In Vitro Mutagenic and Genotoxic Assessment of a Mixture of the Cyanotoxins Microcystin-LR and Cylindrospermopsin
Abstract
1. Introduction
2. Results
2.1. Ames Test
2.2. Micronucleus Test
2.3. Mouse Lymphoma Thymidine-Kinase Assay (MLA)
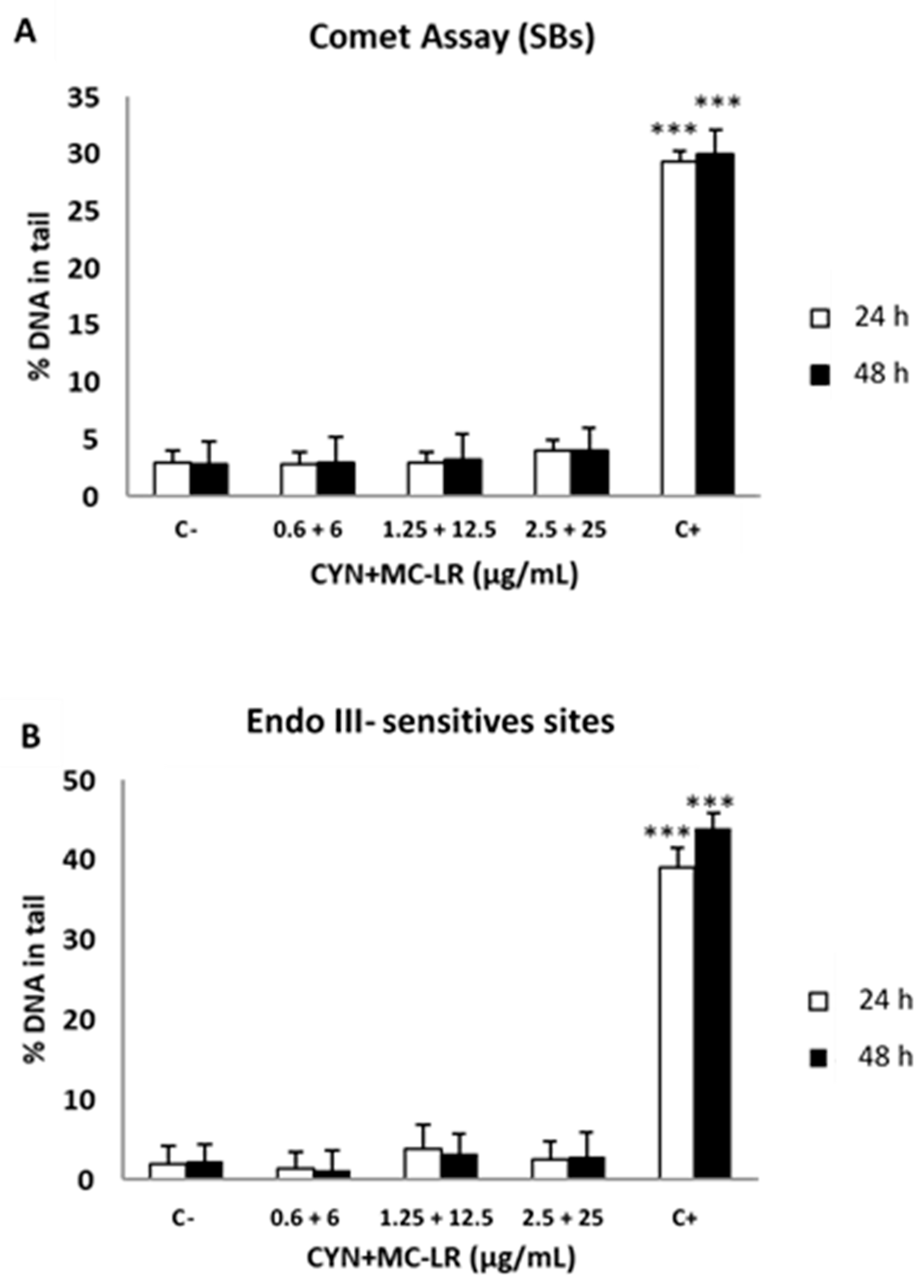
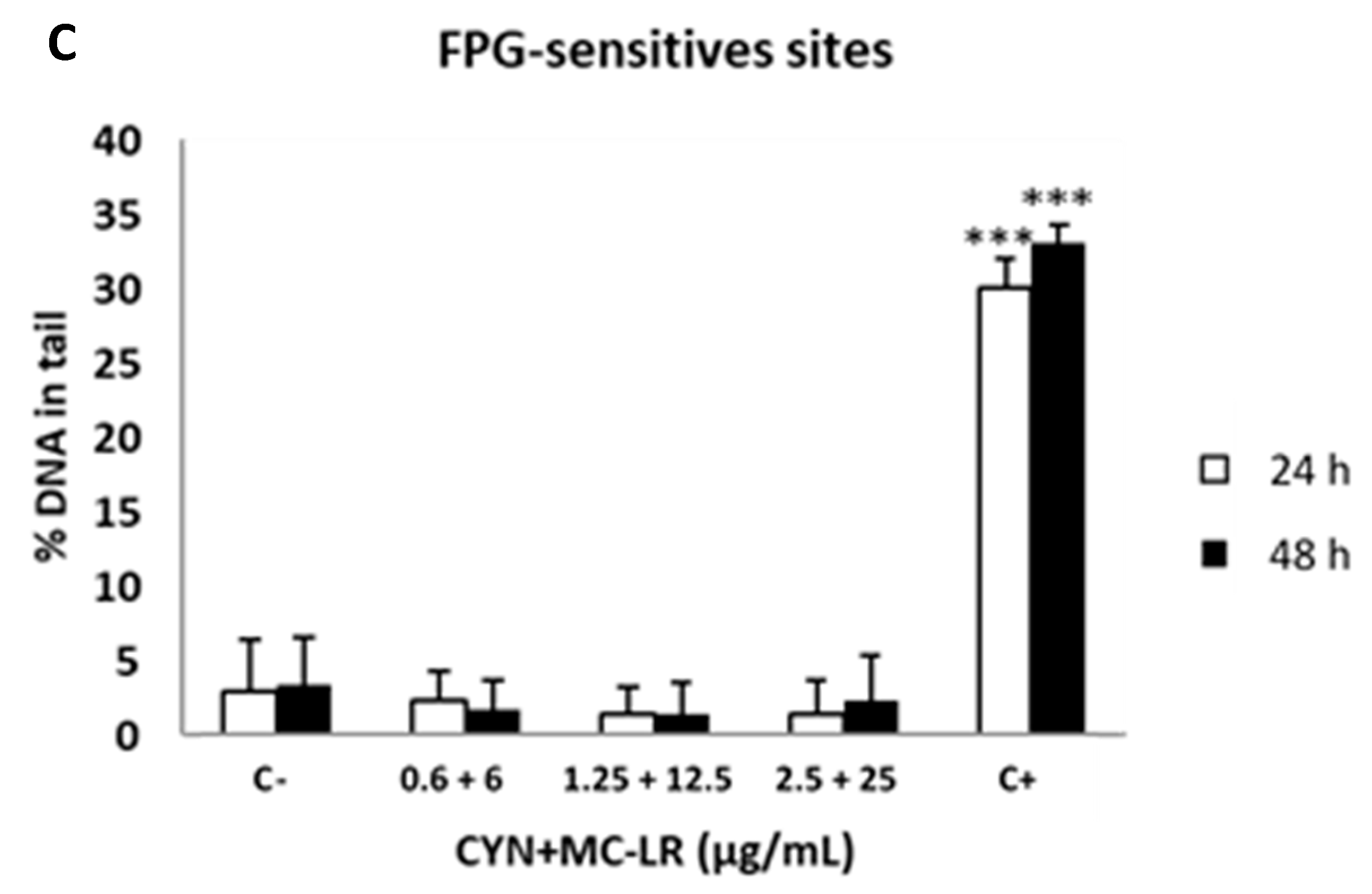
2.4. Standard and Enzyme-Modified Comet Assays
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Cells and Culture Conditions
5.3. Test Solutions
5.4. Bacterial Reverse Mutation Test (Ames Test)
5.5. Micronucleus Test (MN)
5.6. Mouse Lymphoma Thymidine-Kinase Assay (MLA)
5.7. Standard and Enzyme-Modified Comet Assay
5.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef] [PubMed]
- Diez-Quijada, L.; Puerto, M.; Gutierrez-Praena, D.; Llana-Ruiz-Cabello, M.; Jos, A.; Camean, A.M. Microcystin-RR: Occurrence, content in water and food and toxicological studies. A review. Environ. Res. 2019, 168, 467–489. [Google Scholar] [CrossRef]
- Diez-Quijada, L.; Prieto, A.I.; Guzman-Guillen, R.; Jos, A.; Camean, A.M. Occurrence and toxicity of microcystin congeners other than MC-LR and MC-RR: A review. Food Chem. Toxicol. 2019, 125, 106–132. [Google Scholar] [CrossRef] [PubMed]
- Codd, G.A.; Meriluoto, J.; Metcalf, J.S. Introduction: Cyanobacteria, cyanotoxins, their human impact, and risk management. Handb. Cyanobact. Monit. Cyanotoxin Anal. 2016, 1–8. [Google Scholar] [CrossRef]
- Testai, E.; Buratti, F.M.; Funari, E.; Manganelli, M.; Vichi, S.; Arnich, N.; Biré, R.; Fessard, V.; Sialehaamoa, A. Review and analysis of occurrence, exposure and toxicity of cyanobacteria toxins in food. EFSA Support. Publ. 2016, 13, 1–309. [Google Scholar] [CrossRef]
- Roy-Lachapelle, A.; Solliec, M.; Bouchard, M.F.; Sauvé, S. Detection of Cyanotoxins in Algae Dietary Supplements. Toxins 2017, 9, 76. [Google Scholar] [CrossRef]
- Spoof, L.; Catherine, A. Appendix 3: Tables of microcystins and nodularins. Handb. Cyanobact. Monit. Cyanotoxin Anal. 2016, 526–537. [Google Scholar] [CrossRef]
- Fischer, W.J.; Altheimer, S.; Cattori, V.; Meier, P.J.; Dietrich, D.R.; Hagenbuch, B. Organic anion transporting polypeptides expressed in liver and brain mediate uptake of microcystin. Toxicol. Appl. Pharmacol. 2005, 203, 257–263. [Google Scholar] [CrossRef]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef]
- Prieto, A.I.; Jos, A.; Pichardo, S.; Moreno, I.; Cameán, A.M. Protective role of vitamin E on the microcystin-induced oxidative stress in tilapia fish (Oreochromis niloticus). Environ. Toxicol. Chem. 2008, 27, 1152–1159. [Google Scholar] [CrossRef]
- Moreno, I.; Mate, A.; Repetto, G.; Vázquez, C.; Cameán, A.M. Influence of microcystin-LR on the activity of membrane enzymes in rat intestinal mucosa. J. Physiol. Biochem. 2003, 59, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Atencio, L.; Moreno, I.; Prieto, A.I.; Moyano, R.; Molina, A.M.; Camean, A.M. Acute effects of microcystins MC-LR and MC-RR on acid and alkaline phosphatase activities and pathological changes in intraperitoneally exposed tilapia fish (Oreochromis sp.). Toxicol. Pathol. 2008, 36, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Valério, E.; Vasconcelos, V.; Campos, A. New insights on the mode of action of microcystins in animal cells-a review. Mini Rev. Med. Chem. 2016, 16, 1032–1041. [Google Scholar] [CrossRef]
- Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins. Available online: https://www.ncbi.nlm.nih.gov/books/NBK326544/pdf/Bookshelf_NBK326544.pdf (accessed on 10 April 2019).
- Dias, E.; Louro, H.; Pinto, M.; Santos, T.; Antunes, S.; Pereira, P.; Silva, M.J. Genotoxicity of microcystin-LR in in vitro and in vivo experimental models. BioMed. Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Žegura, B. An overview of the mechanisms of microcystin-LR genotoxicity and potential carcinogenicity. Mini Rev. Med. Chem. 2016, 16, 1042–1062. [Google Scholar] [CrossRef]
- Kokociński, M.; Cameán, A.M.; Carmeli, S.; Guzmán-Guillén, R.; Jos, Á.; Mankiewicz-Boczek, J.; Metcalf, J.S.; Moreno, I.M.; Prieto, A.I.; Sukenik, A. Cylindrospermopsin and congeners. Handb. Cyanobact. Monit. Cyanotoxin Anal. 2017, 127–137. [Google Scholar] [CrossRef]
- Chiswell, R.K.; Shaw, G.R.; Eaglesham, G.; Smith, M.J.; Norris, R.L.; Seawright, A.A.; Moore, M.R. Stability of cylindrospermopsin, the toxin from the cyanobacterium, Cylindrospermopsis raciborskii: Effect of pH, temperature, and sunlight on decomposition. Environ. Toxicol. Int. J. 1999, 14, 155–161. [Google Scholar] [CrossRef]
- Falconer, I.R.; Humpage, A.R. Cyanobacterial (blue-green algal) toxins in water supplies: Cylindrospermopsins. Environ. Toxicol. 2006, 21, 299–304. [Google Scholar] [CrossRef]
- Guzmán-Guillén, R.; Puerto, M.; Gutiérrez-Praena, D.; Prieto, A.; Pichardo, S.; Jos, Á.; Campos, A.; Vasconcelos, V.; Cameán, A. Potential use of chemoprotectants against the toxic effects of cyanotoxins: A review. Toxins 2017, 9, 175. [Google Scholar] [CrossRef]
- Hinojosa, M.; Gutiérrez-Praena, D.; Prieto, A.; Guzmán-Guillén, R.; Jos, A.; Cameán, A. Neurotoxicity induced by microcystins and cylindrospermopsin: A review. Sci. Total Environ. 2019, 668, 547–565. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Rzymski, P.; Kokociński, M. Cylindrospermopsin: Water-linked potential threat to human health in Europe. Environ. Toxicol. Pharmacol. 2012, 34, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Pichardo, S.; Devesa, V.; Puerto, M.; Vélez, D.; Cameán, A.M. Intestinal transport of Cylindrospermopsin using the Caco-2 cell line. Toxicol. In Vitro 2017, 38, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Terao, K.; Ohmori, S.; Igarashi, K.; Ohtani, I.; Watanabe, M.; Harada, K.; Ito, E.; Watanabe, M. Electron microscopic studies on experimental poisoning in mice induced by cylindrospermopsin isolated from blue-green alga Umezakia natans. Toxicon 1994, 32, 833–843. [Google Scholar] [CrossRef]
- Froscio, S.M.; Humpage, A.R.; Burcham, P.C.; Falconer, I.R. Cylindrospermopsin-induced protein synthesis inhibition and its dissociation from acute toxicity in mouse hepatocytes. Environ. Toxicol. Int. J. 2003, 18, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Runnegar, M.T.; Kong, S.-M.; Zhong, Y.-Z.; Lu, S.C. Inhibition of reduced glutathione synthesis by cyanobacterial alkaloid cylindrospermopsin in cultured rat hepatocytes. Biochem. Pharmacol. 1995, 49, 219–225. [Google Scholar] [CrossRef]
- Gutiérrez-Praena, D.; Pichardo, S.; Jos, Á.; Cameán, A.M. Toxicity and glutathione implication in the effects observed by exposure of the liver fish cell line PLHC-1 to pure cylindrospermopsin. Ecotoxicol. Environ. Saf. 2011, 74, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Puerto, M.; Jos, A.; Pichardo, S.; Gutiérrez-Praena, D.; Cameán, A.M. Acute effects of pure cylindrospermopsin on the activity and transcription of antioxidant enzymes in tilapia (Oreochromis niloticus) exposed by gavage. Ecotoxicology 2011, 20, 1852–1860. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Rzymski, P.; Karczewski, J. The role of the enzymatic antioxidant system in cylindrospermopsin-induced toxicity in human lymphocytes. Toxicol. In Vitro 2015, 29, 926–932. [Google Scholar] [CrossRef]
- Norris, R.; Seawright, A.; Shaw, G.; Senogles, P.; Eaglesham, G.; Smith, M.; Chiswell, R.; Moore, M. Hepatic xenobiotic metabolism of cylindrospermopsin in vivo in the mouse. Toxicon 2002, 40, 471–476. [Google Scholar] [CrossRef]
- Humpage, A.R.; Fontaine, F.; Froscio, S.; Burcham, P.; Falconer, I.R. Cylindrospermopsin genotoxicity and cytotoxicity: Role of cytochrome P-450 and oxidative stress. J. Toxicol. Environ. Health A 2005, 68, 739–753. [Google Scholar] [CrossRef]
- Humpage, A.R.; Fenech, M.; Thomas, P.; Falconer, I.R. Micronucleus induction and chromosome loss in transformed human white cells indicate clastogenic and aneugenic action of the cyanobacterial toxin, cylindrospermopsin. Mutat. Res. 2000, 472, 155–161. [Google Scholar] [CrossRef]
- Štraser, A.; Filipič, M.; Žegura, B. Genotoxic effects of the cyanobacterial hepatotoxin cylindrospermopsin in the HepG2 cell line. Arch. Toxicol. 2011, 85, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Žegura, B.; Gajski, G.; Štraser, A.; Garaj-Vrhovac, V. Cylindrospermopsin induced DNA damage and alteration in the expression of genes involved in the response to DNA damage, apoptosis and oxidative stress. Toxicon 2011, 58, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Štraser, A.; Metka, F.; Matjaž, N.; Bojana, Ž. Double strand breaks and cell-cycle arrest induced by the cyanobacterial toxin cylindrospermopsin in HepG2 cells. Mar. Drugs 2013, 11, 3077–3090. [Google Scholar]
- Sieroslawska, A.; Rymuszka, A. Cylindrospermopsin induces oxidative stress and genotoxic effects in the fish CLC cell line. J. Appl. Toxicol. 2015, 35, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Pichardo, S.; Cameán, A.M.; Jos, Á.M. In vitro toxicological assessment of cylindrospermopsin: A review. Toxins 2017, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Puerto, M.; Prieto, A.I.; Maisanaba, S.; Gutierrez-Praena, D.; Mellado-Garcia, P.; Jos, A.; Camean, A.M. Mutagenic and genotoxic potential of pure Cylindrospermopsin by a battery of in vitro tests. Food Chem. Toxicol. 2018, 121, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt-Oliveira, M.; Carmo, D.; Piccin-Santos, V.; Moura, A.N.; Aragão-Tavares, N.K.; Cordeiro-Araújo, M.K. Cyanobacteria, microcystins and cylindrospermopsin in public drinking supply reservoirs of Brazil. An. Acad. Bras. Cienc. 2014, 86, 297–310. [Google Scholar] [CrossRef]
- Jančula, D.; Straková, L.; Sadílek, J.; Maršálek, B.; Babica, P. Survey of cyanobacterial toxins in Czech water reservoirs—the first observation of neurotoxic saxitoxins. Environ. Sci. Pollut. Res. Int. 2014, 21, 8006–8015. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Praena, D.; Guzmán-Guillén, R.; Pichardo, S.; Moreno, F.J.; Vasconcelos, V.; Jos, Á.; Cameán, A.M. Cytotoxic and morphological effects of microcystin-LR, cylindrospermopsin, and their combinations on the human hepatic cell line HepG2. Environ. Toxicol. 2018, 34, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Hercog, K.; Maisanaba, S.; Filipič, M.; Jos, Á.; Cameán, A.M.; Žegura, B. Genotoxic potential of the binary mixture of cyanotoxins microcystin-LR and cylindrospermopsin. Chemosphere 2017, 189, 319–329. [Google Scholar] [CrossRef] [PubMed]
- EFSA, S.C. Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA J. 2011, 9. [Google Scholar] [CrossRef]
- OECD Guidelines for the Testing of Chemicals, Bacterial Reverse Mutation Test. Available online: https://www.oecd.org/chemicalsafety/risk-assessment/1948418.pdf (accessed on 1 April 2019).
- OECD Guidelines for the Testing of Chemicals, In Vitro Mammalian Cell Micronucleus Test. Available online: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd-tg487-2014-508.pdf (accessed on 1 April 2019).
- OECD Guidelines for the Testing of Chemicals, In Vitro Mammalian Cell Gene Mutation Tests Using the Thymidine Kinase Gene. Available online: https://www.oecd-ilibrary.org/docserver/9789264264908-en.pdf?expires=1559275406&id=id&accname=guest&checksum=323552A68EC3041C6EA6F9E8A7ACF632 (accessed on 1 April 2019).
- EFSA. Opinion of the Scientific Committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA J. 2005, 3, 282. [Google Scholar] [CrossRef]
- Kamath, G.H.; Rao, K. Genotoxicity guidelines recommended by International Conference of Harmonization (ICH). Methods Mol. Biol. 2013, 1044, 431–458. [Google Scholar] [PubMed]
- EFSA, S.C.; More, S.; Bampidis, V.; Benford, D.; Boesten, J.; Bragard, C.; Halldorsson, T.; Hernandez-Jerez, A.; Hougaard-Bennekou, S.; Koutsoumanis, K. Genotoxicity assessment of chemical mixtures. EFSA J. 2019, 17, 5519. [Google Scholar] [CrossRef]
- Sieroslawska, A. Assessment of the mutagenic potential of cyanobacterial extracts and pure cyanotoxins. Toxicon 2013, 74, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Guerin, M.R. Energy Sources of Polycyclic Aromatic Hydrocarbons. Available online: https://www.osti.gov/servlets/purl/7303055 (accessed on 1 April 2019).
- Abramsson-Zetterberg, L.; Sundh, U.B.; Mattsson, R. Cyanobacterial extracts and microcystin-LR are inactive in the micronucleus assay in vivo and in vitro. Mutat. Res. 2010, 699, 5–10. [Google Scholar] [CrossRef]
- Huang, P.; Xu, A. Genotoxic effects of microcystin-LR in mammalian cells. In Proceedings of the 2009 3rd International Conference on Bioinformatics and Biomedical Engineering, Beijing, China, 11–16 June 2009. [Google Scholar]
- Zhan, L.; Sakamoto, H.; Sakuraba, M.; Wu, D.-S.; Zhang, L.-S.; Suzuki, T.; Hayashi, M.; Honma, M. Genotoxicity of microcystin-LR in human lymphoblastoid TK6 cells. Mutat. Res. 2004, 557, 1–6. [Google Scholar] [CrossRef]
- Moore, M.M.; Honma, M.; Clements, J.; Bolcsfoldi, G.; Cifone, M.; Delongchamp, R.; Fellows, M.; Gollapudi, B.; Jenkinson, P.; Kirby, P. Mouse lymphoma thymidine kinase gene mutation assay: International Workshop on Genotoxicity Tests Workgroup report—Plymouth, UK 2002. Mutat. Res. 2003, 540, 127–140. [Google Scholar] [CrossRef]
- Chen, T.; Harrington-Brock, K.; Moore, M.M. Mutant frequencies and loss of heterozygosity induced by N-ethyl-N-nitrosourea in the thymidine kinase gene of L5178Y/Tk+/−-3.7.2C mouse lymphoma cells. Mutagenesis 2002, 17, 105–109. [Google Scholar] [CrossRef][Green Version]
- Demir, E.; Kaya, B.; Soriano, C.; Creus, A.; Marcos, R. Genotoxic analysis of four lipid-peroxidation products in the mouse lymphoma assay. Mutat. Res. 2011, 726, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Lam, P.; Shaw, G.; Wickramasinghe, W. Genotoxicity investigation of a cyanobacterial toxin, cylindrospermopsin. Toxicon 2002, 40, 1499–1501. [Google Scholar] [CrossRef]
- Bazin, E.; Huet, S.; Jarry, G.; Hégarat, L.L.; Munday, J.S.; Humpage, A.R.; Fessard, V. Cytotoxic and genotoxic effects of cylindrospermopsin in mice treated by gavage or intraperitoneal injection. Environ. Toxicol. 2012, 27, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Žegura, B.; Sedmak, B.; Filipič, M. Microcystin-LR induces oxidative DNA damage in human hepatoma cell line HepG2. Toxicon 2003, 41, 41–48. [Google Scholar] [CrossRef]
- Lankoff, A.; Krzowski, Ł.; Głąb, J.; Banasik, A.; Lisowska, H.; Kuszewski, T.; Góźdź, S.; Wójcik, A. DNA damage and repair in human peripheral blood lymphocytes following treatment with microcystin-LR. Mutat. Res. 2004, 559, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Žegura, B.; Gajski, G.; Štraser, A.; Garaj-Vrhovac, V.; Filipič, M. Microcystin-LR induced DNA damage in human peripheral blood lymphocytes. Mutat. Res. 2011, 726, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Zouaoui, N.; Mallebrera, B.; Berrada, H.; Abid-Essefi, S.; Bacha, H.; Ruiz, M.-J. Cytotoxic effects induced by patulin, sterigmatocystin and beauvericin on CHO–K1 cells. Food Chem. Toxicol. 2016, 89, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Pflugmacher, S.; Wiegand, C.; Oberemm, A.; Beattie, K.A.; Krause, E.; Codd, G.A.; Steinberg, C.E. Identification of an enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin-LR: The first step of detoxication. Biochim. Biophys. Acta 1998, 1425, 527–533. [Google Scholar] [CrossRef]
- Lankoff, A.; Bialczyk, J.; Dziga, D.; Carmichael, W.; Gradzka, I.; Lisowska, H.; Kuszewski, T.; Gozdz, S.; Piorun, I.; Wojcik, A. The repair of gamma-radiation-induced DNA damage is inhibited by microcystin-LR, the PP1 and PP2A phosphatase inhibitor. Mutagenesis 2006, 21, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Nesslany, F. The current limitations of in vitro genotoxicity testing and their relevance to the in vivo situation. Food Chem. Toxicol. 2017, 106, 609–615. [Google Scholar] [CrossRef]
- De La Cruz, A.A.; Hiskia, A.; Kaloudis, T.; Chernoff, N.; Hill, D.; Antoniou, M.G.; He, X.; Loftin, K.; O’Shea, K.; Zhao, C.; et al. A review on cylindrospermopsin: The global occurrence, detection, toxicity and degradation of a potent cyanotoxin. Environ. Sci. Process. Impacts 2013, 15, 1979. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Maisanaba, S.; Prieto, A.I.; Puerto, M.; Gutiérrez-Praena, D.; Demir, E.; Marcos, R.; Cameán, A.M. In vitro genotoxicity testing of carvacrol and thymol using the micronucleus and mouse lymphoma assays. Mutat. Res. 2015, 784, 37–44. [Google Scholar] [CrossRef] [PubMed]
- International Conferences on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use. Available online: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S2_R1/Step4/S2R1_Step4.pdf (accessed on 1 April 2019).
- Honma, M.; Hayashi, M.; Shimada, H.; Tanaka, N.; Wakuri, S.; Awogi, T.; Yamamoto, K.I.; Kodani, N.-U.; Nishi, Y.; Nakadate, M. Evaluation of the mouse lymphoma Tk assay (microwell method) as an alternative to the in vitro chromosomal aberration test. Mutagenesis 1999, 14, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R.; Azqueta, A. Chapter 4: Single-cell gel electrophoresis combined with lesion-specific enzymes to measure oxidative damage to DNA. In Methods Cell Biology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 112, pp. 69–92. [Google Scholar]
- LLana-Ruiz-Cabello, M.; Maisanaba, S.; Puerto, M.; Prieto, A.I.; Pichardo, S.; Jos, Á.; Cameán, A.M. Evaluation of the mutagenicity and genotoxic potential of carvacrol and thymol using the Ames Salmonella test and alkaline, Endo III-and FPG-modified comet assays with the human cell line Caco-2. Food Chem. Toxicol. 2014, 72, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Puerto, M.; Pichardo, S.; Jos, Á.; Cameán, A.M. Comparison of the toxicity induced by microcystin-RR and microcystin-YR in differentiated and undifferentiated Caco-2 cells. Toxicon 2009, 54, 161–169. [Google Scholar] [CrossRef]
- Puerto, M.; Pichardo, S.; Jos, Á.; Cameán, A.M. Microcystin-LR induces toxic effects in differentiated and undifferentiated Caco-2 cells. Arch. Toxicol. 2010, 84, 405–410. [Google Scholar] [CrossRef]
- Gutiérrez-Praena, D.; Pichardo, S.; Jos, Á.; Moreno, F.J.; Cameán, A.M. Biochemical and pathological toxic effects induced by the cyanotoxin Cylindrospermopsin on the human cell line Caco-2. Water Res. 2012, 46, 1566–1575. [Google Scholar] [CrossRef]
| Concentration (µg/mL) | TA97A | TA98 | TA100 | TA102 | TA1535 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −S9 | MI | +S9 | MI | −S9 | MI | +S9 | MI | −S9 | MI | +S9 | MI | −S9 | MI | +S9 | MI | −S9 | MI | +S9 | MI | ||
| Pure CYN-MC-LR mixture | Negative controls | 231 ± 42 | - | 244 ± 5 | - | 21 ± 2 | - | 24 ± 9 | - | 117 ± 25 | - | 135 ± 14 | - | 215 ± 12 | - | 292 ± 11 | - | 293 ± 23 | - | 273 ± 33 | - |
| 0.125–1.25 | 297 ± 37 | 1.4 | 319 ± 51 | 1.3 | 19 ± 2 | 0.9 | 18 ± 8 | 0.8 | 136 ± 40 | 1.2 | 153 ± 21 | 1.1 | 230 ± 36 | 1.1 | 440 ± 29 ** | 1.5 | 327 ± 25 | 1.1 | 376 ± 54 * | 1.3 | |
| 0.25–2.5 | 165 ± 28 | 0.8 | 334 ± 49 ** | 1.4 | 20 ± 1 | 1.0 | 17 ± 7 | 0.7 | 144 ± 12 | 1.2 | 166 ± 24 | 1.2 | 217 ± 29 | 1.0 | 380 ± 33 ** | 1.3 | 311 ± 10 | 1.1 | 411 ± 54 ** | 1.4 | |
| 0.5–5 | 213 ± 15 | 1.0 | 290 ± 58 | 1.2 | 26 ± 9 | 1.3 | 20 ± 10 | 0.9 | 154 ± 13 | 1.3 | 143 ± 19 | 1.1 | 251 ± 17 | 1.2 | 296 ± 18 | 10. | 309 ± 42 | 1.1 | 336 ± 18 * | 1.1 | |
| 1–10 | 168 ± 10 | 0.8 | 234 ± 43 | 1.0 | 21 ± 2 | 1.0 | 19 ± 9 | 1.0 | 146 ± 18 | 1.2 | 130 ± 10 | 1.0 | 134 ± 12 | 0.6 | 383 ± 44 ** | 1.3 | 250 ± 43 | 0.9 | 464 ± 44 ** | 1.6 | |
| 2–20 | 205 ± 31 | 1.0 | 295 ± 25 | 1.2 | 19 ± 5 | 0.9 | 25 ± 8 | 1.0 | 104 ± 31 | 0.9 | 143 ± 19 | 1.1 | 151 ± 1 | 0.7 | 397 ± 32 ** | 1.4 | 276 ± 15 | 0.9 | 476 ± 52 ** | 1.6 | |
| Positive controls | 613 ± 66 ** | 2.9 | 527 ± 19 ** | 2.2 | 883 ± 55 ** | 42.0 | 960 ± 53 ** | 40.9 | 816 ± 11 ** | 7.0 | 583 ± 39 ** | 4.3 | 950 ± 118 ** | 4.4 | 671 ± 22 ** | 2.3 | 833 ± 25 ** | 2.8 | 659 ± 39 ** | 2.2 | |
| MeOH 2% | 176 ± 25 | 0.8 | 316 ± 32 | 1.3 | 17 ± 5 | 0.8 | 25 ± 13 | 1.1 | 92 ± 13 | 0.8 | 87 ± 29 | 0.6 | 192 ± 8 | 0.8 | 280 ± 12 | 0.6 | 313 ± 9 | 1.1 | 233 ± 35 | 0.9 | |
| DMSO | 209 ± 66 | 1.3 | 184 ± 38 | 0.8 | 25 ± 2 | 1.2 | 30 ± 6 | 1.3 | 115 ± 5 | 1.0 | 113 ± 17 | 0.8 | 250 ± 65 | 1.2 | 231 ± 35 | 0.8 | 342 ± 63 | 1.2 | 298 ± 16 | 1.1 | |
| Experimental Group | Absence of S9 | Presence of S9 | ||||||
|---|---|---|---|---|---|---|---|---|
| Exposure Time (h) | Concentrations (µg/mL) | BNMN (%) ± SD | CBPI ± SD | Exposure Time (h) | Concentrations (µg/mL) | BNMN (%) ± SD | CBPI ± SD | |
| Negative control | 24 | - | 2.3 ± 0.5 | 1.9 ± 0.1 | 4 | - | 2.5 ± 1.0 | 1.8 ± 0.1 |
| Positive control | 24 | Mitomycin C 0.0625 | 10.5 ± 4.1 *** | 1.5 ± 0.1 *** | 4 | Cyclophosfamide 8 | 8.3 ± 1.9 ** | 1.8 ± 0.1 |
| Colchicine 0.0125 | 9.6 ± 1.7 *** | 1.8 ± 0.0 | ||||||
| CYN+MC-LR | 24 | 0.084–0.84 | 1.8 ± 1.5 | 1.9 ± 0.0 | 4 | 0.125–1.25 | 4.8 ± 2.6 | 1.8 ± 0.1 |
| 24 | 0.168–1.68 | 2.3 ± 1.0 | 1.9 ± 0.0 | 4 | 0.250–2.5 | 4.0 ± 1.4 | 1.8 ± 0.1 | |
| 24 | 0.336–3.36 | 2.5 ± 0.6 | 1.8 ± 0.0 | 4 | 0.5–5 | 5.8 ± 1.5 | 1.8 ± 0.1 | |
| 24 | 0.672–6.72 | 1.3 ± 0.5 | 1.7 ± 0.1 | 4 | 1–10 | 8.8 ± 4.2 ** | 1.8 ± 0.1 | |
| 24 | 1.35–13.5 | 0.8 ± 1.0 | 1.3 ± 0.3 *** | 4 | 2–20 | 4.8 ± 0.5 | 1.8 ± 0.1 | |
| Concentration (µg/mL) | Relative Total Growth | Percent Plating Efficiency | Mutant Frequency (× 10−6) | MF (S/L) a | IMF (MF-SMF) (× 10−6) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | |
| 0 | 100 | 100 | 91 | 124 | 107 | 152 | 51/56 | 33/41 | - | - |
| 0.04 CYN-0.4 MC | 77 | 90 | 98 | 98 | 126 | 143 | 95/48 | 86/57 | 56 | 70 |
| 0.08 CYN-0.8 MC | 98 | 100 | 93 | 70 | 202 | 157 | 111/91 | 102/55 | 95 | 83 |
| 0.16 CYN-1.6 MC | 82 | 86 | 102 | 82 | 71 | 162 | 44/27 | 100/62 | −14.4 | 89 |
| 0.33 CYN-3.3 MC | 64 | 72 | 98 | 91 | 165 | 150 | 84/81 | 80/70 | 58 | 76 |
| 0.67 CYN-6.7 MC | 57 | 58 | 95 | 88 | 174 | 144 | 106/68 | 60/84 | 67 | 71 |
| MMS (10 µg/mL) | 46 | 70 | 69 | 82 | 728 *** | 738 *** | 407/321 | 424/314 | 621 | 664 |
| Concentration (µg/mL) | Relative Total Growth | Percent Plating Efficiency | Mutant Frequency (× 10−6) | MF (S/L) a | IMF (MF-SMF) (× 10−6) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | |
| 0 | 100 | 100 | 93 | 102 | 155 | 146 | 96/59 | 82/64 | - | - |
| 0.04 CYN-0.4 MC | 96 | 84 | 82 | 84 | 94 | 100 | 43/51 | 42/58 | −61 | −46 |
| 0.08 CYN-0.8 MC | 82 | 72 | 91 | 91 | 95 | 95 | 50/45 | 50/45 | −60 | −51 |
| 0.16 CYN-1.6 MC | 58 | 51 | 95 | 100 | 98 | 95 | 48/50 | 49/47 | −57 | −51 |
| 0.33 CYN-3.3 MC | 58 | 56 | 102 | 100 | 98 | 105 | 56/43 | 60/45 | −57 | −41 |
| 0.67 CYN-6.7 MC | 26 | 31 | 118 | 113 | 120 | 132 | 62/58 | 77/55 | −35 | −14 |
| 1.35 CYN-13.5 MC | 16 | 16 | 130 | 116 | 70 | 91 | 29/41 | 38/53 | −85 | −55 |
| CP (3 µg/mL) | 99 | 81 | 65 | 73 | 480 *** | 433 *** | 228/252 | 213/220 | 325 | 286 |
| Concentration (µg/mL) | Relative Total Growth | Percent Plating Efficiency | Mutant Frequency (× 10−6) | MF (S/L) a | IMF (MF-SMF) (× 10−6) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | |
| 0 | 100 | 100 | 113 | 124 | 170 | 170 | 106/72 | 87/92 | - | - |
| 0.04 CYN-0.4 MC | 103 | 115 | 90 | 87 | 107 | 78.9 | 62/45 | 48/30 | −71 | −100 |
| 0.08 CYN-0.8 MC | 91 | 102 | 102 | 93 | 121 | 124 | 50/71 | 72/52 | −57 | −55 |
| 0.16 CYN-1.6 MC | 79 | 96 | 76 | 108 | 143 | 100 | 81/66 | 56/44 | −35 | −79 |
| 0.33 CYN-3.3 MC | 71 | 74 | 116 | 104 | 115 | 168 | 64/51 | 109/59 | −63 | −12 |
| 0.67 CYN-6.7 MC | 39 | 39 | 127 | 104 | 113 | 195 | 74/39 | 77/118 | −66 | 16 |
| MMS (10 µg/mL) | 52 | 66 | 35 | 34 | 778 *** | 897 *** | 370/408 | 459/438 | 599 | 718 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díez-Quijada, L.; Prieto, A.I.; Puerto, M.; Jos, Á.; Cameán, A.M. In Vitro Mutagenic and Genotoxic Assessment of a Mixture of the Cyanotoxins Microcystin-LR and Cylindrospermopsin. Toxins 2019, 11, 318. https://doi.org/10.3390/toxins11060318
Díez-Quijada L, Prieto AI, Puerto M, Jos Á, Cameán AM. In Vitro Mutagenic and Genotoxic Assessment of a Mixture of the Cyanotoxins Microcystin-LR and Cylindrospermopsin. Toxins. 2019; 11(6):318. https://doi.org/10.3390/toxins11060318
Chicago/Turabian StyleDíez-Quijada, Leticia, Ana I. Prieto, María Puerto, Ángeles Jos, and Ana M. Cameán. 2019. "In Vitro Mutagenic and Genotoxic Assessment of a Mixture of the Cyanotoxins Microcystin-LR and Cylindrospermopsin" Toxins 11, no. 6: 318. https://doi.org/10.3390/toxins11060318
APA StyleDíez-Quijada, L., Prieto, A. I., Puerto, M., Jos, Á., & Cameán, A. M. (2019). In Vitro Mutagenic and Genotoxic Assessment of a Mixture of the Cyanotoxins Microcystin-LR and Cylindrospermopsin. Toxins, 11(6), 318. https://doi.org/10.3390/toxins11060318







