Abstract
Mycotoxins are secondary metabolites, produced by fungi of genera Aspergillus, Penicillium and Fusarium (among others), which produce adverse health effects on humans and animals (carcinogenic, teratogenic and immunosuppressive). In addition, mycotoxins negatively affect the productive parameters of livestock (e.g., weight, food consumption, and food conversion). Epidemiological studies are considered necessary to assist stakeholders with the process of decision-making regarding the control of mycotoxins in processing environments. This study addressed the prevalence in feed ingredients and compound feed of eight different types of toxins, including metabolites produced by Fusarium spp. (Deoxynivalenol/3-acetyldeoxynivalenol, T-2/HT-2 toxins, zearalenone and fumonisins) and two additional toxins (i.e., ochratoxin A (OTA) and aflatoxin M1 (AFM1)) from different fungal species, for over a period of five years. On the subject of Fusarium toxins, higher prevalences were observed for fumonisins (n = 80/113, 70.8%) and DON (n = 212/363, 58.4%), whereas, for OTA, a prevalence of 40.56% was found (n = 146/360). In the case of raw material, mycotoxin contamination exceeding recommended values were observed in cornmeal for HT-2 toxin (n = 3/24, 12.5%), T-2 toxin (n = 3/61, 4.9%), and ZEA (n = 2/45, 4.4%). In contrast, many compound feed samples exceeded recommended values; in dairy cattle feed toxins such as DON (n = 5/147, 3.4%), ZEA (n = 6/150, 4.0%), T-2 toxin (n = 10/171, 5.9%), and HT-2 toxin (n = 13/132, 9.8%) were observed in high amounts. OTA was the most common compound accompanying Fusarium toxins (i.e., 16.67% of co-occurrence with ZEA). This study also provided epidemiological data for AFM1 in liquid milk. The outcomes unveiled a high prevalence of contamination (i.e., 29.6–71.1%) and several samples exceeding the regulatory threshold. Statistical analysis exposed no significant climate effect connected to the prevalence of diverse types of mycotoxins.
Keywords:
Fusarium mycotoxins co-contamination; ochratoxin A; feed prevalence and safety; HPLC analysis Key Contribution:
This study generated essential epidemiological and toxicological evidence about the individual and combined occurrence of Fusarium mycotoxins and ochratoxin A in feedstuffs in Costa Rica. These findings portray imperative implications for all stakeholders linked to the feed industry as well as supplies for improving the management of mycotoxins in animal production.
1. Introduction
Mycotoxins are toxic fungal metabolites that can be found in feed ingredients and compound feeds [1,2]. Due to their compositions, they are detrimental to animal and human health [3,4,5,6,7,8]. Currently, more than 400 different types of mycotoxins have been identified [9]. However, Fusarium toxins are among the most commonly monitored as they are acknowledged to present serious health concerns [7,10]. Under certain conditions, some fungi can produce several toxins simultaneously [11,12,13].
In feed production, ca. 60% of the formulation consists of cornmeal, soybean meal, and their derivates [14,15]. In Costa Rica, cereal production represents 38% of the agricultural sector imports [16], where its main suppliers are the United States and Brazil with 84% and 15% contribution, respectively [17]. In this regard, corn imports have increased from 738,539.97 to 781,903.54 metric tons from 2015 to 2017 [18]. On the other hand, soybean imports have risen to 309,897.97 metric tons per year, even though 83% of the soybean meal used as a feedstuff comes from national production [18]. Furthermore, only 38% of the products destined for animal consumption are from national origin, representing a total feed production of 1,238,243 metric tons in 2017. Approximately 45%, 27%, 20%, and 4% of this production is intended to be destined to poultry, higher ruminants, swine, and pets (i.e., cats and dogs), respectively [18]. That is, import and export of animal feed and feed ingredients play an essential part in the co-occurrence of various types of mycotoxins in the finished feed [19,20]. Hence, co-occurrence could be a far more certain and prevalent issue in real mycotoxin feed analysis [11,12,20,21,22,23].
Mycotoxin metabolites retain toxicity and thus must be surveilled [24,25]. Mycotoxins and their metabolites have several implications for animal and human health. Some are identified/classified as teratogenic, genotoxic, carcinogenic, and immunotoxic. The ingestion of contaminated feed affects animal health and may reduce productivity in animals, generating economic losses [26]. Some mycotoxins ingested and metabolized by productive animals could be accumulated in different organs and tissues reaching the food chain through meat, milk, or eggs [24,27,28]. In Costa Rica, during 2018, consumption of these commodities was estimated in 58.7 kg (i.e., 14.3, 15.4, and 29 kg year−1 for cattle, pork, and chicken, respectively), 215 L, and 218 units per capita, individually [18].
In this regard, epidemiological information tends to be more comprehensive when exploring data from several toxins simultaneously [29]. Accurate mycotoxin data about their presence in feeds are paramount for stakeholders’ decision-making process towards the risk management in their manipulation [30]. Numerous reports have explicitly documented the incidence of mycotoxins in feeds, especially in Europe [11,31,32], USA [33], Asia [31], and China [34]. Nowadays, there are insufficient reports oriented to describe the incidence of mycotoxins in feed in Costa Rica. The emphasis has been made towards the investigation of aflatoxins [35,36].
Herein, the prevalent data from feed and feed ingredient samples of eight different toxins, mainly produced by Fusarium spp. (deoxynivalenol/3-acetyldexoynivalenol (DON/3-ADON), T-2/HT-2 toxins, zearalenone (ZEA) and fumonisins (FB1 and FB2)), but also ochratoxin A (OTA), during five years are provided. Finally, in the same period, we analyzed the behavior of AFM1 in liquid milk.
2. Results
2.1. Fusarium Toxins Present in Animal Feed
The highest prevalence of Fusarium toxins during the analyzed period (2012–2017) was observed for fumonisin and DON in 70.8% (n = 80/113) and 58.4% (n = 212/363) of the cases, respectively. For FB1 + FB2 the prevalence ranged from 27.8% (n = 5/18) in 2013 to 85.2% (n = 23/27) in 2014, with a maximum concentration of 53,580 µg kg−1 observed in 2015. The prevalence for DON ranged from 42.0% (n = 40/94) in 2016 to 79.3% (n = 69/87) in 2014, with a maximum concentration of 151,060 µg kg−1 presented in 2013 (Table 1). Lower prevalences of 21.2% (n = 45/212) and 36.1% (n = 97/269) with a maximum mycotoxin level of 16,100 µg kg−1 (in 2015) and 12,500 µg kg−1 (in 2014) were observed for 3-acetyldeoxynivalenol and HT-2, respectively (Table 1). Concentration-wise and among periods, ZEA and T-2 toxin increased meaningfully in 2017 and 2013, respectively. For HT-2, OTA, DON, 3-ADON, FB1, FB2, and FB1 + FB2, no differences were observed.

Table 1.
Mycotoxin presence and concentration in animal feedstuff commercialized in Costa Rica.
2.2. Mycotoxin Prevalence in Feed Ingredients
In the matter of feed ingredients, cornmeal exceeded guideline values for HT-2 toxin (n = 3/24, 12.5%), T-2 toxin (n = 3/61, 4.9%), and ZEA (n = 2/45, 4.4%) (Table 2). In a soybean meal, merely HT-2 toxin (n = 1/6, 16.7%) was detected in this situation, and just one sample of wheat had an excessive amount of DON (n = 1/8, 12.5%) (Table 2). With reference to other raw materials, of less inclusion, such as rice byproducts, palm oil byproducts, of the citrus industry, as well as forages, silages, and hays (treated as a whole group), there are no regulatory guidelines to establish an acceptance parameter. However, it is interesting to notice that, in the groups described above, they share as a common feature a high prevalence of DON (i.e., 66.7%) (Table 2).

Table 2.
Mycotoxin contamination levels for feed ingredients. a
2.3. Mycotoxin Prevalence in Compound Feed
Among compound feeds, beef cattle feed presented only a few samples above the guideline level (specifically, T-2 and HT-2 toxin, n = 2/63, 3.2%). Dairy cattle feed presented the highest number of samples that surpassed the recommended levels of mycotoxins (n = 34/105, 32.4%), specifically DON (n = 5/147, 3.4%), ZEA (n = 6/150, 4.0%), T-2 toxin (n = 10/171, 5.8%) and HT-2 (n = 13/132, 9.8%) (Table 3). Poultry feed presented only 10 samples exceeding the guidelines, for DON (n = 2/14, 14.3%), FB1 (n = 1/7, 14.3%), HT-2 toxin (n = 1/15, 6.7%), and OTA (n = 1/9, 11.1%). Cat and dog food also showed values above legal thresholds for fumonisins (n = 6/13, 46.1%), with a maximum of 18,910 µg kg−1 (Table 3). The second highest prevalence was observed connected with swine feed (n = 14/71, 19.7%) with the mycotoxins ZEA (n = 2/18, 11.2%), FB1 (n = 2/9, 22.2%), and DON (n = 6/17, 35.3%) infringing the respective recommended guidelines (Table 2). Fish feed also exceeded thresholds for DON (n = 2/16, 12.5%). Finally, in horse feed, Fumonisin B2 was found (n = 1/26, 3.8%) (Table 3).

Table 3.
Mycotoxin contamination levels for compound animal feed. a
2.4. Geographical Distribution and Climate Influence for Fusarium Toxins Present in Animal Feed
Geographical and national toxin hotspot distribution was similar for those toxins produced by Fusarium species (Figure 1A–G). A completely different profile was observed when studying OTA and AFM1. Interestingly, only 3-ADON and HT-2 toxins prevailed during the rainy season. For other toxins, there were no differences in the levels of contamination between the dry season and the rainy season (Table 4). As expected, the co-occurrence of two different toxins was the most common situation (i.e., n = 141/279, 50.5%) (Table 5). Therefore, as the number of simultaneous toxins increased, co-occurrence was less likely to be found (Table 5). In the case of the parent compound–metabolite comparison, the most common combination was the pair T-2/HT-2 toxin with (n = 66/155) 42.6% of prevalence, followed by FB1/FB2 (n = 23/137, 16.8%) and DON/3-ADON (n = 18/177, 10.2%) (Table 5).
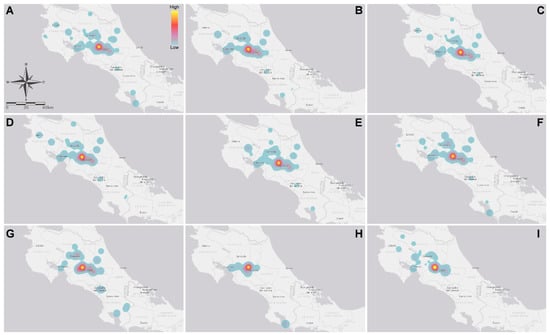
Figure 1.
Heat map representing the geographical origin of samples and the mycotoxin concentration: (A) DON; (B) 3-ADON; (C) T-2 toxin; (D) HT-2 toxin; (E) ZEA; (F) FB1; (G) FB2; (H) OTA; and (I) AFM1.

Table 4.
Seasonal prevalence and behavior per toxin.

Table 5.
Mycotoxin co-occurrence in the sample totals.
2.5. OTA Prevalence in Animal Feeds
Referring to OTA, the total prevalence from 2012 to 2017 was 40.6% (n = 146/360), ranging from 16.3% (n = 8/49) in 2013 to 76.6% (n = 49/64) in 2015. The maximum OTA reported level was 1810 µg kg−1, in 2016 (Table 1). Only one sample exceeded the maximal advisory level for ochratoxin; this sample corresponded to poultry feed where the recommended concentration is 100 µg kg−1. The overall OTA prevalence in non-traditional ingredients, poultry, and fish feed was of 56.3%, 44.4%, and 66.7%, respectively (Table 2 and Table 3). Furthermore, in May and September, the highest global concentrations of OTA were presented, corresponding to the rainy season releasing an evident difference compared with the findings of the dry season (Table 4). As the presence of OTA involves other toxin-producing fungi (other than Fusarium), co-occurrence with other metabolites is a possibility. The most prevalent Fusarium toxins present in feed (different from OTA), in decreasing order of incidence, were ZEA, DON + FB1, FB1, and T-2 toxin with (n = 17/102) 16.7%, (n = 14/102) 13.7%, and (n = 12/102) 11.8% of incidence, respectively (Table 5). As expected, OTA incidence had a completely different geographical/spatial (Figure 1H) and thermo/temporal (Figure 2H) distribution, when compared with the other toxins.
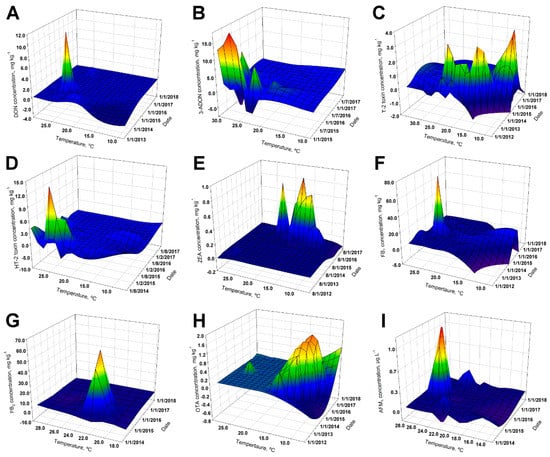
Figure 2.
3D mesh graphs representing the relationship among mycotoxin concentration, mean temperature, and sample date: (A) DON; (B) 3-ADON; (C) T-2 toxin; (D) HT-2 toxin; (E) ZEA; (F) FB1; (G) FB2; (H) OTA; and (I) AFM1.
2.6. Aflatoxin M1 in Liquid Milk
Water buffalo milk and butter samples were also analyzed for the presence of Aflatoxin M1. Water buffalo (Bubalus bubalis) milk samples (n = 2) were reported below the limit of quantification (i.e., 0.014 µg kg−1) and butter (n = 3) ranged from 0.021 to 0.024 µg kg−1. Even though 2016 was the year with the lowest number of analyzed samples, it was also the year when fewer samples surpassed the 0.05 µg kg−1 threshold (Table 6). An increase in AFM1 prevalence with 71.1% and 63.2%, respectively (Table 6), was observed during 2014 and 2017. Excluding three samples from 2015, there were no other samples surpassing the US FDA threshold of 0.5 µg kg−1, thus representing a very small overall percentage for the four years of the study (i.e., n = 3/175, 1.7%). It was studied/monitored that, consistently, higher concentrations of AFM1 were obtained during March, August, and September (Table 6 and Figure 2I).

Table 6.
Prevalence and epidemiological data regarding AFM1 in fresh bovine milk for four years.
3. Discussion
3.1. Mycotoxin Prevalence between 2013 and 2017 in Animal Feed
Most of the studied toxins (except for 3-ADON, FB1, and HT-2) had prevalences higher than 40% during the five years. The average concentrations found in the different toxins in animal feed did not vary between one year and another, except for ZEA and T-2. The drastic increase of ZEA concentrations during 2017 was observed in corn meal and sorghum silo. There is a prior documented avidity of Fusarium spp. to produce ZEA when using moderately alkaline cereals (e.g., maize) as substrates [43]. A general drop in annual temperature may have provoked this upsurge in ZEA contamination. For example, Fusarium graminearum has demonstrated that conditions of pH 9 and incubation temperature of 15.05 °C are required to favor ZEA production [44]. Interestingly, the most toxicologically relevant levels for ZEA were encountered at relatively low temperatures (i.e., near 15 °C). Despite a relatively high prevalence for mycotoxins (i.e., between 46% and 99%, except for FB1 + FB2 and DON), the positive samples possessed comparatively low concentrations (Table 1) based on guidance values for mycotoxins in animal feeds within the European Union (see Appendix A Table A1 and Table A2) [37,38]. This relatively low toxicological burden could be associated with the control of mycotoxin in animal feed and raw materials that were established in the country since 2007. This control policy covers the majority of the toxins analyzed in this study added to the control of imported raw materials, before its distribution. In coherence to what has been stated, since 2013, proficient manufacturing practices have been evaluated and audited by regulation in animal feed plants. These proficient practices involve the management of raw materials and storage measures, among others, contributing to the reduction of mycotoxin contamination [45].
However, some of the samples were observed with concentrations above the established guidelines with potentially adverse effects on animal health and productivity. It is worth of mentioning the fact that human health could be affected through the consumption of foods of animal origin contaminated with mycotoxins or their metabolites [24,27,28].
3.2. Mycotoxin Prevalence in Compound Feed and Feed Ingredients
3.2.1. Prevalence in Feed Ingredients
Vegetable ingredients may represent from 80% to 100% of the feed (e.g., in ruminants, animal origin ingredients are prohibited) [14,46,47]. For these vegetable-based formulations, corn and soybean meal may represent up to 60% of the input [14,15]. Costa Rican soybean meal and corn, as well as other relevant ingredients, are imported [18]. Quality grain assessment is a degree-based classification. Usually, grade 2 or 3 corn is purchased for feed production [18]. At least 97.9% of the samples contain around 3% of cracked material, and 36.2% of the samples exhibited higher moisture content (i.e., 17%); both factors promote the proliferation of fungi [48]. Toxin-wise, AFB1, and DON were assayed and are regulated according to FDA criteria. Only 1.9% samples exceeded levels for AFB1 but none for DON [49]. The data reveal coherence with the obtained results (Table 2). Notwithstanding, a high prevalence for DON was detected and reported by other researchers both for corn and wheat [49]. Conversely, a relatively lower incidence was found in OTA, different from what was conveyed elsewhere [50].
3.2.2. Prevalence in Cattle Feeds
In both dairy and meat cattle, forage, hay, and silage input must not be underplayed, especially in countries where extensive feeding systems based on grazing cattle predominate. Considering Costa Rica a particular case, 85% and 95.9% of the dairy and beef cattle are based on grazing farming, respectively [51]. Relatively favorable toxin profiles were still found in the tested samples. Thereby, surveillance efforts have been focused on compound feed. Generally speaking, ruminants are relatively less sensitive toward the effects of mycotoxins as rumen bacteria play a detoxification role [35,38]. For example, for DON (prevalence of 70.0% and 55.1% in beef cattle feed and dairy cattle feed, respectively), Charmley and collaborators determined that concentrations of 6000 μg kg−1 neither affect feed intake nor are biotransferred to the milk [36,52].
3.2.3. Prevalence in Compound Feed destined for Poultry and Swine
Mycotoxin effects over monogastric animals are varied, depending on the species and physiological and productive stage [53]. For example, in pigs, fumonisin feed contamination is related to pulmonary, hepatic and cardiovascular lesions [54] while DON has been associated with a reduction of productive parameters and feed efficiency [54]. Besides, pigs are especially sensitive to ZEA, as it is directly related to reproductive disorders and low fertility rates [55]. Mycotoxin findings in poultry feed are also worrisome as birds are noticeably susceptible to molecules such as DON. For example, in broilers, trichothecene exposure (e.g., DON), through feed, increases mortality, reduces immune function, and impairs weight gain [56].
3.2.4. Prevalence in Pet Food
Mycotoxins in pet foods have already been reported by other countries, including industrialized ones (e.g., Portugal, USA, England, and Brazil) [57]. Mainly, Fusarium and Penicillium toxins have been described [51]. An elevated prevalence was described for DON and FB1 (50.0% and 93.3%, respectively) [58]. Mycotoxicosis in pets is associated with chronic disease, liver and kidney damage, and cancer [58]. Finding mycotoxins in thermally treated foods is not uncommon as mycotoxins molecules can withstand relatively elevated temperature; low toxin reduction will occur during extrusion. Fungi colonization of pet extruded food is expected to be low as it possesses relatively low values of moisture and water activity [58,59]. Mycotoxin in pet foods may represent an additional burden to humans due to the pet closeness with their owners.
3.2.5. Prevalence in Fish Feed
Presence of mycotoxins in fish feed is another proof of an industry which has progressively substituted animal protein sources for vegetable ones [60,61]. In this regard, DON, OTA, and ZEA have been said to be responsible for weight loss, exacerbated feed conversion, and increased susceptibility to infection and disease in fish [61,62]. In line with the data reported herein, a recent report revealed that commercial fish feed samples were frequently contaminated with DON (i.e., over 80% of the samples) with mean concentrations of 289 μg kg−1 [49]. Levels as low as 4.5 mg DON kg−1 feed have already confirmed adverse effects in productive parameters and increased mortality in some fish. even in a relatively short period [62].
3.3. Geographical Distribution and Climate Influence for Fusarium Toxins Present in Animal Feed
A different spatial distribution profile was observed for AFM1 and OTA, which are not produced by Fusarium species. Fusarium species have the potential of simultaneously producing the remainder of the toxins assayed [63,64]. OTA is a toxin produced by several fungal species including Aspergillus ochraceus, A. carbonarius, A. niger and Penicillium verrucosum [65]. On the other hand, AFM1 is not only produced by Aspergillus species but it is also a product of metabolism [66]. Our data not only demonstrate that most sampling weight is centered on the Costa Rican Central Valley plateau, but the largest concentrations also occur therein (geographical zones with a high average relative humidity of 82%). The data also demonstrate that the intricate climate in tropical countries (such as Costa Rica) predicts the behavior of mycotoxin contamination as more challenging.
3.4. Aflatoxin M1 in Liquid Milk
Milk is not only a staple commodity by itself, but it can accompany other potentially contaminated products (e.g., coffee, tea, or chocolate). Additionally, although AFM1 is the most studied toxin in milk, other toxins have been described as well [67]. Other dairy products are derived from this raw material (e.g., cheese). Although processing is involved, these other dairy products can carry by themselves aflatoxin metabolites as well (see, for example, [68]). During 2017 alone, milk consumption was calculated to be 212 kg per capita [18]. Assuming the worst-case scenario (a sample with the highest concentration of 0.989 µg kg−1), a Costa Rican citizen could be exposed up to 210 µg AFM1 per year. Similarly, a Jersey calf weighing 25–30 kg at birth would be fed with 10% of its live weight with contaminated milk (from 2.5 to 3 kg of milk per day) [69]. Reiteratively, this means a daily exposure of 2.5–3 µg AFM1 per day. Milk weaning can occur at ten weeks old [70]. Milk consumption level exposure is estimated to be 0.023 ng AFM1 per kg body weight per day when a maximum level of 0.5 μg kg−1 is used.
Much higher average concentrations of AFM1 have been documented in other Latin-American countries [71]. Interestingly, AFB1 (the parent compound of AFM1) has been reported to be present in milk samples [71]). Besides the toxic burden that AFB1 and AFM1 have in the liver, recent evidence suggests that kidney toxicity is a certainty [66]. On the other hand, considerably low (i.e., 0.037 µg kg−1) AFM1 levels in milk have been recently reported, although prevalence rates are also relatively high (i.e., 38.8%), [71]. Other Latin-American countries have reported similar percentages [72,73,74,75], and recent prevalence studies have been published in industrialized countries [76,77,78,79]. Epidemiological studies [1] and risk assessment [80,81,82] are paramount to reduce mycotoxin exposure to both humans and animals.
Aflatoxin-contaminated feed must also be monitored to avoid feeding dairy cows with contaminated batches [83]. For instance, the association among most aflatoxin-contaminated feed ingredients and prevalence has been detailed [36,73]. Although the samples reported herein come from a highly industrialized sector, similar prevalence has been reported in fresh milk from small farms [84]. Consistent with our results, the seasonal distribution does not seem to affect AFM1 prevalence [71], probably because Costa Rica has a tropical climate. In general, Costa Rica has relatively high temperatures (19–30°C), humidity (60–91%) and abundant rainfall (1400–4500 mm per year) during a great part of the year (i.e., two distinct seasons), in opposition to an Iranian study exhibited a lower prevalence of AFM1 in bovine milk during spring [85]. Seasonal variations (i.e., during rainy season) were also described for milk from other species (i.e., sheep, goat, and camel) [81]. Other researchers have not documented a clear tendency regarding AFM1 occurrence during seasons [73]. It has been suggested, however, that climate change can bear an impact on human exposure to aflatoxins and health [85]. Finally, the burden of AFM1 exposure for a human can be twice as much as breast milk contamination, as has also been well documented [86]. Although some methods for reducing AFM1 contamination are available [87], pre- and post-harvest strategies are still the most effective strategies [88].
4. Conclusions
Toxicologically relevant concentrations were found during the five-year survey as some sample concentrations exceeded the regulatory guidelines. Fumonisin and deoxynivalenol feed contamination is worrisome since these toxins have the capacity of being found in significant levels in these matrices, and, in our case, higher levels of toxins are found in the Central Valley of the country. Therefore, surveillance programs should be expanded to the outermost productive regions of the country to suppress sampling bias, if existing any. Thermopluvial conditions do not seem to have a considerable effect on toxin levels, although some metabolites actually seem to behave concurrently. Fusarium metabolites must be stridently monitored as it is clear that contamination in feed and feed ingredients is unfortunately common; this is especially true for fumonisins and T-2. Feed manufacturers, farmers (both in the field and storage facilities) and pet owners alike should be educated as to the proper conditions for food storage to avoid mycotoxin-producing fungal colonization. Toxin metabolite analysis and co-occurrence are paramount for complete surveillance of toxin feeds, and efficiently execute systems for the control and reduction of mycotoxins, as well as their metabolites in feeds. In addition, a strict control of AFM1 in milk is necessary, because the prevalence of AFM1 in milk is considerable and several samples exceeded the regulatory thresholds. It must be remembered that milk is the raw material for a wide variety of dairy products (butter, cheese, and yogurt, among others), therefore, the exposure of the population to this mycotoxin is increased.
5. Materials and Methods
5.1. Reagents
An analytical standard with certified concentrations, dissolved in acetonitrile, for DON, 3-ADON, T-2 (TSL-314), HT-2 (TSL-333), ZEA (TSL-401), FB1, FB2 (TSL-202), and OTA (TSL-504) was purchased from Trilogy® Analytical Laboratory Inc (Washington, MO, USA). All standards have an initial concentration of 100 mg L−1, except for FB2 that was at 30 mg L−1. Additionally, a naturally contaminated reference material (TRMT100, cornmeal) was used as a quality control sample (TS-108, Washington, MO, USA). Acetonitrile (ACN) and methanol (MeOH), both chromatographic grade, were purchased from J.T. Baker (Avantor Materials, Center Valley, PA, USA). Ultrapure water (type I, 0.055 µS cm−1 at 25°C, 5 µg L−1 TOC) was obtained using an A10 Milli-Q Advantage system and an Elix 35 system (Merck KGaA, Darmstadt, Germany).
5.2. Sampling
A total of n = 487 different feedstuffs of ca. 5 kg were collected during 2013–2017 by government inspectors from n = 107 Costa Rican feed manufacturers, as part of a countrywide surveillance program. Sample collection was composed of compound feed and feed ingredients, as follows: dairy cattle feed 28.9% (n = 141), cornmeal 9.9% (n = 48), citrus pulp 5.5% (n = 27), cattle feed 5.5% (n = 27), pig feed 5.3% (n = 26), calf feed 4.3% (n = 21), palm kernel meal 4.1% (n = 20), fish feed (Tilapia) 3.7% (n = 18), poultry feed 3.5% (n = 17), distillers dried grains 3.5% (n = 17), hay 3.3% (n = 16), dog food 3.3% (n = 16), wheat middlings 2.9% (n = 14), soybean meal 2.7% (n = 13), layer hen feed 2.0% (n = 10), horse feed 1.8% (n = 9), forage 1.8% (n = 7), pineapple byproducts 1.2% (n = 6), cassava meal 1.2% (n = 6), sorghum meal 0.6% (n = 3), rodent feed 0.6% (n = 3), ground roasted coffee 0.6% (n = 3), banana peel 0.6% (n = 3), rice bran 0.4% (n = 2), chamomile flowers 0.4% (n = 2), soybean hulls 0.2% (n = 1), shrimp feed 0.2% (n = 1), rice meal 0.2% (n = 1), rabbit feed 0.2% (n = 1), hydrolyzed feather meal 0.2% (n = 1), fish feed (snapper, n = 1), fish feed (salmon and trout, n = 1), corn silage (n = 1), and corn gluten (n = 1). Selection of feed and feed ingredients to be tested, number of samples, sampling sites, and specific toxins to assay (per matrix) were chosen by feed control officials. The selection considered the most common feedstuffs used in Costa Rica, import and export regulations, contamination risk factors, the productivity of the feed industry, and the risk for human and animal health associated with each feed or feed ingredient. Sampling was performed following the Association of American Feed Control Officials (AAFCO) recommendations for mycotoxin test object collection [89], and samples were taken from silos and storage reservoirs from feed manufacturing plants. All samples were quartered and sieved (1 mm particle size) [89]. Additionally, n = 180 dairy samples (mostly liquid bovine milk) from n = 13 different Costa Rican dairy farms were assayed; 50 mL subsamples were processed from 500 mL samples.
5.3. Reference Methods for Toxin Determination
Mycotoxins were assayed using the following methods: DON/3-ADON [90], T-2 and HT-2 toxins [91], ZEA AOAC 976.22, fumonisins AOAC 995.15, and OTA AOAC 991.44. AFM1 was assayed according to the methods in [36,92] for milk and butter, respectively.
5.4. Chromatographic System and Conditions
All analytes were assayed using HPLC. Equipment consisted of an Agilent 1260 Infinity series HPLC with a quaternary pump (G1311B), a column compartment (G1316A), a variable wavelength and fluorescence detector (G1314B and G1321B) and an autosampler system (G1329A) (Agilent Technologies, Santa Clara, CA, USA). Peak separation was accomplished using a 5 mm Agilent Zorbax Eclipse C18 column (3.0 × 150 mm, 5 µm) except for T-2/HT-2 toxin analyses for which a Luna® Phenyl-Hexyl column (4.6 × 150 mm, 5 µm) was used (Phenomenex, Torrance, CA, USA). All analytes, except AFM1, were extracted using Immunoaffinity columns (R-biopharm Rhöne Ltd, Darmstadt, Germany).
5.4.1. DON/3-ADON
DONPREP® (R-biopharm) columns were used for sample extraction. Briefly, 200 mL of purified H2O was added to 25 g of test portion. The mixture was dispersed using an Ultra-Turrax® (T25, IKA Works GmbH & Co, Staufen, Germany) at 8000 rpm. The supernatant was filtered by gravity over an ashless filter paper (Grade 541, Whatman®, GE Healthcare Life Sciences, Marlborough, MA, USA). Subsequently, an exact 2 mL aliquot from the supernatant was transferred to the IAC column and passed at 1 mL min−1 using an SPE 12 port vacuum manifold (57044, Visiprep™, Supelco Inc., Bellefonte, PA, USA) at 15 mm Hg vacuum. After a washing step using 2× 10 mL water, the columns were left to dry and then four MeOH fractions of 500 µL were passed through the IAC. The total volume recovered was concentrated to dryness under vacuum at 60°C. The sample was reconstituted with MeOH to 300 µL and transferred to an analytical HPLC conical vial insert (5182-0549, Agilent Technologies, Santa Clara, CA, USA) before injection into the chromatograph.
Gradient mode starting at 80:20 H2O, Solvent A/CH3OH, Solvent B as per chromatographic conditions. The rest of the program was as follows: at 0.5 min 80% A, at 5.50 min 90% A, at 10 min 90% A, at 11 min 80% A, and at 15 min 80% A. DON and 3-ADON absorption at 220 nm was exploited for detection purposes. Linear calibration curves ranging from 1.25 to 10.00 µg mL−1 were prepared during quantification. The limit of quantification for DON/3-ADON was 10.00 and 40.00 μg kg−1.
5.4.2. T-2 and HT-2 Toxin
The extraction was similarly performed as detailed for DON/3ADON using an EASI-EXTRACT® T-2 and HT-2 IAC (R-biopharm). Extraction solvent consisted in 125 mL of MeOH/H2O (90:10) and 2.5 g of NaCl. An aliquot of 5 mL 10-fold diluted in PBS (1.37 mol L−1) was passed through the column. Precolumn derivatization was performed after the evaporation step using 50 µL of 4-dimethylaminopyridine (107700, Sigma-Aldrich, St. Louis, Mo, USA) and 50 µL of 1-anthroyl cyanide (017-12101, FUJIFILM (Wako Pure Chemical Corporation, Osaka, Japan) both at 1 mg mL−1 in toluene (TX0737, Sigma-Aldrich). Gradient mode started at 70:30 CH3CN, Solvent A/H2O, Solvent B as per chromatographic conditions. The rest of the program was as follows: at 5 min 70% A, at 15 min 70% A, at 25 min 85% A, at 27 min 100% A, at 32 min 100% A, and at 35 min 70% A. Flow rate was set at 1 mL min−1. Adduct fluorescence was measured at λex = 381 and λem = 470 nm. Linear calibration curves ranging from 125.00 to 1000.00 µg L−1 were prepared during quantification. The limit of quantification for T-2 and HT-2, was 5.00 and 3.00 μg kg−1, respectively.
5.4.3. ZEA
Extraction was performed using 100 mL of CH3CN/H2O 60:40 and an EASI-EXTRACT® ZEARALENONE IAC (R-biopharm). Isocratic mode using a 40:10:50 CH3CN/CH3OH/H2O mixture at a flow rate of 0.7 mL min−1 was used as per chromatographic conditions. ZEA natural fluorescence (at λex = 236, λem = 464 nm) was exploited for detection purposes. Linear calibration curves ranging from 300.00 to 1200.00 µg L−1 were prepared during quantification. The limit of quantification was 0.072 μg kg−1.
5.4.4. FB1 and FB2
Extraction was performed using 100 mL of CH3CN/MeOH/H2O (25:25:50) and FUMONIPREP® IAC (R-biopharm). Fumonisin derivatization was based on the reaction with o-phthalaldehyde (Millipore Sigma, P0657) and 2-mercaptoethanol (Millipore Sigma, 97622) as stated on the reference method. However, pre-column derivatization was performed in situ in the autosampler injector, according to Bartolomeo and Maisano (2006), but increasing the sample and OPA reagent volume 5-fold. Adduct fluorescence was measured at λex = 335 and λem = 440 nm. Isocratic mode using MeOH/0.1 mol L−1 NaH2PO4 (77:23), adjusted to apparent pH 3.3 with H3PO4, was used at a 0.8 mL min−1 flow rate. The limit of quantification was 0.05 μg kg−1 for both FB1 and FB2.
5.4.5. OTA
Extraction was performed using 100 mL of CH3CN/H2O 60:40 and an OCRAPREP® IAC column. OTA elution from column and resuspension after evaporation was achieved using a 98:2 MeOH and acetic acid solution to ensure OTA protonation. Isocratic mode using a 50:50 H2O/CH3CN mixture using 0.2 mol L−1 trifluoroacetic acid, pH = 2.1 (74564 Millipore Sigma) at a flow rate of 0.7 mL min−1 was used as per chromatographic conditions. OTA natural fluorescence (at λex = 247, λem = 480 nm) was exploited for detection purposes. Linear calibration curves ranging from 2.50 to 40 µg L−1 were prepared during quantification. The limit of quantification was 0.011 μg kg−1.
5.4.6. AFM1 in Milk and Butter
AflaStar® M1 (Romer Labs Diagnostic GmbH, Tulln an der Donau, Austria) columns were used for sample extraction. An exact 50 mL of raw or processed milk, previously homogenized and filtered by gravity over an ashless filter paper, was transferred to the IAC column. After a washing step using 3× 10 mL of water, the columns were left to dry and eluted using MeOH and concentrated as described above in 5.4.1. Isocratic mode using a 10:35:55 CH3CN/CH3OH/H2O mixture at a flow rate of 0.6 mL min−1 was used as per chromatographic conditions. AFM1 natural fluorescence (at λex = 365, λem = 455 nm) was exploited for detection purposes. Linear calibration curves ranging from 0.50 to 2.00 µg L−1 were prepared during quantification. The limit of quantification was 0.014 μg kg−1.
In the case of the butter samples, the preparation was performed according to the method in [84]. Briefly, 25 mL of aqueous methanol (70 mL/100 mL) was added to 5 g of butter. Afterwards, the solution was extracted by mixing gently for 10 min at room temperature using sonication. The extract was filtered through a paper filter, and 15 mL of distilled water was added to 5 mL of filtered solution. After that, 0.25 mL of Tween 20 were added and dispersed for 2 min, followed by the entire amount of the sample solution (20 mL) passing over the IAC.
5.5. Data Analysis
For Table 1, Table 2 and Table 3, prevalence is expressed as the ratio between the total of assays above the limit of detection and the total of assays performed for each toxin. Descriptive statistics displayed in Table 1 are expressed without considering samples below the limit of detection. Heat maps used in Figure 1 were rendered using ArcGIS Pro v2.2 (EsriTM, Redlands, CA, USA). For each contaminant, Spearman Rank Order tests were applied to assess the association among the toxin concentration and climatic variables (i.e., precipitation, rainy days and temperature). In this particular case, toxin levels below the limit of detection were considered zero for association purposes; this analysis was performed using SigmaPlot 14 (Systat Software Inc., San Jose, CA, USA). Sampling date was linked to mean monthly values and data were retrieved from the closest climatological station to the sampling region. Meteorological data were provided by the Costa Rican National Weather Service (https://www.imn.ac.cr/boletin-meteorologico).
Author Contributions
Conceptualization, F.G.-C., and M.A.-C.; methodology, A.L., G.C., and F.G.-C.; validation, F.G.-C.; formal analysis, F.G.-C. and A.L.; resources, M.A.-C., G.C., and F.G.-C.; data curation, A.M. and F.G.-C.; writing—original draft preparation, A.M., A.L. and F.G.-C.; writing—review and editing, F.G.-C.; visualization, F.G.-C. and A.L.; supervision, F.G.-C.; project administration, M.A.-C. and G.C.; and funding acquisition, A.M.
Funding
The University of Costa Rica funded this research through grants ED-427 and ED-428, and the APC was supported by the Office of the Vice Provost for Research of the University of Costa Rica.
Acknowledgments
Geovanna Méndez is acknowledged for tabulating the data that corresponds to the year 2017. Special thanks to Mauricio Redondo-Solano and María Sabrina Sánchez for their suggestions, revising the manuscript and for language editing.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Indicative Levels for T-2 and HT-2 in Cereals and Cereal products according to UE a.
Table A1.
Indicative Levels for T-2 and HT-2 in Cereals and Cereal products according to UE a.
| Matrix | Indicative Levels for the Sum of T-2 and HT-2 (µg kg−1) from Which Onwards/above Which Investigations Should be Performed, Certainly in Case of Repetitive Findings |
|---|---|
| Unprocessed Cereals | |
| Barley (including malting barley) and maize | 200 |
| Oats (with husk) | 1000 |
| Wheat, rye and other cereals | 100 |
| Cereal Products for Feed and Compound Feed | |
| Oat milling products (husks) | 2000 |
| Other cereal products | 500 |
| Compound feed with the exception of feed for cats | 250 |
a Based on Reference [38] and according to 2013/165/EU. Please see notes contained in each recommendation.

Table A2.
Relevant guidance values for each mycotoxin in products intended for animal feed according to UE a.
Table A2.
Relevant guidance values for each mycotoxin in products intended for animal feed according to UE a.
| Mycotoxin | Products Intended for Animal Feed | Guidance Value in mg kg−1 Relative to a Feedstuff with a Moisture Content of 12 g/100 g |
|---|---|---|
| Deoxynivalenol | Feed materials | |
| Cereals and cereal products with the exception | 8 | |
| Cereals and cereal products with the exception | 12 | |
| Compound feed (exception of compound feed for pigs, calves (<4 months), lambs, kids and dogs) | 5 | |
| Compound feed for pigs | 0.9 | |
| Compound feed for calves (<4 months), lambs, kids and dogs | 2 | |
| Zearalenone | Feed materials | |
| Cereals and cereal products with the exception of maize byproducts | 2 | |
| Maize byproducts | 3 | |
| Compound feed for: | ||
| Piglets, gilts (young sows), puppies, kittens, dogs and cats for reproduction | 0.1 | |
| Adult dogs and cats other than for reproduction | 0.2 | |
| Sows and fattening pigs | 0.25 | |
| Calves, dairy cattle, sheep (including lamb) and goats (kids) | 0.5 | |
| Ochratoxin A | Feed materials | |
| Cereals and cereal products | 0.25 | |
| Compound feed for | ||
| Pigs | 0.05 | |
| Poultry | 0.1 | |
| Cats and dogs | 0.01 | |
| Fumonisin FB1 + FB2 | Feed materials | |
| Maize and maize products | 60 | |
| Compound feed for | ||
| Pigs, horses (Equidae), rabbits and pet animals | 5 | |
| Fish | ||
| Poultry, calves (<4 months), lambs and kids | 20 | |
| Adult ruminants (> 4 months) and mink | 50 | |
| T2 + HT-2 | Compound Feed for Cats | 0.05 |
a Based on Reference [37] and according to 2006/576/EC, 2016/1319, and definitions stated in 68/2013/EC. Please see notes contained in each recommendation.
References
- Tola, M.; Kebede, B. Occurrence, Importance and Control of Mycotoxins: A Review. Cogent Food Agric. 2016. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, D. Worldwide occurrence of mycotoxins in cereals and cereal derived food products: Public Health Perspectives of their Co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef] [PubMed]
- Arcella, D.; Gergelova, P.; Innocenti, M.L.; Steinkellner, H. Human and animal dietary exposure to T-2 and HT-2. EFSA J. 2017, 15, 4972. [Google Scholar]
- Chen, S.S.; Li, Y.-H.; Lin, M.-F. Chronic Exposure to the Fusarium Mycotoxin Deoxynivalenol: Impact on Performance, Immune Organ, and Intestinal Integrity of Slow-Growing Chickens. Toxins 2017, 9, 334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, J.; Zhu, C.-C.; Tang, F.; Cui, X.-S.; Kim, N.-H. Exposure to HT-2 toxins causes oxidative stress-induced apoptosis/autophagy in porcine oocytes. Sci. Rep. 2016, 6, 33904. [Google Scholar] [CrossRef]
- Ismail, Z.; Basha, E.A.; Al-Nabulsi, F. Mycotoxins in animal feed, hazardous to both animals and human health. J. Vet. Med. Res. 2018, 5, 1145. [Google Scholar]
- Bertero, A.; Moretti, A.; Spicer, L.J.; Caloni, F. Fusarium Molds and Mycotoxins: Potential Species-Specific Effects. Toxins 2018, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, A.; Keese, C.; Meyer, U.; Starke, A.; Wrenzycki, C.; Dänicke, S.; Rehage, J. Chronic Effects of Fusarium Mycotoxins in rations with or without Increased Concentrate Proportion on the Insulin Sensitivity in Lactating Dairy Cows. Toxins 2018, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Ashiq, S. Natural Occurrence of Mycotoxins in Food and Feed: Pakistan Perspective. Compr. Rev. Food Sci. Food Saf. 2015, 14, 159–175. [Google Scholar] [CrossRef]
- Antonissen, G.; Van Immerseel, F.; Pasmans, F.; Ducatelle, R.; Haesebrouck, F.; Timbermont, L.; Verlinden, M.; Janssens, G.P.J.; Eeckhaut, V.; Eekhpout, M.; et al. The mycotoxin deoxynivalenol predisposes for the development of Clostridium perfringens-induced necrotic enteritis in broiler chickens. PLoS ONE 2014, 9, e108775. [Google Scholar] [CrossRef] [PubMed]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicoau, A.; Aprodu, I.; Puel, O.; et al. Current Situation of Mycotoxin Contamination and Co-occurrence in Animal Feed-Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.; Pardo, A.; Pose, G. Mycotoxigenic Fungi and Natural Co-Occurrence of Mycotoxins in Rainbow Trout (Oncorhynchus mykiss) Feeds. Toxins 2015, 7, 4595–4609. [Google Scholar] [CrossRef] [PubMed]
- Ismaeil, A.A.; Papenbrock, J. Mycotoxins: Producing Fungi and Mechanisms of Phytotoxicity. Agriculture 2015, 5, 492–537. [Google Scholar] [CrossRef]
- Rostagno, H.; Teixeira, L.; Hannas, M.; Lopes, J.; Kazue, N.; Perazzo, F.; Saraiva, A.; Teixeira de Abreu, M.; Borges, P.; Flávia De Oliveira, R.; et al. Tablas Brasileñas Para aves y Cerdos, 4th ed.; Universidad Federal de Viçosa, Departamento de Zootecnia: Viçosa, Brasil, 2017; pp. 28–256. [Google Scholar]
- De Blass, C.; Mateos, G.G.; García-Rebollar, P. Tablas FEDNA de Composición y Valor Nutritivo de Alimentos Para la Fabricación de Piensos Compuestos, 3rd ed.; Fundación Española para el Desarrollo de la Nutrición Animal: Madrid, Spain, 2010; Available online: http://www.fundacionfedna.org/ingredientes-para-piensos (accessed on 4 February 2019).
- SEPSA [Secretaría Ejecutiva de Planificación Agropecuaria]. Informe de Comercio Exterior del Sector Agropecuario 2016–2017. 2018. Available online: http://www.sepsa.go.cr/docs/2018-004-Informe_Comercio_Exterior_Sector_Agropecuario_2016-2017.pdf (accessed on 4 February 2019).
- Chacón, M. Evolución del Cultivo de Maíz en Costa Rica. Oficina Nacional de Semillas, 2017. Available online: http://ofinase.go.cr/certificacion-de-semillas/certificacion-de-semillas-de-maiz/evolucion-cultivo-maiz/ (accessed on 4 February 2019).
- CIAB [Cámara de Industriales de Alimentos Balanceados]. Situación Actual de Alimentos Balanceados—Informe Anual. 2018. Available online: https://www.ciabcr.com/charlas/Nutrici%C3%B3n%20Animal%202018/Charlas/Carl_Oroz.pdf (accessed on 4 February 2019).
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhang, L.; Su, Y.-T.; Xie, W.-M.; Zhang, N.-Y.; Dai, J.-F.; Wang, Y.; Rajput, S.A.; Qi, D.-S.; Karrow, N.A.; et al. Individual and Combined Occurrence of Mycotoxins in Feed Ingredients and Complete Feeds in China. Toxins 2018, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Chagwa, R.; Abia, W.; Msagati, T.; Nyoni, H.; Ndleve, K.; Njobeh, P. Multi-Mycotoxin Occurrence in Dairy Cattle Feeds from the Gauteng Province of South Africa: A Pilot Study Using UHPLC-QTOF-MS/MS. Toxins 2018, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.T.; Petta, T.; Rottinghaus, G.E.; Bordin, K.; Gomes, G.A.; Oliveira, C.A.F. Co-occurrence of mycotoxins in maize food and maize-based feed from small-scale farms in Brazil: A pilot study. Mycotoxin Res. 2018. [Google Scholar] [CrossRef]
- Ul Hassan, Z.; Al Thani, R.; Atia, F.A.; Al Meer, S.; Migheli, Q.; Jaoua, S. Co-occurrence of mycotoxins in commercial formula milk and cereal-based baby food in Qatar. Food Addit. Contam. Part B 2018, 11, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Bozzo, G.; Bonerba, E.; Ceci, E.; Valeriana, C.; Tantillo, G. Determination of ochratoxin A in eggs and target tissues of experimentally drugged hens using HPLC–FLD. Food Chem. 2011, 126, 1278–1282. [Google Scholar]
- Payros, D.; Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I.P. Toxicology for deoxynivalenol and its acetylated and modified forms. Arch. Toxicol. 2016, 90, 2931–2957. [Google Scholar] [CrossRef]
- Munkvold, G.P.; Arias, S.; Taschl, I.; Gruber-Dorninger, C. Chapter 9: Mycotoxin in Corn: Occurrence, Impacts and Management. In Corn; AACC International Press: St. Paul, MN, USA, 2019; pp. 235–287. [Google Scholar] [CrossRef]
- Guerre, P. Fusariotoxins in Avian Species: Toxicokinetics, Metabolism, and Persistence in Tissues. Toxins 2015, 7, 2289–2305. [Google Scholar] [CrossRef] [PubMed]
- Alshannaq, A.; Yu, J.H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Envron. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed]
- Krska, R.; Sulyok, M.; Berthiller, F.; Schuhmacher, R. Mycotoxin testing: From Multi toxin analysis to metabolomics. JSM Mycotoxins 2017, 67, 11–16. [Google Scholar] [CrossRef]
- Van der Fels-Klerx, H.J.; Adamse, P.; Punt, A.; van Asselt, E.D. Data analyses and modeling for risk-based monitoring of mycotoxins in animal feed. Toxins 2018, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Streit, E.; Naehrer, K.; Rodrigues, I.; Schatzmayr, G. Mycotoxin occurrence in feed and feed raw materials worldwide: Long-term analysis with special focus on Europe and Asia. J. Sci. Food Agric. 2013, 93, 2892–2899. [Google Scholar] [CrossRef]
- Pinotti, L.; Ottoboni, M.; Giormini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin Contamination in the EU Feed Supply Chain: A Focus on Cereal Byproducts. Toxins 2016, 8, 45. [Google Scholar] [CrossRef]
- Rodrigues, I.; Naehrer, K. A Thre-Year Survey on the Worldwide Occurrence of mycotoxins in feedstuff and feed. Toxins 2012, 4, 663–675. [Google Scholar] [CrossRef]
- Selvaraj, J.N.; Wang, Y.; Zhou, L.; Zhao, Y.; Xing, F.; Dai, X.; Liu, Y. Recent mycotoxin survey data and advanced mycotoxin detection techniques reported from China: A review. Food Addit. Contam. Part A 2015, 32, 440–452. [Google Scholar] [CrossRef]
- Granados-Chinchilla, F.; Molina, A.; Chavarría, G.; Alfaro-Cascante, M.; Bogantes, D.; Murillo-Williams, A. Aflatoxins occurrence through the food chain in Costa Rica: Applying the One Health approach to mycotoxin surveillance. Food Control 2017, 82, 217–226. [Google Scholar] [CrossRef]
- Chavarría, G.; Granados-Chinchilla, F.; Alfaro-Cascante, M.; Molina, A. Detection of Aflatoxin Mn milk, cheese and sour cream samples from Costa Rica using enzyme-assisted extraction and HPLC. Food Addit. Contam. Part B 2015, 8, 128–135. [Google Scholar] [CrossRef]
- EU Commission Recommendations (2006/576/EC) of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2016, 229, 7–9.
- EU Commission Recommendations (2013/165/EU) of March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products. Off. J. Eur. Union 2013, 91, 12–15.
- FAO (Food and Agriculture Organization of the United Nations). Aquaculture Feed and Fertilizer Resources Information System. 2018. Available online: http://www.fao.org/fishery/affris/species-profiles/nile-tilapia/tables/en/ (accessed on 19 July 2018).
- INRA. Equine Nutrition, 1st ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2015; pp. 97–120. [Google Scholar]
- FEDIAF. Nutritional Guidelines for Complete and Complementary Pet Food for Cats and Dog; FEDIAF: Brussels, Belgium, 2016; pp. 1–102. [Google Scholar]
- Martínez Marín, A.L. Inclusion of feeds in pelleted concentrates intended for stall-fed leisure horses. Arch. Zootec 2008, 57, 115–122. [Google Scholar]
- Milani, J.M. Ecological conditions affecting mycotoxin production in cereals: A review. Vet. Med. 2013, 58, 405–411. [Google Scholar] [CrossRef]
- Wu, L.; Qiu, L.; Zhang, H.; Sun, J.; Hu, X.; Wang, B. Optimization for the Production of Deoxynivalenol and Zearalenone by Fusarium graminearum Using Response Surface Methodology. Toxins 2017, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- CODEX. Code of Practice for the Prevention and Reduction of Mycotoxin Contamination in Cereals; CAC/RCP 51-2003; CODEX: Rome, Italy, 2014. [Google Scholar]
- Pettersson, H. Mycotoxin contamination of animal feed. In Woodhead Publishing Series in Food Science, Technology and Nutrition, Animal Feed Contamination; Fink-Gremmels, J., Ed.; Woodhead Publishing: Cambridge, UK, 2012; ISBN 9781845697259. [Google Scholar] [CrossRef]
- Leiva, A.; Granados-Chinchilla, F.; Redondo-Solano, M.; Arrieta-González, M.; Pineda Salazar, E.; Molina, A. Characterization of the animal by-product meal industry in Costa Rica: Manufacturing practices through the production chain and food safety. Poult. Sci. 2018, 97, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- U.S. Grains Council. 2017/2018 Corn Harvest Quality Report 2018. Available online: https://grains.org/corn_report/corn-harvest-quality-report-2017-2018/ (accessed on 4 February 2019).
- Xie, S.; Zheng, L.; Wan, M.; Niu, J.; Liu, Y.; Tian, L. Effect of deoxynivalenol on growth performance, histological morphology, anti-oxidative ability and immune response of juvenile Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2018, 82, 442–452. [Google Scholar] [CrossRef]
- Di Stefano, V. Occurrence & Risk of OTA Food and Feed in Food and Feed. Ref. Modul. Food Sci. 2018. [Google Scholar] [CrossRef]
- INEC (Instituto Nacional de Estadística y Censos). Encuesta Nacional Agropecuaria 2017: Resultados Generales de las Actividades Ganaderas Vacuna y Porcina. 2019. Available online: http://www.inec.go.cr/multimedia/encuesta-nacional-agropecuaria-2017-datos-de-la-ganaderia-vacuna-y-porcina (accessed on 4 February 2019).
- Charmley, E.; Trenholm, H.L.; Thompson, B.K.; Vudathala, D.; Nicholson, J.W.G.; Prelusky, B.D.; Charmley, L.L. Influence of level of deoxynivalenol in the diet of dairy cows on feed intake, milk production, and its composition. J. Dairy Sci. 1993, 76, 3580–3587. [Google Scholar] [CrossRef]
- CAST. Mycotoxins: Risks in Plant, Animal, and Human Systems. Council for Agricultural Science and Technology; CAST: Ames, IA, USA, 2003; pp. 1–217. [Google Scholar]
- Focht Müller, L.K.; Paiano, D.; Gugel, J.; Lorenzetti, W.R.; Morais Santurio, J.; de Castro Tavernari, F.; Micotti da Gloria, E.; Baldissera, M.D.; Da Silva, A.S. Post-weaning piglets fed with different levels of fungal mycotoxins and spray-dried porcine plasma have improved weight gain, feed intake and reduced diarrhea incidence. Microb. Pathog. 2018, 117, 259–264. [Google Scholar] [CrossRef]
- Dänicke, S.; Winkler, J. Invited review: Diagnosis of zearalenone (ZEN) exposure of farm animals and transfer of its residues into edible tissues (carry over). Food Chem. Toxicol. 2015, 84, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Hogan, N.S. Performance effects of feed-borne Fusarium mycotoxins on broiler chickens: Influences of timing and duration of exposure. Anim. Nutr. 2018, 5, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Boermans, H.J.; Leung, M.C.K. Mycotoxins and the pet food industry: Toxicological evidence and risk assessment. Int. J. Food Microbiol. 2007, 119, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Gazzotti, T.; Biagi, G.; Pagliuca, G.; Pinna, C.; Scardilli, M.; Grandi, M.; Zaghini, G. Occurrence of mycotoxins in extrude commercial dog food. Anim. Feed Sci. Technol. 2015, 202, 81–89. [Google Scholar] [CrossRef]
- Atungulu, G.G.; Mohammadi-Shad, Z.; Wilson, S. Chapter 2—Mycotoxin Issues in Pet. In Food and Feed Safety Systems and Analysis; Academic Press: Cambridge, MA, USA, 2018; pp. 25–44. [Google Scholar]
- García-Herranz, V.; Valdehita, A.; Navvas, J.M.; Fernández-Cruz, M.L. Cytotoxicity against fish and mammalian cells lines and endocrine activity of the mycotoxins beauvericin, deoxynivalenol and ochratoxin-A. Food Chem. Toxicol. 2019. [Google Scholar] [CrossRef]
- Gonçalves, R.A.; Navarro-Guillén, C.; Gilannejad, N.; Días, J.; Schatzmayr, D.; Bichl, G.; Czabany, T.; Moyano, F.J.; Rema, P.; Yúfera, M.; et al. Impact of deoxynivalenol on rainbow trout: Growth performance, digestibility, key gene expression regulation and metabolism. Aquaculture 2018, 490, 362–372. [Google Scholar] [CrossRef]
- Woźny, M.; Obremski, K.; Hliwa, P.; Gomulka, P.; Rożyński, R.; Wojtacha, P.; Florczyk, M.; Segner, H.; Brzuzan, P. Feed contamination with zearalenone promotes growth but affects the immune system of rainbow trout. Fish Shellfish Immunol. 2019, 84, 680–694. [Google Scholar]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef]
- Shi, W.; Tan, Y.; Wang, S.; Gardiner, D.M.; De Saeger, S.; Liao, Y.; Wang, C.; Fan, Y.; Wang, Z.; Wu, A. Mycotoxigenic Potentials of Fusarium Species in Various Culture Matrices Revealed by Mycotoxin Profiling. Toxins 2017, 9, 6. [Google Scholar] [CrossRef]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 Years of Research. Toxins 2016, 8, 191. [Google Scholar] [CrossRef]
- Li, H.; Xing, L.; Zhang, M.; Wang, J.; Zheng, N. The Toxic Effects of Aflatoxin B1 and Aflatoxin M1 on Kidney through Regulating L-Proline and Downstream Apoptosis. BioMed Res. Int. 2018, 2018, 9074861. [Google Scholar] [CrossRef] [PubMed]
- Becker-Algeri, T.A.; Castagnaro, D.; de Bartoli, K.; de Souza, C.; Drunkler, D.A.; Badiale-Furlong, E. Mycotoxins in Bovine Milk and Dairy Products: A Review. J. Food Sci. 2016, 81, R545–R552. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Martínez, A.; Camarillo-Hernández, E.; Carvajal-Moreno, M.; Vargas-Ortiz, M.; Wesolek, N.; Del Carmen, G.; Jimenes, R.; García Alvarado, M.A.; Roudot, A.-C.; Salgado Cervantes, M.A.; et al. Assessment of Aflatoxin M1 and M2 exposure risk through Oaxaca cheese consumption in southeastern Mexico. Int. J. Environ. Health Res. 2018, 28, 202–213. [Google Scholar]
- US Jersey. A Quality Heifer. Cornell University, 2008. Available online: https://www.usjersey.com/Portals/0/AJCA/2_Docs/QualityHeiferBrochure.pdf (accessed on 27 February 2019).
- Franklin, S.T.; Amaral-Phillips, D.M.; Jackson, J.A.; Campbell, A.A. Health and performance of Holstein calves that suckled or were hand-fed colostrum and were fed one of three physical forms of starter. J. Dairy Sci. 2003, 86, 2145–2153. [Google Scholar] [CrossRef]
- Scaglioni, P.T.; Becker-Algeri, T.; Drunkler, D.; Badiale-Furlong, E. Aflatoxin B1 and M1 in milk. Anal. Chim. Acta 2014, 829, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Michlig, N.; Signorini, M.; Gaggiotti, M.; Chiericatti, C.; Basílico, J.C.; Repetti, M.R.; Beldomenico, H.R. Risk factors associated with the presence of aflatoxin M1 in raw bulk milk from Argentina. Food Control 2016, 64, 151–156. [Google Scholar] [CrossRef]
- Quevedo-Garza, P.A.; Amador-Espejo, G.G.; Cantú-Martínez, P.C.; Trujillo-Mesa, J.A. Aflatoxin M1 occurrence in fluid milk commercialized in Monterrey, Mexico. J. Food Saf. 2018, 38, e12507. [Google Scholar] [CrossRef]
- Peña-Rodas, O.; Martinez-Lopez, R.; Hernandez-Rauda, R. Occurrence of Aflatoxin M1 in cow milk in El Salvador: Results from a two-year survey. Toxicol. Rep. 2018, 5, 671–678. [Google Scholar] [CrossRef]
- Sibaja, K.V.; Gonçalves, K.D.M.; Garcia, S.D.O.; Feltrin, A.C.P.; Noguiera, W.V.; Badiale-Furlong, E.; Garda-Buffon, J. Aflatoxin M1 and B1 in Colombian milk powder and estimated risk exposure. Food Addit. Contam. Part B 2019, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.Z.; Jinap, S.; Pirouz, A.A.; Faizal, A.R.A. Aflatoxin M1 in milk and dairy products, occurrence and recent challenges: A review. Trends Food Sci. Technol. 2015, 46, 110–119. [Google Scholar] [CrossRef]
- Yoon, B.R.; Hong, S.-Y.; Cho, S.M.; Lee, K.R.; Kim, M.; Chung, S.H. Aflatoxin M1 levels in dairy products from South Korea determined by high-performance liquid chromatography with fluorescence detection. J. Food Nutr. Res. 2016, 55, 171–180. [Google Scholar]
- Assunçao, R.; Martins, C.; Viegas, S.; Viegas, C.; Jakobsen, L.S.; Pires, S.; Alvito, P. Climate change and the health impact of aflatoxins exposure in Portugal—An overview. Food Addit. Contam. Part A 2018, 35, 1610–1621. [Google Scholar] [CrossRef]
- Bellio, A.; Bianchi, D.M.; Gramaglia, M.; Loria, A.; Nucera, D.; Gallina, S.; Gili, M.; Decastelli, L. Aflatoxin M1 in Cow’s Milk: Method Validation for Milk Sampled in Northern Italy. Toxins 2016, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.Y.; Watson, S.; Routledge, M.N. Aflatoxin Exposure and Associated Human Health Effects, a Review of Epidemiological Studies. Food Saf. 2016, 4, 14–27. [Google Scholar] [CrossRef]
- Milićević, D.R.; Spirić, D.; Radičević, T.; Velebit, B.; Steganović, S.; Milojević, L.; Janković, S. A review of the current situation of aflatoxin M1 in cow’s milk in Serbia: Risk assessment and regulatory aspects. Food Addit. Contam. Part A 2017, 34, 1617–1631. [Google Scholar] [CrossRef] [PubMed]
- Ahlberg, S.; Grace, D.; Kiarie, G.; Kirino, Y.; Lindahl, J. A Risk Assessment of Aflatoxin M1 Exposure in Low Mid-Income Dairy Consumers in Kenya. Toxins 2018, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Walte, H.-G.; Schwake-Anduschus, C.; Geisen, R.; Fritsche, J. Afaltoxin: Food chain transference from feed to food. J. Verbr. Lebensm 2016, 11, 295–297. [Google Scholar] [CrossRef]
- Gonçalves, L.; Dalla Rosa, A.; Gonzales, S.L.; Feltes, M.M.C.; Badiale-Furlong, E.; Dors, G.C. Incidence of aflatoxin M1 in fresh milk from small farms. Food Sci. Technol. 2017, 37, 11–15. [Google Scholar]
- Fallah, A.A.; Fazlollahi, R.; Emami, A. Seasonal study of aflatoxin M1 contamination in milk of four dairy species in Yazd, Iran. Food Control 2016, 68, 77–82. [Google Scholar] [CrossRef]
- Cantú-Cornelio, F.; Aguilar-Toalá, J.E.; de León-Rodríguez, C.I.; Esparza-Romero, J.; Vallejo-Córdoba, A.F.; García, H.S.; Hernández-Mendoza, A. Occurrence and factors associated with the presence of aflatoxin M1 in breast milk samples of nursing mothers in central Mexico. Food Control 2016, 62, 16–22. [Google Scholar] [CrossRef]
- Naeimipour, F.; Aghajani, J.; Kojuri, S.A.; Ayoubi, S. Useful Approaches for Reducing Aflatoxin M1 Content in Milk and Dairy Products. Biomed. Biotechnol. Res. J. 2018, 2, 94–99. [Google Scholar]
- Udomkun, P.; Wiredu, A.N.; Nagle, M.; Müller, J.; Vanlauwe, B.; Bandyopadhyay, R. Innovative technologies to manage aflatoxins in foods and feeds and the profitability of application—A review. Food Control 2017, 76, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Association of American Feed Control Officials (AAFCO). Feed Inspector’s Manual, 7th ed.; AAFCO Inspection and Sampling Committee: Atlanta, GA, USA, 2017. [Google Scholar]
- Czerwiecki, L.; Wilczyńska, G. Determination of Deoxynivalenol in cereal by HPLC-UV. Mycotoxin Res. 2003, 19, 31–34. [Google Scholar] [CrossRef]
- Visconti, A.; Lattanzio, V.M.T.; Pascale, M.; Haidukowski, M. Analysis of T-2 and HT-2 toxins in cereal grains by immunoaffinity clean-up and liquid chromatography with fluorescence detection. J. Chromatogr. A 2005, 1075, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Aydemir Atasever, M.; Atasever, M.; Özturan, K.; Urçar, S. Determination of Aflatoxin M1 level in Butter Samples Consumed in Erzurum, Turkey. Kafkas Univ. Vet. Fak. Derg. 2010, 16 (Suppl. A), S159–S162. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).