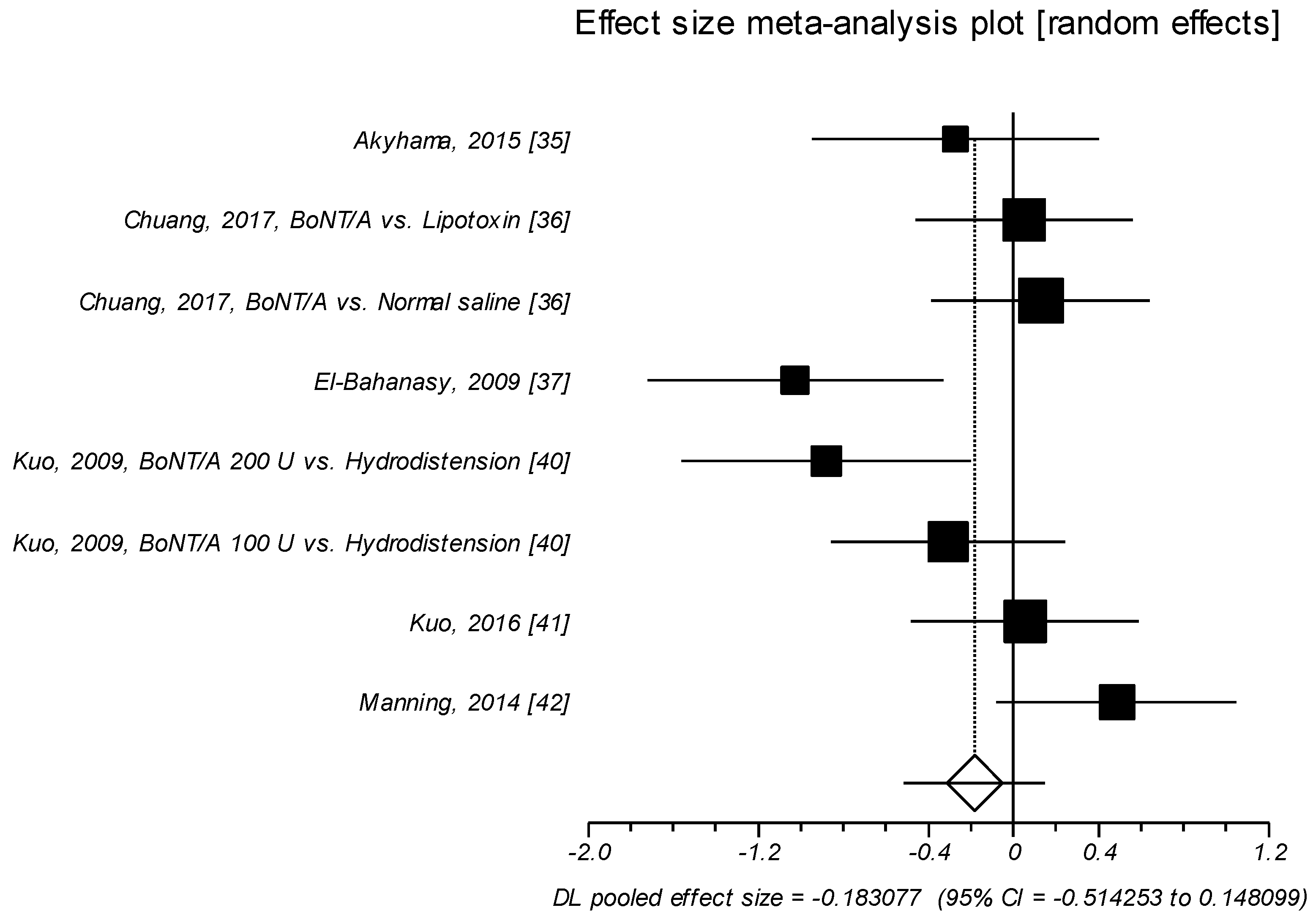
2.1.4. Effect Size of Standardized Mean Difference on Daytime Urinary Frequency
2.1.5. Effect Size of Standardized Mean Difference on Nocturia
2.1.6. Effect Size of Standardized Mean Difference on Functional Bladder Capacity
2.1.7. Effect Size of Standardized Mean Difference on Maximum Flow Rate
2.1.8. Effect Size of Standardized Mean Difference on Post Void Urinary Residual Volume (PVR)
2.2. Side Effects
3. Discussion
4. Materials and Methods
4.1. Literature Search
4.2. Inclusion and Exclusion Criteria
4.3. Assessment of Results
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doggweiler, R.; Whitmore, K.E.; Meijlink, J.M.; Drake, M.J.; Frawley, H.; Nordling, J.; Hanno, P.; Fraser, M.O.; Homma, Y.; Garrido, G. A standard for terminology in chronic pelvic pain syndromes: Areport from the chronic pelvic pain working group of the international continence society. Neurourol. Urodyn. 2017, 36, 984–1008. [Google Scholar] [CrossRef]
- van de Merwe, J.P.; Nordling, J.; Bouchelouche, K.; Cervigni, M.; Daha, L.K.; Elneil, S.; Fall, M.; Hohlbrugger, G.; Irwin, P.; Mortensen, S. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: An ESSIC proposal. Eur. Urol. 2008, 53, 60–67. [Google Scholar] [CrossRef]
- Rovner, E.; Propert, K.J.; Bresinger, C.; Wein, A.J.; Foy, M.; Kirkemo, A.; Landis, J.M.; Kusek, J.W.; Nyberg, L.M. Treatment used in women with interstitial cystitis: The interstitial cystitis data base (ICDB) study experience. The Interstitial Cystitis Data Base Study Group. Urology 2000, 56, 940–945. [Google Scholar] [CrossRef]
- Clemens, J.Q.; Mullins, C.; Ackerman, A.L.; Bavendam, T.; van Bokhoven, A.; Ellingson, B.M.; Harte, S.E.; Kutch, J.J.; Lai, H.H.; Martucci, K.T.; et al. MAPP Research Network Study Group. Urologic chronic pelvic pain syndrome: Insights from the MAPP Research Network. Nat. Rev. Urol 2019, 16, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Pontari, M.A. Chronic prostatitis/chronic pelvic pain syndrome and interstitial cystitis: Are they related? Curr. Urol. Rep. 2006, 4, 141–146. [Google Scholar] [CrossRef]
- Simpson, D.M.; Justin, C.M.; Robert, H.D. (Eds.) Peripheral and central mechanisms of neuropathic pain. In Neuropathic Pain –Mechanisms, Diagnosis and Treatment; Oxford Publisher: Oxford, UK, 2012; pp. 14–24. [Google Scholar]
- Aoki, R.K.; Francis, J. Update on the nociceptive mechanism hypothesis and related disorders. Park. Relat. Disord. 2011, 17, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Jhang, J.F.; Kuo, H.C. Novel Treatment of Chronic Bladder Pain Syndrome and Other Pelvic Pain Disorders by OnabotulinumtoxinA Injection. Toxins 2015, 7, 2232–2250. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.D.; Kuo, Y.C.; Kuo, H.C. Intravesical onabotulinumtoxinA injections for refractory painful bladder syndrome. Pain Physician 2012, 15, 197–202. [Google Scholar]
- Chermansky, C.J.; Chancellor, M.B. Use of Botulinum Toxin in Urologic Diseases. Urology 2016, 91, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.M.; Chung, M.E. Botulinum toxin for neuropathic pain: A review of the literature. Toxins 2015, 7, 3127–3154. [Google Scholar] [CrossRef] [PubMed]
- Pellett, S.; Yaksh, T.; Ramachandran, R. Current status and future directions of botulinum neurotoxins for targeting pain processing. Toxins 2015, 7, 4519–4563. [Google Scholar] [CrossRef] [PubMed]
- Courseau, M.; Salle, P.V.; Ranoux, D.; de Pouilly Lachatre, A. Efficacy of Intra-Articular Botulinum Toxin in Osteoarticular Joint Pain: A Meta-Analysis of Randomized Controlled Trials. Clin. J. Pain 2018, 34, 383–389. [Google Scholar] [PubMed]
- Luvisetto, S.; Gazerani, P.; Cianchetti, C.; Pavone, F. Botulinum Toxin Type a as a Therapeutic Agent against Headache and Related Disorders. Toxins 2015, 7, 3818–3844. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.; Kumar, A.; Jabbari, B. Abobotulinum Toxin A in the Treatment of Chronic Low Back Pain. Toxins 2016, 8, 374. [Google Scholar] [CrossRef]
- Rivera Día, R.C.; Lotero, M.A.A.; Suarez, M.V.A.; Saldarriaga, S.E.; Martínez, M.G. Botulinum toxin for the treatment of chronic pain. Review of the evidence. Colomb. J. Anesth. 2014, 42, 205–213. [Google Scholar] [CrossRef]
- Lew, M.F. Review of the FDA-approved uses of botulinum toxins, including data suggesting efficacy in pain reduction. Clin. J. Pain 2002, 18, S142–S146. [Google Scholar] [CrossRef] [PubMed]
- Lovati, C.; Giani, L. Action mechanisms of Onabotulinum toxin-A: Hints for selection of eligible patients. Neurol. Sci. 2017, 38, 131–140. [Google Scholar] [CrossRef]
- Chiu, B.; Tai, H.C.; Chung, S.D.; Birder, L.A. Botulinum Toxin A for Bladder Pain Syndrome/Interstitial Cystitis. Toxins 2016, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Willett, O.; Thompkins, T.; Hermann, R.; Ramanathan, S.; Cornett, E.M.; Fox, C.J.; Kaye, A.D. Botulinum Toxin: Pharmacology and Therapeutic Roles in Pain States. Curr. Pain Headache Rep. 2016, 20, 15. [Google Scholar] [CrossRef]
- Antonucci, F.; Rossi, C.; Gianfranceschi, L.; Rossetto, O.; Caleo, M. Long-distance retrograde effects of botulinum neurotoxin A. J. Neurosci. 2008, 28, 3689–3696. [Google Scholar] [CrossRef] [PubMed]
- Bomba-Warczak, E.; Vevea, J.D.; Brittain, J.M.; Figueroa-Bernier, A.; Tepp, W.H.; Johnson, E.A.; Yeh, F.L.; Chapman, E.R. Interneuronal Transfer and Distal Action of Tetanus Toxin and Botulinum Neurotoxins A and D in Central Neurons. Cell Rep. 2016, 16, 1974–1987. [Google Scholar] [CrossRef]
- Caleo, M.; Restani, L. Direct central nervous system effects of botulinum neurotoxin. Toxicon 2018, 147, 68–72. [Google Scholar] [CrossRef]
- Marinelli, S.; Vacca, V.; Ricordy, R.; Uggenti, C.; Tata, A.M.; Luvisetto, S.; Pavone, F. The analgesic effect on neuropathic pain of retrogradely transported botulinum neurotoxin A involves Schwann cells and astrocytes. PLoS ONE 2012, 7, e47977. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.J.; Purkiss, J.R.; Foster, K.A. Sensitivity of embryonic rat dorsal root ganglia neurons to Clostridium botulinum neurotoxins. Toxicon 2000, 38, 245–258. [Google Scholar] [CrossRef]
- Durham, P.L.; Cady, R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: Implications for migraine therapy. Headache 2004, 44, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Cheng, J.; Zhuang, Y.; Qu, W.; Muir, J.; Liang, H.; Zhang, D. Botulinum toxin type A reduces hyperalgesia and TRPV1 expression in rats with neuropathic pain. Pain Med. 2013, 14, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Yoshimura, N.; Huang, C.C.; Wu, M.; Chiang, P.H.; Chancellor, M.B. Intraprostatic botulinum toxin A injection inhibits cyclooxygenase-2 expression and suppresses prostatic pain on capsaicin induced prostatitis model in rat. J. Urol. 2008, 180, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Kuo, H.C. Intravesical botulinum toxin A injections plus hydrodistension can reduce nerve growth factor production and control bladder pain in interstitial cystitis. Urology 2007, 70, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Q.; Wu, Q.; Chen, Y.; Wu, P. Intravesical botulinum toxin A injections for bladder pain syndrome/interstitial cystitis: A systematic review and meta-analysis of controlled studies. Med. Sci Monit 2016, 22, 3257–3267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shim, S.R.; Cho, Y.J.; Shin, I.S.; Kim, J.H. Efficacy and safety of botulinum toxin injection for interstitial cystitis/bladder pain syndrome: A systematic review and meta-analysis. Int. Urol. Nephrol. 2016, 48, 1215–1227. [Google Scholar] [CrossRef]
- Zhang, W.; Deng, X.; Liu, C.; Wang, X. Intravesical treatment for interstitial cystitis/painful bladder syndrome: A network meta-analysis. Int. Urogynecol. J. 2017, 28, 515–525. [Google Scholar] [PubMed]
- Tirumuru, S.; A.-Kurdi, D.; Latthe, P. Intravesical botulinum toxin A injections in the treatment of painful bladder syndrome/interstitial cystitis: A systematic review. Int. Urogynecol. J. 2010, 21, 1285–1300. [Google Scholar] [PubMed]
- O’Leary, M.P.; Sant, G.R.; Fowler, F.J., Jr.; Whitmore, K.E.; Spolarich-Kroll, J. The interstitial cystitis symptom index and problem index. Urology 1997, 49, 58–63. [Google Scholar] [PubMed]
- Akiyama, Y.; Nomiya, A.; Niimi, A.; Yamada, Y.; Fujimura, T.; Nakagawa, T.; Fukuhara, H.; Kume, H.; Igawa, Y.; Homma, Y.; et al. Botulinum toxin type A injection for refractory interstitial cystitis: A randomized comparative study and predictors of treatment response. Urology 2015, 22, 835–841. [Google Scholar]
- Chuang, Y.C.; Kuo, H.C. A prospective, multicenter, double-blinded, randomized trial of bladder instillation of liposome formulation onabotulinumtoxin A for interstitial cystitis/bladder pain syndrome. J. Urol. 2017, 198, 376–382. [Google Scholar] [CrossRef]
- El-Bahnasy, A.; Farahat, Y.; El-Bendary, M.; Taha, M.R.; El-Damhogy, M.; Mourad, S. A randomized controlled trial of bacillus Calmette-Guerin and botulinum toxin A for the treatment of refractory interstitial cystitis. UroToday Int. J. 2009, 2. [Google Scholar] [CrossRef]
- Gottsch, H.P.; Miller, J.L.; Yang, C.C.; Berger, R.E. A Pilot study of botulinum toxin for interstitial cystitis/painful bladder syndrome. Neurourol. Urodyn. 2011, 30, 93–96. [Google Scholar] [PubMed]
- Kasyan, G.; Pushkar, D. Randomized controlled trial for efficacy of botulinum toxin type A in treatment of patients suffering bladder pain syndrome/interstitial cystitis with Hunners’ Lesions: Preliminary results. J. Urol. 2012, 187, e335–e336. [Google Scholar] [CrossRef]
- Kuo, H.C.; Chancellor, M.B. Comparison of botulinum toxin type A injections plus hydrodistention with hydrodistention alone for the treatment of refractory interstitial cystitis/painful bladder syndrome. BJUI Int. 2009, 104, 657–661. [Google Scholar]
- Kuo, H.C.; Jiang, Y.H.; Tsai, Y.C.; Kuo, Y.C. Intravesical botulinum toxin-A injections reduces bladder pain of interstitial cystitis/bladder pain syndrome refractory to conventional treatment-A prospective, multicenter, randomized, double-blind, placebo-controlled clinical trial. Neurourol. Urodyn. 2016, 35, 609–614. [Google Scholar] [PubMed]
- Manning, J.; Dwyer, P.; Rosamilia, A.; Colyvas, K.; Murray, C.; Fitzgerald, E. A multicenter, prospective, randomized, double-blind study to measure the treatment effectiveness of abobotulinum A (AboBTXA) among women with refractory interstitial cystitis/bladder pain syndrome. Int. Urogynecol. J. 2014, 25, 593–599. [Google Scholar] [PubMed]
- Pinto, R.A.; Costa, D.; Morgado, A.; Pereira, P.; Charrua, A.; Silva, J.; Cruz, F. Intratrigonal onabotulinumtoxinA improves bladder symptoms and quality of life in patients with bladder pain syndrome/interstitial cystitis: A pilot, single center, randomized, double-blind, placebo controlled trial. J. Urol. 2018, 199, 998–1003. [Google Scholar] [PubMed]
- Taha Rasheed, M.; Farahat, A.; Bahanasy, M.; Bindary, A.; Tatawy, H. A prospective, randomized study of intravesical pentosan polysulfate and botulinum toxin-A for the treatment of painful bladder syndrome/Interstitial cystitis. Eur. Urol. Suppl. 2010, 9, 213. [Google Scholar]
- Gillenwater, J.Y.; Wein, A.J. Summary of the National Institute of Arthritis, Diabetes, Digestive and Kidney Diseases Workshop on Interstitial Cystitis, National Institutes of Health, Bethesda, Maryland, August 28–29, 1987. J. Urol. 1988, 140, 203–206. [Google Scholar] [CrossRef]
- Oelke, M.; De Wachter, S.; Drake, M.J.; Giannantoni, A.; Kirby, M.; Orme, S.; Rees, J.; van Kerrebroeck, P.; Everaert, K. A practical approach to the management of nocturia. Int. J. Clin. Pract. 2017, 71, e13027. [Google Scholar]
- Hsieh, P.F.; Chiu, H.C.; Chen, K.C.; Chang, C.H.; Chou, EC. Botulinum toxin A for the Treatment of Overactive Bladder. Toxins 2016, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Giannantoni, A.; Bini, V.; Dmochowski, R.; Hanno, P.; Curtis Nickel, J.; Proietti, S.; Wyndaele, J.J. Contemporary management of the painful bladder: A systematic review. Eur. Urol. 2012, 61, 29–53. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, DG. Measuring inconsistency in meta-analysis. BMJ 2003, 327, 557–560. [Google Scholar]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 13, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Jadad, A.R. Randomised Controlled Trials; Blackwell Publishing: London, UK, 1998. [Google Scholar]


| Author, Year, Treatment | Js | Diagnostic Criteria | Design | Total No. of Pts | Females/ Males | Patients’ Age, (y) Mean ± SD or Mean (min-ax) | Disease Duration (y) Mean ± SD | Active Agent | Control Agent | No. of Pts atBaseline Active Agent /Control | No. of Pts at Follow-Up Active Agent /Control | Follow-up, months |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akyhama, 2015, [35] | 3 | NIDDK | RCT | 34 | 26/8 | 64.9 ± 13.7 | 6.6 ± 4.4 | Onab 100 U i.ve, immediate injection | Onab 100 U i.ve 1-month delayed injection | 18/16 | 18/16 | 1 |
| Chuang, 2017, [36] BoNT/A vs. Lipotoxin | 5 | ESSIC | RCT | 90 | 80/10 | 52.5 ± 4.2 | 7.2 ± 6.0 | Onab 200 U i.ve | Lipotoxin i.ve | 28/31 | 28/31 | 1 |
| Chuang 2017, [36] BoNT/A vs. Normal Saline | 5 | ESSIC | RCT | 90 | 80/10 | 52.5 ± 4.2 | 7.2 ± 6.0 | Onab 200 U i.ve | Normal saline i.ve | 28/31 | 28/31 | 1 |
| Gottsch, 2011, [38] | 3 | Clinical | RCT | 20 | 20/0 | 45.8 (22–62) | 16.5 (2–30) | Onab 50 U peri-urethral | Normal saline i.ve | 9/11 | 9/11 | 3 |
| El-Bahanasy, 2009, [37] | 2 | NIDDK | RCT | 36 | 36/0 | NA | NA | Onab 300 U i.ve | BCG i.ve | 18/18 | 18/16 (at 1 month) | 5.5 vs. 5.75 |
| Kasyan, 2012, [39] | 2 | Clinical, cystoscopic | RCT | 32 | 32/0 | NA | NA | Onab 100 U i.ve | Hydrodistension | 15/17 | 15/17 | 3 |
| Kuo, 2009, [40] BoNT/A 200 U vs. Hydrodistension | 5 | NIDDK | RCT | 38 | 31/7 | 49.1 (26–83) | 8 ± 5 | Onab 200 U i.ve | Hydrodistension | 15/23 | 15/23 | 3 |
| Kuo, 2009, [40] BoNT/A 100 U vs. Hydrodistension | 5 | NIDDK | RCT | 52 | 45/7 | 50.1 (26–83) | 8 ± 5 | Onab 100 U i.ve | Hydrodistension | 29/23 | 29/23 | 3 |
| Kuo, 2016, [41] | 5 | NIDDK | RCT | 60 | 52/8 | 51.5 (20–82) | 5 ± 2.8 | Onab 100 U i.ve | Normal saline i.ve | 40/20 | 40/20 | 2 |
| Manning, 2014, [42] | 3 | NIDDK | RCT | 50 | 50/0 | 53.5 | 13.5 ± 6.75 | Abob 500 U i.ve + hydrodistension | Normal saline i.ve + hydrodistension | 25/25 | 25/25 | 3 |
| Pinto, 2018, [43] | 5 | ESSIC | RCT | 19 | 19/0 | 45.8 ± 10.5 | NA | Onab 100 U i.ve | Normal saline i.ve | 11/10 | 10/9 | 3 |
| Taha-Rasheed, 2010, [44] | 2 | NIDDK | RCT | 28 | 28/0 | NA | NA | BoNT/A 300 U i.ve | PPS | 14/14 | 14/14 | 4.75 vs. 5.25 |
| ICSI | Active Agent, Baseline | Control Agent, Baseline | Active Agent, f-up | Control Agent, f-up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study, Treatment | Mean | SD | No. of pts | Mean | SD | No. of pts | Mean | SD | No. of pts | Mean | SD | No. of pts |
| Akyhama, 2015, [35] | 14.2 | 3.6 | 18 | 12.6 | 4.3 | 16 | −3.1 * | 3.9 | 18 | −0.8 * | 3.4 | 16 |
| Chuang, 2017, BoNT/A vs. Lipotoxin [36] | 11.9 | 3.93 | 28 | 12.4 | 4.12 | 31 | 8.29 | 3.68 | 28 | 8.42 | 4.35 | 31 |
| Chuang, 2017, BoNT/A vs. Normal saline [36] | 11.9 | 3.93 | 28 | 11.2 | 3.4 | 31 | 8.29 | 3.68 | 28 | 7.45 | 3.66 | 31 |
| Gottsch, 2011, [38] | 35.2 | 3.9 | 9 | 29.6 | 8 | 11 | 31.3 | 7.5 | 9 | 27.7 | 7.3 | 11 |
| Kasyan, 2012, [39] | 14.5 | 2.3 | 15 | 13.8 | 3.7 | 17 | 9.4 | 2.9 | 15 | 8.8 | 3.3 | 17 |
| Kuo, 2009, BoNT/A 200 U vs. Hydrodistension [40] | 13.9 | 2.53 | 15 | 12.8 | 3.41 | 23 | 8.9 | 5.58 | 15 | 9.87 | 4.85 | 23 |
| Kuo, 2009, BoNT/A 100 U vs. Hydrodistension [40] | 12.5 | 2.15 | 29 | 12.8 | 3.41 | 23 | 8.17 | 4.06 | 29 | 9.87 | 4.85 | 23 |
| Kuo, 2016, [41] | 13.3 | 3.8 | 40 | 12.3 | 3.9 | 20 | 8.8 | 4.2 | 40 | 9.8 | 5.1 | 20 |
| Manning, 2014, [42] | 13.2 | 2.6 | 26 | 13.9 | 2.8 | 27 | 10.5 | 4.4 | 25 | 12.3 | 4.5 | 25 |
| Pinto, 2018, [43] | 15.7 | 3.3 | 11 | 13.6 | 2.3 | 10 | −9 * | 4.7 | 10 | −2 * | 4.6 | 9 |
| ICPI | Active Agent, Baseline | Control Agent, Baseline | Active Agent, f-up | Control Agent, f-up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study, Treatment | Mean | SD | No. of pts | Mean | SD | No. of pts | Mean | SD | No. of pts | Mean | SD | No. of pts |
| Akyhama, 2015 [35] | 12.1 | 2.5 | 18 | 10.6 | 3.2 | 16 | −2.9 * | 3.6 | 18 | −0.1 * | 3.1 | 16 |
| Chuang, 2017, BoNT/A vs. Lipotoxin [36] | 11.4 | 3.92 | 28 | 11.8 | 3.9 | 31 | 8.64 | 4.3 | 28 | 8.42 | 5.44 | 31 |
| Chuang, 2017, BoNT/A vs. Normal saline [36] | 11.4 | 3.92 | 28 | 11 | 4.14 | 31 | 8.64 | 4.3 | 28 | 7.97 | 4.42 | 31 |
| Kasyan, 2012, [39] | 12.4 | 2.4 | 15 | 11.9 | 3.1 | 17 | 7.3 | 2.1 | 15 | 6.8 | 2.5 | 17 |
| Kuo, 2009, BoNT/A 200 U vs. Hydrodistension [40] | 12.3 | 1.4 | 15 | 11.1 | 2.6 | 23 | 7.13 | 4.52 | 15 | 8.57 | 4.59 | 23 |
| Kuo, 2009, BoNT/A 100 U vs. Hydrodistension [40] | 11.1 | 2.05 | 29 | 11.1 | 2.6 | 23 | 6.93 | 3.58 | 29 | 8.57 | 4.59 | 23 |
| Kuo, 2016, [41] | 12.6 | 3 | 40 | 11.7 | 3.8 | 20 | 7.6 | 4.2 | 40 | 8.4 | 4.8 | 20 |
| Manning, 2014, [42] | 13.6 | 2.54 | 25 | 13.7 | 2.66 | 25 | 9.9 | 4 | 25 | 12.8 | 4 | 25 |
| Pinto, 2018, [43] | 13.3 | 1.5 | 10 | 12.2 | 2.2 | 9 | −7.1 * | 4.6 | 10 | −1 * | 4.6 | 9 |
| VAS/Likert Scale | Active Agent, Baseline | Control Agent, Baseline | Active Agent, f-up | Control Agent, f-up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study, Treatment | Mean | SD | No. of pts | Mean | SD | No. of pts | Mean | SD | No. of pts | Mean | SD | No. of pts |
| Akyhama, 2015 [35] | 7.3 | 1.7 | 18 | 6.3 | 2.5 | 16 | −2.2 * | 2 | 18 | −0.1 * | 2.1 | 16 |
| Chuang, 2017, BoNT/A vs. Lipotoxin [36] | 4.5 | 3.22 | 28 | 4.84 | 2.34 | 31 | 2.57 | 2.54 | 28 | 3.19 | 2.71 | 31 |
| Chuang, 2017, BoNT/A vs. Normal saline [36] | 4.5 | 3.22 | 28 | 4.32 | 2.65 | 31 | 2.57 | 2.54 | 28 | 2.55 | 2.23 | 31 |
| El-Bahanasy, 2009 [37] | 5.8 | 1.39 | 18 | 5.4 | 1.23 | 18 | 0.22 | 0.43 | 18 | 1.06 | 0.77 | 18 |
| Kasyan, 2012 [39] | 9.3 | 0.9 | 15 | 8.7 | 1.2 | 17 | 5.8 | 2.4 | 15 | 6.1 | 1.8 | 17 |
| Kuo, 2009, BoNT/A 200 U vs. Hydrodistension [40] | 5.47 | 2.1 | 15 | 4.3 | 2.6 | 23 | 2.47 | 2.1 | 15 | 3.52 | 3.07 | 23 |
| Kuo, 2009, BoNT/A 100 U vs. Hydrodistension [40] | 4.83 | 2.21 | 29 | 4.3 | 2.6 | 23 | 2.97 | 1.99 | 29 | 3.52 | 3.07 | 23 |
| Kuo, 2016 [41] | 5.3 | 2.6 | 40 | 3.7 | 2.9 | 20 | 2.7 | 2.7 | 40 | 2.8 | 2.5 | 20 |
| Pinto, 2018 [43] | 6.8 | 1.2 | 11 | 6.8 | 0.8 | 10 | −3.8 * | 2.5 | 10 | −1.6 * | 2.1 | 9 |
| Daytime Urinary Frequency | Active Agent, Baseline | Control Agent, Baseline | Active Agent, f-up | Control Agent, f-up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study, Treatment | Mean | SD | No. of pts | Mean | SD | No. of pts | Mean | SD | No. of pts | Mean | SD | No. of pts |
| Akyhama, 2015 [35] | 18.6 | 8 | 18 | 22.6 | 13.1 | 16 | −2.9 * | 5.1 | 18 | −1 * | 2.5 | 16 |
| Chuang, 2017, BoNT/A vs. Lipotoxin [36] | 12.8 | 5.2 | 28 | 14.3 | 7.09 | 31 | 11.5 | 4.82 | 28 | 13.5 | 7.77 | 31 |
| Chuang, 2017, BoNT/A vs. Normal saline [36] | 12.8 | 5.2 | 28 | 12.9 | 6.6 | 31 | 11.5 | 4.82 | 28 | 11.5 | 4.82 | 31 |
| El-Bahanasy, 2009 [37] | 16.8 | 2.6 | 18 | 16.7 | 3.2 | 18 | 5.3 | 1.14 | 18 | 11.5 | 2.34 | 18 |
| Kuo, 2009, BoNT/A 200 U vs. Hydrodistension [40] | 14.2 | 5.44 | 15 | 11.6 | 4.36 | 23 | 9.4 | 3.22 | 15 | 9.96 | 3.97 | 23 |
| Kuo, 2009, BoNT/A 100 U vs. Hydrodistension [40] | 13 | 4.69 | 29 | 11.6 | 4.36 | 23 | 9.72 | 4.03 | 29 | 9.96 | 3.97 | 23 |
| Kuo, 2016 [41] | 14.3 | 6 | 40 | 13.7 | 9.1 | 20 | 10.5 | 5.1 | 40 | 12.4 | 9.6 | 20 |
| Manning, 2014 [42] | 13.5 | 7.1 | 25 | 12.5 | 5.4 | 25 | 10.4 | 5.8 | 25 | 11.4 | 4.4 | 25 |
| Nocturia | Active Agent, Baseline | Control Agent, Baseline | Active Agent, f-up | Control Agent, f-up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study, Treatment | Mean | SD | No. of pts | Mean | SD | No. of pts | Mean | SD | No. of pts | Mean | SD | No. of pts |
| Akyhama, 2015 [35] | 4.2 | 3.1 | 18 | 5.1 | 4.8 | 16 | −0.6 * | 2.4 | 18 | −0.1 * | 0.6 | 16 |
| Chuang, 2017, BoNT/A vs. Lipotoxin [36] | 2.96 | 1.48 | 28 | 3.46 | 2.35 | 31 | 2.74 | 1.58 | 28 | 3.13 | 3.04 | 31 |
| Chuang, 2017, BoNT/A vs. Normal saline [36] | 2.96 | 1.48 | 28 | 3.15 | 1.65 | 31 | 2.74 | 1.58 | 28 | 2.71 | 2.02 | 31 |
| El-Bahanasy, 2009 [37] | 6.3 | 1.8 | 18 | 6.06 | 6.06 | 18 | 0.28 | 0.48 | 18 | 2.78 | 1.08 | 18 |
| Kuo, 2009, BoNT/A 200 U vs. Hydrodistension [40] | 6.33 | 6.96 | 15 | 3.7 | 2.03 | 23 | 3.13 | 2.47 | 15 | 3.52 | 2.15 | 23 |
| Kuo, 2009, BoNT/A 100 U vs. Hydrodistension [40] | 3.41 | 2.16 | 29 | 3.7 | 2.03 | 23 | 2.59 | 1.97 | 29 | 3.52 | 2.15 | 23 |
| Kuo, 2016 [41] | 3.5 | 1.3 | 40 | 4.3 | 2.6 | 20 | 2.8 | 1.3 | 40 | 3.5 | 2.3 | 20 |
| Manning, 2014 [42] | 3.2 | 1.6 | 25 | 3.2 | 2.6 | 25 | 3.3 | 2.2 | 25 | 2.3 | 1.7 | 25 |
| Bladder Capacity | Active Agent, Baseline | Control Agent, Baseline | Active Agent, f-up | Control Agent, f-up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study, Treatment | Mean | SD | No. of pts | Mean | SD | No. of pts | Mean | SD | No. of pts | Mean | SD | No. of pts |
| Akyhama, 2015 [35] | 201.9 | 131.6 | 18 | 145.3 | 73.3 | 16 | 35 * | 78.5 | 18 | −10 * | 43.4 | 16 |
| Chuang, 2017, BoNT/A vs. Lipotoxin [36] | 309 | 144 | 28 | 262 | 114 | 31 | 315 | 118 | 28 | 307 | 110 | 31 |
| Chuang, 2017, BoNT/A vs. Normal saline [36] | 309 | 144 | 28 | 298 | 134 | 31 | 315 | 118 | 28 | 332 | 169 | 31 |
| Kuo, 2009, BoNT/A 200 U vs. Hydrodistension [40] | 113.9 | 58 | 15 | 134 | 72.4 | 23 | 190.8 | 80.6 | 15 | 145.5 | 77.4 | 23 |
| Kuo, 2009, BoNT/A 100 U vs. Hydrodistension [40] | 161 | 97.4 | 29 | 134 | 72.4 | 23 | 189 | 78.8 | 29 | 145.5 | 77.4 | 23 |
| Kuo, 2016 [41] | 158.1 | 97.7 | 40 | 127.5 | 57.3 | 20 | 219.6 | 103.6 | 40 | 189 | 99.4 | 20 |
| Manning, 2014 [42] | 242 | 166 | 25 | 233 | 96 | 25 | 273 | 152 | 25 | 210 | 84 | 25 |
| Maximum Flow Rate | Active Agent, Baseline | Control Agent, Baseline | active Agent, f-up | Control Agent, f-up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study, Treatment | Mean | SD | No. of pts | Mean | SD | No. of pts | Mean | SD | No. of pts | Mean | SD | No. of pts |
| Chuang, 2017, BoNT/A vs. Lipotoxin [36] | 13.7 | 7.6 | 28 | 14.4 | 8.07 | 31 | 21.2 | 9.31 | 28 | 17.1 | 8.97 | 31 |
| Chuang, 2017, BoNT/A vs. Normal saline [36] | 13.7 | 7.6 | 28 | 14.7 | 6.86 | 31 | 21.2 | 9.31 | 28 | 19.7 | 10.6 | 31 |
| Kasyan, 2012 [39] | 24.2 | 4.6 | 15 | 21.9 | 3.8 | 17 | 14.6 | 13.1 | 15 | 26.9 | 9.8 | 17 |
| Kuo, 2009, BoNT/A 200 U vs. Hydrodistension [40] | 10.2 | 6.48 | 15 | 13.1 | 5.95 | 23 | 11.5 | 7.26 | 15 | 13.6 | 5.62 | 23 |
| Kuo, 2009, BoNT/A 100 U vs. Hydrodistension [40] | 14.1 | 6.1 | 29 | 13.1 | 5.95 | 23 | 15.1 | 4.54 | 29 | 13.6 | 5.62 | 23 |
| Kuo, 2016 [41] | 10.7 | 5.4 | 40 | 10.4 | 3.8 | 20 | 12.1 | 8.6 | 40 | 9.9 | 4.2 | 20 |
| PVR | Active Agent, Baseline | Control Agent, Baseline | Active Agent, f-up | Control Agent, f-up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study, treatment | Mean | SD | No. of pts | Mean | SD | No. of pts | Mean | SD | No. of pts | Mean | SD | No. of pts |
| Akyhama, 2015 [35] | 43.2 | 39.3 | 18 | 32.4 | 16.2 | 16 | 13 * | 43.4 | 18 | 13.1 * | 28.2 | 16 |
| Chuang, 2017, BoNT/A vs. Lipotoxin [36] | 33.9 | 55.6 | 28 | 52.7 | 59.7 | 31 | 24.7 | 25.4 | 28 | 24.9 | 27.3 | 31 |
| Chuang, 2017, BoNT/A vs. Normal saline [36] | 33.9 | 55.6 | 28 | 58.6 | 98 | 31 | 24.7 | 25.4 | 28 | 31.2 | 39.2 | 31 |
| Kasyan, 2012 [39] | 13.1 | 4.3 | 15 | 12.3 | 3.6 | 17 | 23.2 | 3.3 | 15 | 13 | 2.6 | 17 |
| Kuo, 2009, BoNT/A 200 U vs. Hydrodistension [40] | 13.3 | 41.2 | 15 | 38.7 | 79.3 | 23 | 82.7 | 155.6 | 15 | 30.2 | 50.5 | 23 |
| Kuo, 2009, BoNT/A 100 U vs. Hydrodistension [40] | 30.4 | 53.2 | 29 | 38.7 | 79.3 | 23 | 66.7 | 106.5 | 29 | 30.2 | 50.5 | 23 |
| Kuo, 2016 [41] | 22.7 | 48.2 | 40 | 61.8 | 91 | 20 | 86.1 | 115.3 | 40 | 64.7 | 101.9 | 20 |
| Author, Year, Treatment | Hematuria No. of Patients | Dysuria No. of Patients | Large PVR No. of Patients | Urinary Retention No. of Patients | UTIs No. of Patients | Time to Onset |
|---|---|---|---|---|---|---|
| Akyhama, 2015, [35] | 1 (all participants) | 10 (all participants) | 3 (> 100 mL) (all participants) | None | 2 (all participants) | Between week 1 and month 3 |
| Chuang, 2017, [36] BoNT/A vs. Lipotoxin | None | 2 (active group) 1 (control group) | None | None | None | Within 4 weeks |
| Chuang, 2017, [36] BoNT/A vs. Normal Saline | None | 1 (control group) | None | None | None | Within 4 weeks |
| Gottsch, 2011, [38] | None | None | None | None | None | Within 3 months |
| El-Bahanasy, 2009, [37] | 1 (control group) | 3 (active group) 5 (control group) | None | None | 1 (active group) 2 (control group) | Immediately after the injection |
| Kasyan, 2012, [39] | Not described | Not described | Not described | Not described | Not described | Not described |
| Kuo, 2009, [40] BoNT/A 200 U vs. Hydrodistension | 2 (active group) | 7 (active group) | 5 (active group) | 2 (active group) | 3 (active group) | Dysuria: between weeks 4 and 8 |
| Kuo, 2009, [40] BoNT/A 100 U vs. Hydrodistension | None | 3 (active Group) 1 (control group) | 2 (active group) | 1 (active group) | None | Dysuria: between weeks 4 and 8 |
| Kuo, 2016, [41] | 2 | 16 (active group) 1 (control group) | None | 1 (active group) | 1 (active group) 1 (control group) | Within the first 2 weeks |
| Manning, 2014, [42] | None | None | None | None | 7 (active group) 5 (control group) | At some time after the injection, up to month 3 |
| Pinto, 2018, [43] | None | None | None | None | 3 (active group) 2 (control group) | Between weeks 4 and 12 |
| Taha-Rasheed, 2010, [44] | Not described | Not described | Not described | Not described | Not described | Not described |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannantoni, A.; Gubbiotti, M.; Bini, V. Botulinum Neurotoxin A Intravesical Injections in Interstitial Cystitis/Bladder Painful Syndrome: A Systematic Review with Meta-Analysis. Toxins 2019, 11, 510. https://doi.org/10.3390/toxins11090510
Giannantoni A, Gubbiotti M, Bini V. Botulinum Neurotoxin A Intravesical Injections in Interstitial Cystitis/Bladder Painful Syndrome: A Systematic Review with Meta-Analysis. Toxins. 2019; 11(9):510. https://doi.org/10.3390/toxins11090510
Chicago/Turabian StyleGiannantoni, Antonella, Marilena Gubbiotti, and Vittorio Bini. 2019. "Botulinum Neurotoxin A Intravesical Injections in Interstitial Cystitis/Bladder Painful Syndrome: A Systematic Review with Meta-Analysis" Toxins 11, no. 9: 510. https://doi.org/10.3390/toxins11090510
APA StyleGiannantoni, A., Gubbiotti, M., & Bini, V. (2019). Botulinum Neurotoxin A Intravesical Injections in Interstitial Cystitis/Bladder Painful Syndrome: A Systematic Review with Meta-Analysis. Toxins, 11(9), 510. https://doi.org/10.3390/toxins11090510






