Binding to The Target Cell Surface Is The Crucial Step in Pore Formation of Hemolysin BL from Bacillus cereus
Abstract
1. Introduction
2. Results
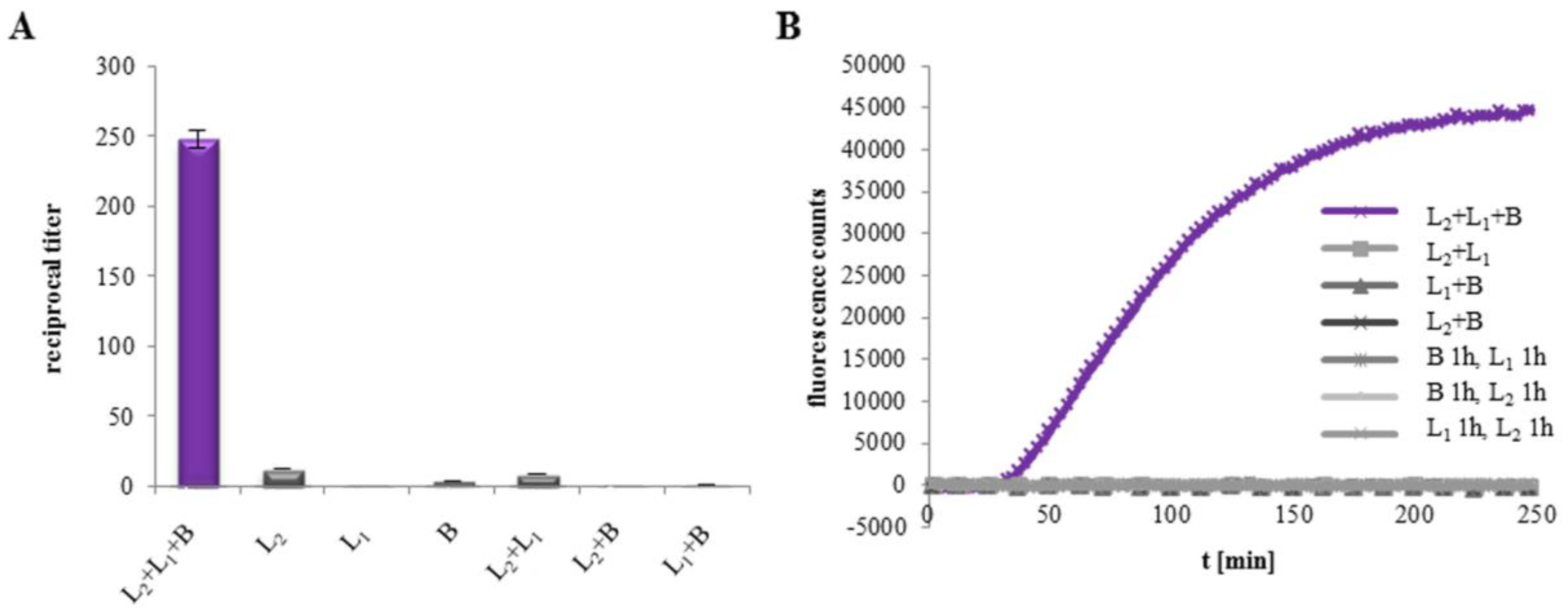
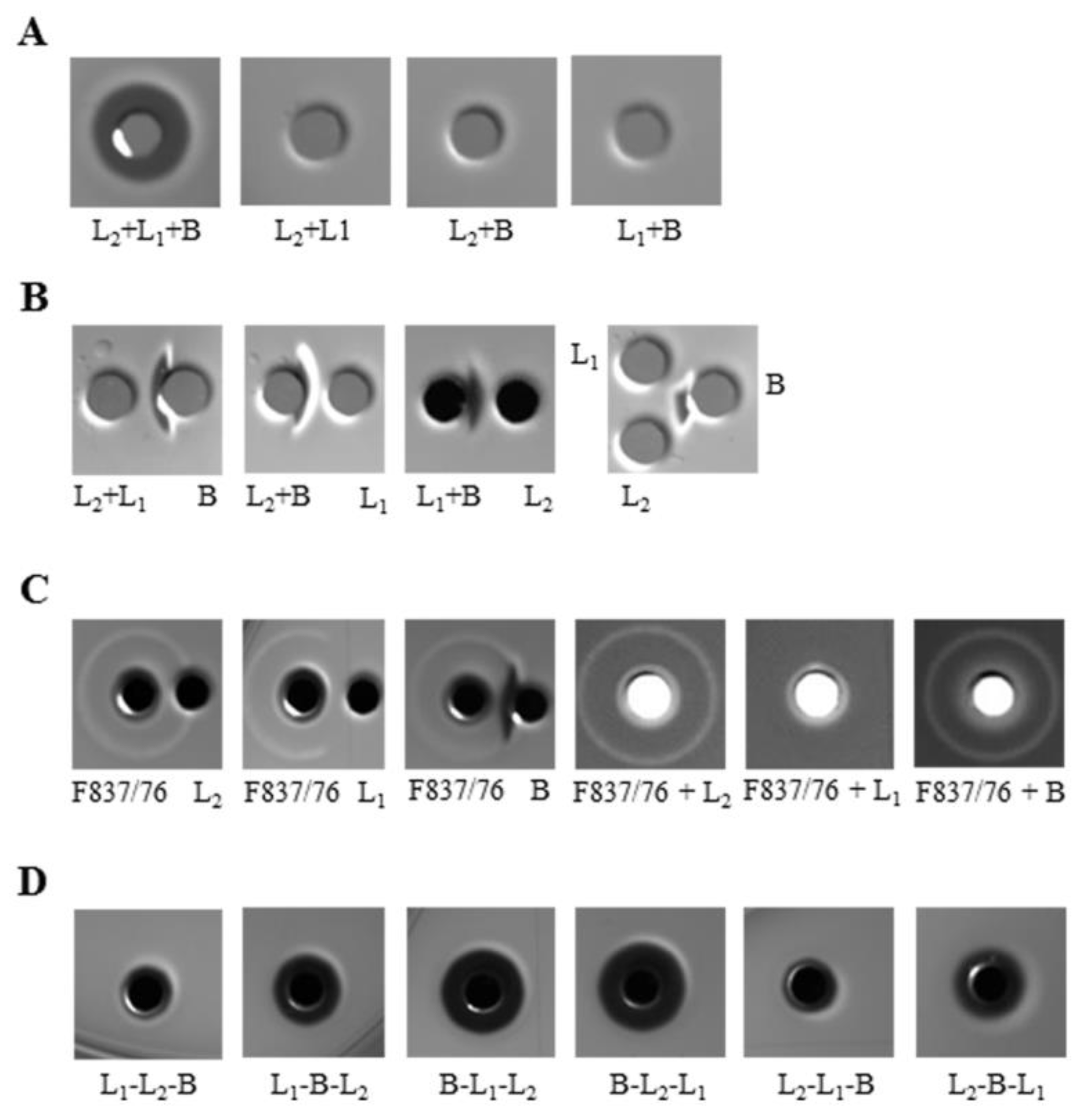
2.1. All Three Hbl Components Are Necessary for Cytotoxicity and Pore Formation
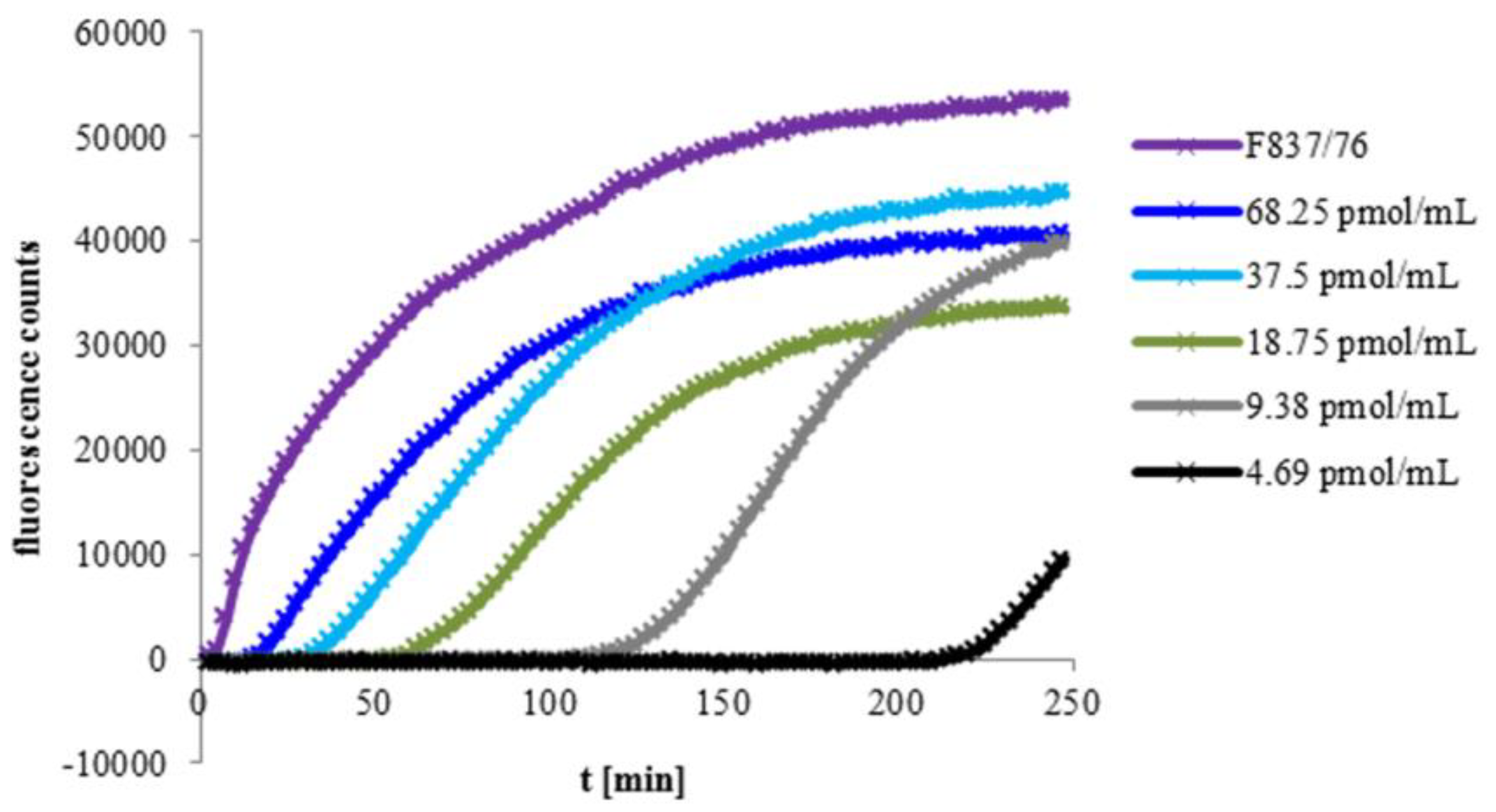
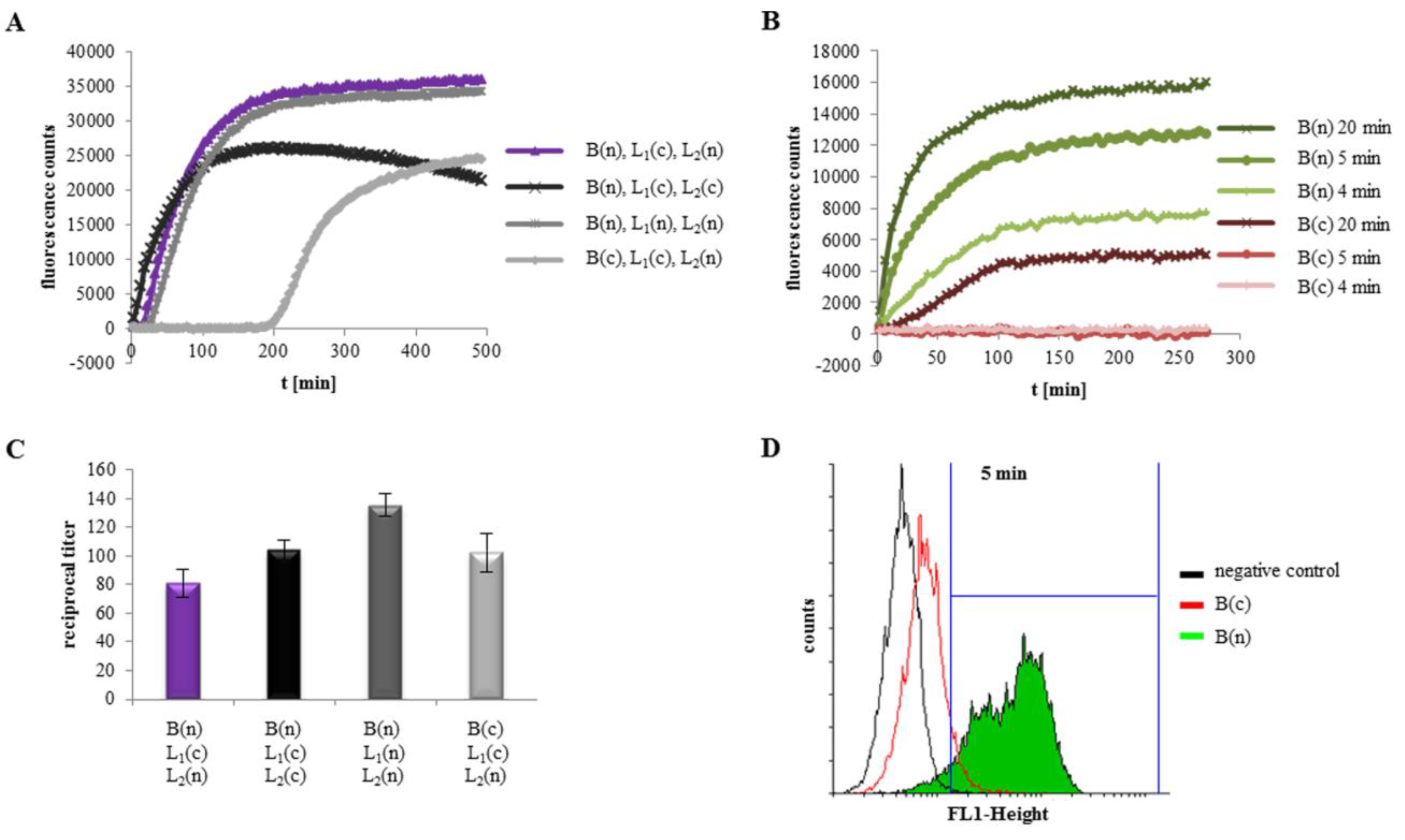
2.2. Velocity of Pore Formation Depends on rHbl Concentration
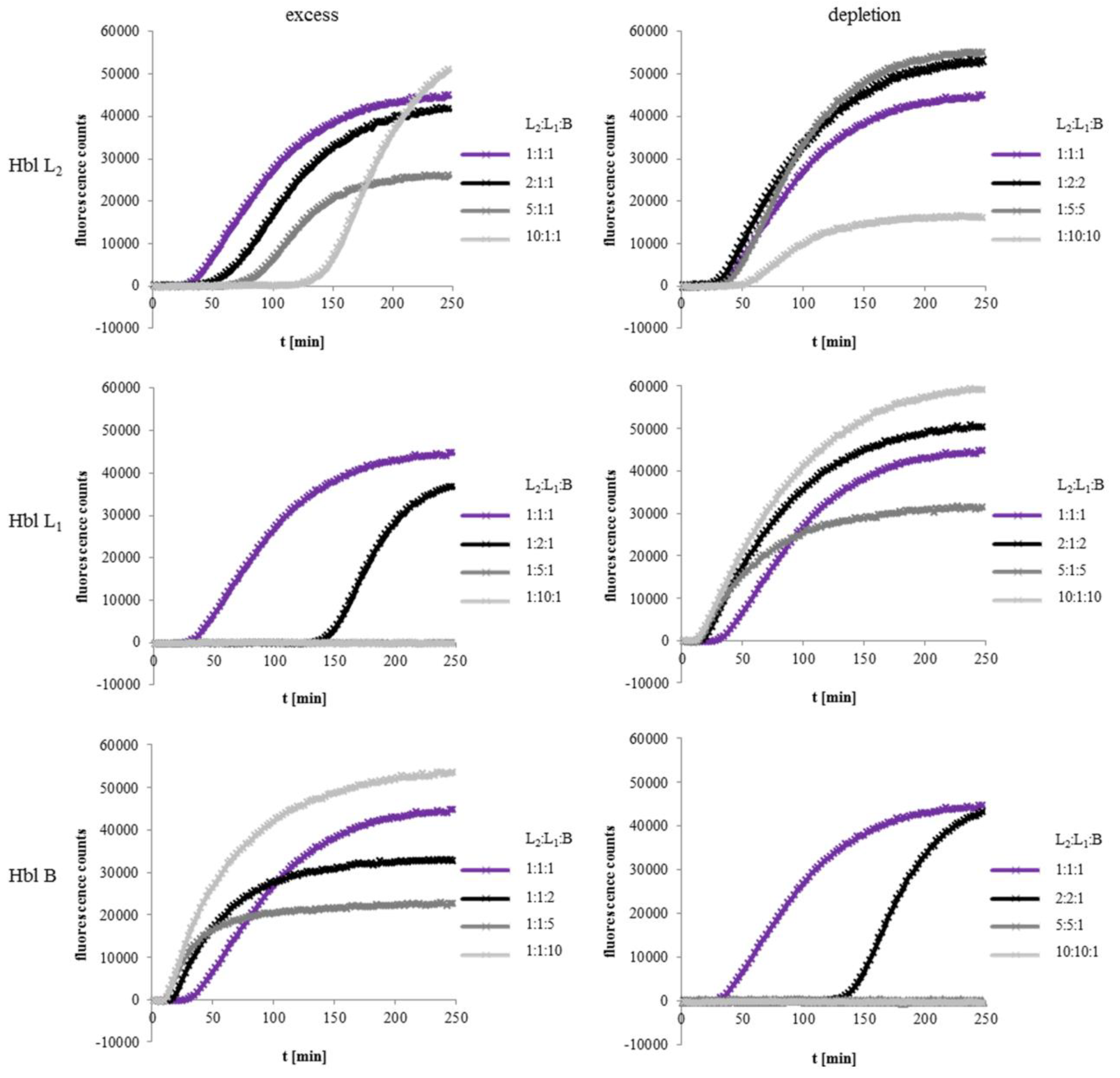
2.3. Excess of rHbl B Accelerates, Excess of rHbl L1 Hinders Pore Formation
2.4. Hbl Activity Is Due to Sequential Binding of The Components B - L1 - L2 as Well as Their Concerted Action
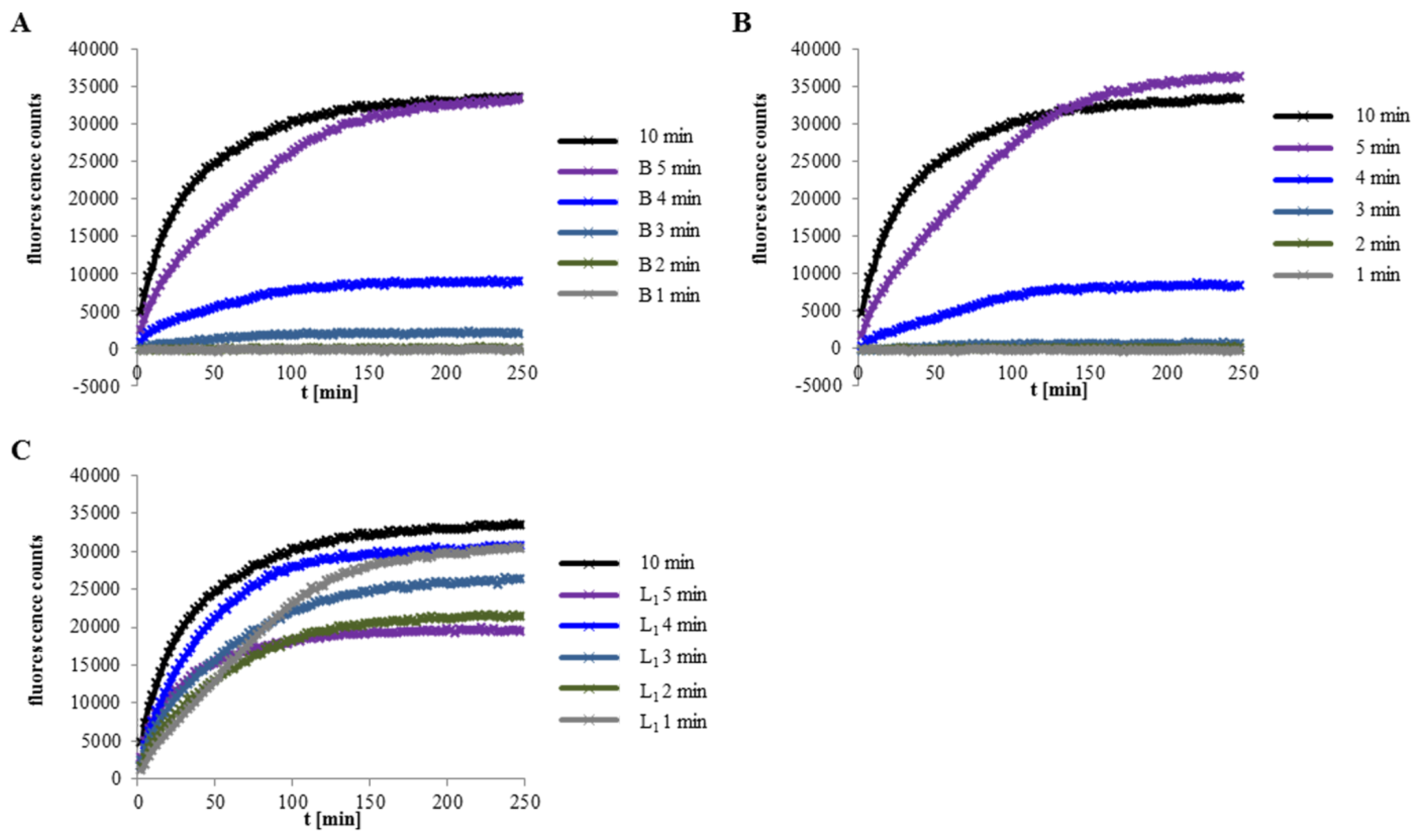
2.5. Binding of Hbl B to The Cell Surface Is The Crucial Step for Pore Formation
2.6. rHbl B Needs A Free C-Terminus for Optimum Cell Binding
2.7. Hemolytic Activity of rHbl Confirms Results on Vero Cells
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Production of Purified, Recombinant (r) Hbl Components
4.3. Cell Line and Culture Conditions
4.4. Cytotoxicity Assays
4.5. Flow Cytometry Analyses
4.6. Determination of Hemolytic Activity
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- European Food Safety Authority. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks 2011. EFSA J. 2013, 11, 3129. [Google Scholar]
- Mead, P.S.; Slutsker, L.; Dietz, V.; McCaig, L.F.; Bresee, J.S.; Shapiro, C.; Griffin, P.M.; Tauxe, R.V. Food-related illness and death in the United States. Emerg. Infect. Dis. 1999, 5, 607–625. [Google Scholar] [CrossRef]
- Scallan, E.; Griffin, P.M.; Angulo, F.J.; Tauxe, R.V.; Hoekstra, R.M. Foodborne illness acquired in the United States--unspecified agents. Emerg. Infect. Dis. 2011, 17, 16–22. [Google Scholar] [CrossRef]
- Stenfors Arnesen, L.P.; Fagerlund, A.; Granum, P.E. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 2008, 32, 579–606. [Google Scholar] [PubMed]
- Lund, T.; Granum, P.E. Characterisation of a non-haemolytic enterotoxin complex from Bacillus cereus isolated after a foodborne outbreak. FEMS Microbiol. Lett 1996, 141, 151–156. [Google Scholar] [CrossRef]
- Beecher, D.J.; Schoeni, J.L.; Wong, A.C. Enterotoxic activity of hemolysin BL from Bacillus cereus. Infect. Immun. 1995, 63, 4423–4428. [Google Scholar] [PubMed]
- From, C.; Pukall, R.; Schumann, P.; Hormazábal, V.; Granum, P.E. Toxin-producing ability among Bacillus spp. outside the Bacillus cereus group. Appl. Environ. Microbiol. 2005, 71, 1178–1183. [Google Scholar]
- Guinebretiére, M.H.; Broussolle, V.; Nguyen-The, C. Enterotoxigenic profiles of food-poisoning and food-borne Bacillus cereus strains. J. Clin. Microbiol. 2002, 40, 3053–3056. [Google Scholar] [CrossRef]
- Moravek, M.; Dietrich, R.; Buerk, C.; Broussolle, V.; Guinebretière, M.H.; Granum, P.E.; Nguyen-The, C.; Märtlbauer, E. Determination of the toxic potential of Bacillus cereus isolates by quantitative enterotoxin analyses. FEMS Microbiol. Lett. 2006, 257, 293–298. [Google Scholar]
- Wehrle, E.; Moravek, M.; Dietrich, R.; Bürk, C.; Didier, A.; Märtlbauer, E. Comparison of multiplex PCR, enzyme immunoassay and cell culture methods for the detection of enterotoxinogenic Bacillus cereus. J. Microbiol. Methods 2009, 78, 265–270. [Google Scholar] [PubMed]
- Ryan, P.A.; Macmillan, J.D.; Zilinskas, B.A. Molecular cloning and characterization of the genes encoding the L1 and L2 components of hemolysin BL from Bacillus cereus. J. Bacteriol. 1997, 179, 2551–2556. [Google Scholar] [CrossRef] [PubMed]
- Agaisse, H.; Gominet, M.; Okstad, O.A.; Kolstø, A.B.; Lereclus, D. PlcR is a pleiotropic regulator of extracellular virulence factor gene expression in Bacillus thuringiensis. Mol. Microbiol. 1999, 32, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Gohar, M.; Faegri, K.; Perchat, S.; Ravnum, S.; Økstad, O.A.; Gominet, M.; Kolstø, A.-B.; Lereclus, D. The PlcR virulence regulon of Bacillus cereus. PLoS ONE 2008, 3, e2793. [Google Scholar] [CrossRef] [PubMed]
- Van der Voort, M.; Kuipers, O.P.; Buist, G.; de Vos, W.M.; Abee, T. Assessment of CcpA-mediated catabolite control of gene expression in Bacillus cereus ATCC 14579. BMC Microbiol. 2008, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Esbelin, J.; Armengaud, J.; Zigha, A.; Duport, C. ResDE-dependent regulation of enterotoxin gene expression in Bacillus cereus: Evidence for multiple modes of binding for ResD and interaction with Fnr. J. Bacteriol. 2009, 191, 4419–4426. [Google Scholar] [CrossRef] [PubMed]
- Esbelin, J.; Jouanneau, Y.; Armengaud, J.; Duport, C. ApoFnr binds as a monomer to promoters regulating the expression of enterotoxin genes of Bacillus cereus. J. Bacteriol. 2008, 190, 4242–4251. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, K.; Clavel, T.; Schmitt, P.; Duport, C. Fnr mediates carbohydrate-dependent regulation of catabolic and enterotoxin genes in Bacillus cereus F4430/73. Res. Microbiol. 2010, 161, 30–39. [Google Scholar] [CrossRef]
- Beecher, D.J.; Macmillan, J.D. Characterization of the components of hemolysin BL from Bacillus cereus. Infect. Immun. 1991, 59, 1778–1784. [Google Scholar]
- Schoeni, J.L.; Wong, A.C. Heterogeneity observed in the components of hemolysin BL, an enterotoxin produced by Bacillus cereus. Int. J. Food Microbiol 1999, 53, 159–167. [Google Scholar] [CrossRef]
- Beecher, D.J.; Wong, A.C. Tripartite haemolysin BL: Isolation and characterization of two distinct homologous sets of components from a single Bacillus cereus isolate. Microbiology 2000, 146 Pt 6, 1371–1380. [Google Scholar] [CrossRef]
- Lindbäck, T.; Okstad, O.A.; Rishovd, A.L.; Kolstø, A.B. Insertional inactivation of hblC encoding the L2 component of Bacillus cereus ATCC 14579 haemolysin BL strongly reduces enterotoxigenic activity, but not the haemolytic activity against human erythrocytes. Microbiology 1999, 145 Pt 11, 3139–3146. [Google Scholar]
- Clair, G.; Roussi, S.; Armengaud, J.; Duport, C. Expanding the known repertoire of virulence factors produced by Bacillus cereus through early secretome profiling in three redox conditions. Mol. Cell Proteomics 2010, 9, 1486–1498. [Google Scholar] [CrossRef]
- Mathur, A.; Feng, S.; Hayward, J.A.; Ngo, C.; Fox, D.; Atmosukarto, I.I.; Price, J.D.; Schauer, K.; Märtlbauer, E.; Robertson, A.A.B.; et al. A multicomponent toxin from Bacillus cereus incites inflammation and shapes host outcome via the NLRP3 inflammasome. Nat. Microbiol. 2019, 4, 362–374. [Google Scholar] [CrossRef]
- Beecher, D.J.; Wong, A.C. Tripartite hemolysin BL from Bacillus cereus. Hemolytic analysis of component interactions and a model for its characteristic paradoxical zone phenomenon. J. Biol. Chem. 1997, 272, 233–239. [Google Scholar] [CrossRef]
- Sastalla, I.; Fattah, R.; Coppage, N.; Nandy, P.; Crown, D.; Pomerantsev, A.P.; Leppla, S.H. The Bacillus cereus Hbl and Nhe tripartite enterotoxin components assemble sequentially on the surface of target cells and are not interchangeable. PLoS ONE 2013, 8, e76955. [Google Scholar] [CrossRef]
- Madegowda, M.; Eswaramoorthy, S.; Burley, S.K.; Swaminathan, S. X-ray crystal structure of the B component of Hemolysin BL from Bacillus cereus. Proteins 2008, 71, 534–540. [Google Scholar] [CrossRef]
- Mueller, M.; Grauschopf, U.; Maier, T.; Glockshuber, R.; Ban, N. The structure of a cytolytic alpha-helical toxin pore reveals its assembly mechanism. Nature 2009, 459, 726–730. [Google Scholar] [CrossRef]
- Tzokov, S.B.; Wyborn, N.R.; Stillman, T.J.; Jamieson, S.; Czudnochowski, N.; Artymiuk, P.J.; Green, J.; Bullough, P.A. Structure of the hemolysin E (HlyE, ClyA, and SheA) channel in its membrane-bound form. J. Biol. Chem. 2006, 281, 23042–23049. [Google Scholar] [CrossRef]
- Beecher, D.J.; Wong, A.C. Improved purification and characterization of hemolysin BL, a hemolytic dermonecrotic vascular permeability factor from Bacillus cereus. Infect. Immun. 1994, 62, 980–986. [Google Scholar]
- Tausch, F.; Dietrich, R.; Schauer, K.; Janowski, R.; Niessing, D.; Märtlbauer, E.; Jessberger, N. Evidence for Complex Formation of the Bacillus cereus Haemolysin BL Components in Solution. Toxins 2017, 9, 288. [Google Scholar] [CrossRef]
- Heilkenbrinker, U.; Dietrich, R.; Didier, A.; Zhu, K.; Lindbäck, T.; Granum, P.E.; Märtlbauer, E. Complex formation between NheB and NheC is necessary to induce cytotoxic activity by the three-component Bacillus cereus Nhe enterotoxin. PLoS ONE 2013, 8, e63104. [Google Scholar] [CrossRef]
- Tirado, S.M.; Yoon, K.J. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003, 16, 69–86. [Google Scholar] [CrossRef]
- Zhu, K.; Didier, A.; Dietrich, R.; Heilkenbrinker, U.; Waltenberger, E.; Jessberger, N.; Märtlbauer, E.; Benz, R. Formation of small transmembrane pores: An intermediate stage on the way to Bacillus cereus non-hemolytic enterotoxin (Nhe) full pores in the absence of NheA. Biochem. Biophys. Res. Commun. 2016, 469, 613–618. [Google Scholar] [CrossRef]
- Granum, P.E.; O’Sullivan, K.; Lund, T. The sequence of the non-haemolytic enterotoxin operon from Bacillus cereus. FEMS Microbiol. Lett. 1999, 177, 225–229. [Google Scholar] [CrossRef]
- Phung, D.; Granum, P.E.; Dietrich, R.; Märtlbauer, E.; Hardy, S.P. Inhibition of cytotoxicity by the Nhe cytotoxin of Bacillus cereus through the interaction of dodecyl maltoside with the NheB component. FEMS Microbiol. Lett. 2012, 330, 98–104. [Google Scholar] [CrossRef]
- Fagerlund, A.; Lindbäck, T.; Storset, A.K.; Granum, P.E.; Hardy, S.P. Bacillus cereus Nhe is a pore-forming toxin with structural and functional properties similar to the ClyA (HlyE, SheA) family of haemolysins, able to induce osmotic lysis in epithelia. Microbiology 2008, 154 Pt 3, 693–704. [Google Scholar] [CrossRef]
- Lindbäck, T.; Fagerlund, A.; Rødland, M.S.; Granum, P.E. Characterization of the Bacillus cereus Nhe enterotoxin. Microbiology 2004, 150 Pt 12, 3959–3967. [Google Scholar] [CrossRef]
- Didier, A.; Dietrich, R.; Märtlbauer, E. Antibody Binding Studies Reveal Conformational Flexibility of the Bacillus cereus Non-Hemolytic Enterotoxin (Nhe) A.-Component. PLoS ONE 2016, 11, e0165135. [Google Scholar] [CrossRef]
- Lindbäck, T.; Hardy, S.P.; Dietrich, R.; Sødring, M.; Didier, A.; Moravek, M.; Fagerlund, A.; Bock, S.; Nielsen, C.; Casteel, M.; et al. Cytotoxicity of the Bacillus cereus Nhe enterotoxin requires specific binding order of its three exoprotein components. Infect. Immun. 2010, 78, 3813–3821. [Google Scholar] [CrossRef]
- Jessberger, N.; Dietrich, R.; Bock, S.; Didier, A.; Märtlbauer, E. Bacillus cereus enterotoxins act as major virulence factors and exhibit distinct cytotoxicity to different human cell lines. Toxicon 2014, 77, 49–57. [Google Scholar] [CrossRef]
- Fagerlund, A.; Lindbäck, T.; Granum, P.E. Bacillus cereus cytotoxins Hbl, Nhe and CytK are secreted via the Sec translocation pathway. BMC Microbiol. 2010, 10, 304. [Google Scholar] [CrossRef]
- Økstad, O.A.; Gominet, M.; Purnelle, B.; Rose, M.; Lereclus, D.; Kolstø, A.B. Sequence analysis of three Bacillus cereus loci carrying PIcR-regulated genes encoding degradative enzymes and enterotoxin. Microbiology 1999, 145 Pt 11, 3129–3138. [Google Scholar]
- Dietrich, R.; Fella, C.; Strich, S.; Märtlbauer, E. Production and characterization of monoclonal antibodies against the hemolysin BL enterotoxin complex produced by Bacillus cereus. Appl. Environ. Microbiol. 1999, 65, 4470–4474. [Google Scholar]
- Jessberger, N.; Krey, V.M.; Rademacher, C.; Böhm, M.E.; Mohr, A.K.; Ehling-Schulz, M.; Scherer, S.; Märtlbauer, E. From genome to toxicity: A combinatory approach highlights the complexity of enterotoxin production in Bacillus cereus. Front. Microbiol. 2015, 6, 560. [Google Scholar]
- Dietrich, R.; Moravek, M.; Bürk, C.; Granum, P.E.; Märtlbauer, E. Production and characterization of antibodies against each of the three subunits of the Bacillus cereus nonhemolytic enterotoxin complex. Appl. Environ. Microbiol. 2005, 71, 8214–8220. [Google Scholar] [CrossRef]

| Sample | FL1 Positive (%) |
|---|---|
| mAb 1G8 (anti Hbl B) | 8.26 ± 0.71 |
| rHbl B | 2.05 ± 0.28 |
| rHbl B + 1G8 | 97.46 ± 0.59 |
| mAb 1B8 (anti Hbl B) | 2.03±0.05 |
| rHbl B + 1B8 | 39.93±1.97 |
| rHbl B + L1 + 1B8 | 82.31±3.25 |
| rHbl B + L2 + 1B8 | 29.62±0.78 |
| mAb 1H9 (anti Hbl L2) | 1.25 ± 0.35 |
| L2+1H9 | 3.5 ± 0.35 |
| B + L2+1H9 | 3.02 ± 0.06 |
| L1 + L2+1H9 | 3.33 ± 0.09 |
| B+L1 + L2+1H9 | 93.66 ± 0.55 |
| Sample | FL1 Positive (%) | |
|---|---|---|
| mAb 1G8 (anti Hbl B) | 0.48 ± 0.15 | |
| rHbl B (n) | 5 min | 93.38 ± 1.38 |
| 15 min | 93.44 ± 2.29 | |
| 30 min | 93.04 ± 4.1 | |
| rHbl B (c) | 5 min | 8.92 ± 2.36 |
| 15 min | 100 ± 5.94 | |
| 30 min | 100 ± 7.05 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jessberger, N.; Dietrich, R.; Schwemmer, S.; Tausch, F.; Schwenk, V.; Didier, A.; Märtlbauer, E. Binding to The Target Cell Surface Is The Crucial Step in Pore Formation of Hemolysin BL from Bacillus cereus. Toxins 2019, 11, 281. https://doi.org/10.3390/toxins11050281
Jessberger N, Dietrich R, Schwemmer S, Tausch F, Schwenk V, Didier A, Märtlbauer E. Binding to The Target Cell Surface Is The Crucial Step in Pore Formation of Hemolysin BL from Bacillus cereus. Toxins. 2019; 11(5):281. https://doi.org/10.3390/toxins11050281
Chicago/Turabian StyleJessberger, Nadja, Richard Dietrich, Stefanie Schwemmer, Franziska Tausch, Valerie Schwenk, Andrea Didier, and Erwin Märtlbauer. 2019. "Binding to The Target Cell Surface Is The Crucial Step in Pore Formation of Hemolysin BL from Bacillus cereus" Toxins 11, no. 5: 281. https://doi.org/10.3390/toxins11050281
APA StyleJessberger, N., Dietrich, R., Schwemmer, S., Tausch, F., Schwenk, V., Didier, A., & Märtlbauer, E. (2019). Binding to The Target Cell Surface Is The Crucial Step in Pore Formation of Hemolysin BL from Bacillus cereus. Toxins, 11(5), 281. https://doi.org/10.3390/toxins11050281





