Acute Kidney Injury Induced by Bothrops Venom: Insights into the Pathogenic Mechanisms
Abstract
1. Introduction
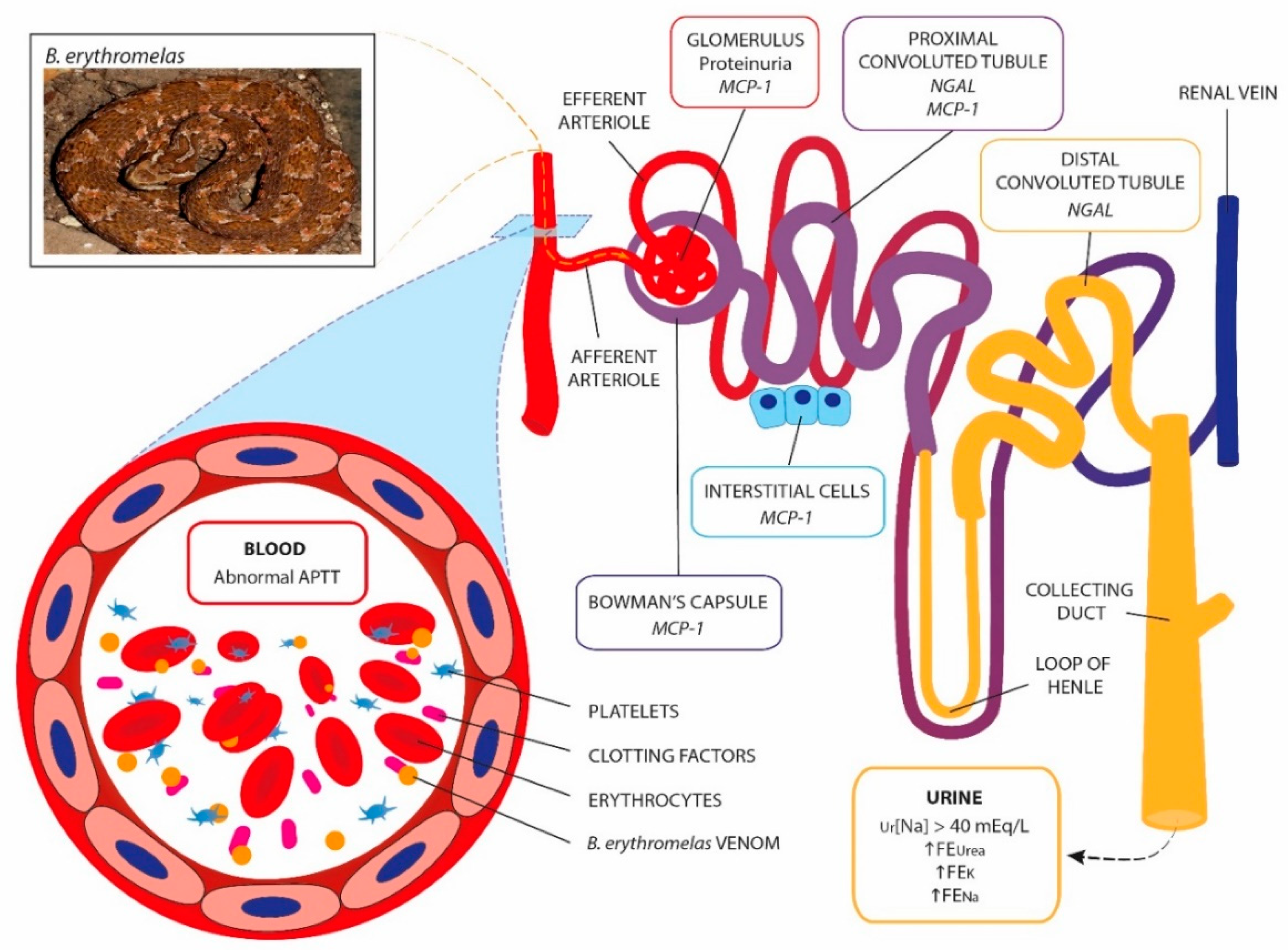
2. Results
3. Discussion
4. Methods
4.1. Sample Collection and Laboratory Assays
4.2. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Otero, R.; Gutiérrez, J.; Maria, B.M.; Edison, D.; Orlando, R.; Jorge, L.A.; Francisco, G.; Alvaro, T.; Fidel, C.; Libia, M.R.; et al. Complications of Bothrops, Porthidium, and Bothriechis snakebites in Colombia. A clinical and epidemiological study of 39 cases attended in a university hospital. Toxicon 2002, 40, 1107–1114. [Google Scholar] [CrossRef]
- Pacheco, U.P.; Zortéa, M. Snakebites in southwestern Goiás State, Brazil. J. Venom. Anim. Toxins Incl. Trop. Dis. 2008, 14, 141–151. [Google Scholar] [CrossRef]
- Sitprija, V.; Sitprija, S. Renal effects and injury induced by animal toxins. Toxicon 2012, 60, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Jorge, R.J.; Jorge, A.R.; de Menezes, R.R.; Mello, C.P.; Lima, D.B.; Silveira, J.A.; Alves, N.T.Q.; Marinho, A.D.; Ximenes, R.M.; Corrêa-Netto, C.; et al. Differences between renal effects of venom from two Bothrops jararaca populations from southeastern and southern Brazil. Toxicon 2017, 125, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Dantas, R.T.; Jorge, A.R.; Jorge, R.J.; Menezes, R.R.; Lima, D.B.; Torres, A.F.; Toyama, M.H.; Monteiro, H.S.; Martins, A.M. l-amino acid oxidase from Bothrops marajoensis causes nephrotoxicity in isolated perfused kidney and cytotoxicity in MDCK renal cells. Toxicon 2015, 104, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Sgrignolli, L.R.; Mendes, G.E.F.; Carlos, C.P.; Burdmann, E.A. Acute kidney injury caused by Bothrops snake venom. Nephron Clin. Pract. 2011, 119, c131–c137. [Google Scholar] [CrossRef] [PubMed]
- Fundacao Nacional de Saude F. [Guidance of Diagnosis and Treatment of Accidents by Venomous Animals] Manual de Diagnostico e Tratamento de Acidentes por Animais Peconhentos. 2001; pp. 37–44. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/funasa/manu_peconhentos.pdf (accessed on 22 January 2019).
- Slagboom, J.; Kool, J.; Harrison, R.A.; Casewell, N.R. Haemotoxic snake venoms: Their functional activity, impact on snakebite victims and pharmaceutical promise. Br. J. Haematol. 2017, 177, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Sitprija, V. Snakebite nephropathy. Nephrology 2006, 11, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.A.; Martin-Cleary, C.; Gutierrez, E.; Toldos, O.; Blanco-Colio, L.M.; Praga, M.; Ortiz, A.; Egido, J. AKI associated with macroscopic glomerular hematuria: clinical and pathophysiologic consequences. Clin. J. Am. Soc. Nephrol. 2012, 7, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Maduwage, K.; Isbister, G.K. Current treatment for venom-induced consumption coagulopathy resulting from snakebite. PLoS Negl. Trop. Dis. 2014, 8, e3220. [Google Scholar] [CrossRef] [PubMed]
- Sartim, M.A.; Cezarette, G.N.; Jacob-Ferreira, A.L.; Frantz, F.G.; Faccioli, L.H.; Sampaio, S.V. Disseminated intravascular coagulation caused by moojenactivase, a procoagulant snake venom metalloprotease. Int. J. Biol. Macromol. 2017, 103, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Pardal, P.P.D.O.; Souza, S.M.; Monteiro, M.R.D.C.D.C.; Fan, H.W.; Cardoso, J.L.C.; França, F.O.S.; Tomy, S.C.; Sano-Martins, I.S.; de Sousa-e-Silva, M.C.; Colombini, M.; et al. Clinical trial of two antivenoms for the treatment of Bothrops and Lachesis bites in the north eastern Amazon region of Brazil. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 28–42. [Google Scholar] [CrossRef]
- Sartim, M.A.; Costa, T.R.; Laure, H.J.; Espindola, M.S.; Frantz, F.G.; Sorgi, C.A.; Cintra, C.A.; Arantes, E.C.; Faccioli, L.H.; Rosa, J.C.; Sampaio, S.V.; et al. Moojenactivase, a novel pro-coagulant PIIId metalloprotease isolated from Bothrops moojeni snake venom, activates coagulation factors II and X and induces tissue factor up-regulation in leukocytes. Arch. Toxicol. 2016, 90, 1261–1278. [Google Scholar] [CrossRef] [PubMed]
- Pinho, F.M.; Yu, L.; Burdmann, E.A. Snakebite-induced acute kidney injury in Latin America. Semin. Nephrol. 2008, 28, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Aung, W.; Kyaw, K.; Hla, B.; Aye, S.; Naing, S.; Kyaw, A.; Sw, T. Renal involvement in Russell’s viper bite patients without disseminated intravascular coagulation. Trans. R. Soc. Trop. Med. Hyg. 1998, 92, 322–324. [Google Scholar] [CrossRef]
- Kubo, A.I.; Iwano, M.; Kobayashi, Y.; Kyoda, Y.; Isumi, Y.; Maruyama, N.; Samejima, K.; Dohi, Y.; Minamino, N.; Yonemasu, K. In vitro effects of Habu snake venom on cultured mesangial cells. Nephron 2002, 92, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Aung, W.; Kyaw, A.; Win, T.; Kun, S.; Hlaing, T.T. Urinary NAG as an early indicator of renal damage in Russell’s viper bite envenomation. Trans. R. Soc. Trop. Med. Hyg. 1996, 90, 169–172. [Google Scholar] [CrossRef]
- Aung, W.; Kyaw, A.; Win, T.; Kyaw, K.P.; Hla, B.; Hlaing, T.T. Isoenzyme profile of urinary NAG in Russell’s viper bite patients with renal damage. Clin. Chim. Acta 1997, 264, 251–254. [Google Scholar] [CrossRef]
- KDIGO. KDIGO: Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Jorge, R.J.; Monteiro, H.S.; Goncalves-Machado, L.; Guarnieri, M.C.; Ximenes, R.M.; Borges-Nojosa, D.M.; Luna, K.P.; Zingali, R.B.; Corrêa-Netto, C.; Gutiérrez, J.M.; et al. Venomics and antivenomics of Bothrops erythromelas from five geographic populations within the Caatinga ecoregion of northeastern Brazil. J. Proteom. 2015, 114, 93–114. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, P.A.; Mattoni, C.I.; Leynaud, G.C.; Scrocchi, G.J. Morphology, phylogeny and taxonomy of South American bothropoid pitvipers (Serpentes, Viperidae). Zool. Scr. 2012, 41, 109–124. [Google Scholar] [CrossRef]
- Snakebite Envenoming [Internet]. 2017. Available online: http://www.who.int/mediacentre/factsheets/fs337/en/ (accessed on 14 February 2019).
- Albuquerque, P.L.; Silva Junior, G.B.; Jacinto, C.N.; Lima, J.B.; Lima, C.B.; Amaral, Y.S.; Veras, M.S.; Mota, R.M.; Daher, E.F. Acute kidney injury after snakebite accident treated in a Brazilian tertiary care centre. Nephrology 2014, 19, 764–770. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz, M.R.; de Sousa, B.B.; da Cunha Pereira, D.F.; Mamede, C.C.N.; Matias, M.S.; de Morais, N.C.G.; de Oliveira Costa, J.; de Oliveira, F. The role of platelets in hemostasis and the effects of snake venom toxins on platelet function. Toxicon 2017, 133, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.B.; Gomes, M.S.; de Souza, D.L.; Gimenes, S.N.; Castanheira, L.E.; Borges, M.H.; Rodrigues, R.S.; Yoneyama, K.A.G.; Brandeburgo, M.I.H.; Rodrigues, V.M. Molecular cloning and pharmacological properties of an acidic PLA2 from Bothrops pauloensis snake venom. Toxins 2013, 5, 2403–2419. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.F.; Zdenek, C.N.; Dobson, J.S.; Op den Brouw, B.; Coimbra, F.; Gillett, A.; Del-Rei, T.H.M.; Chalkidis, H.M.; Sant’Anna, S.; Teixeira-da-Rocha, M.M.; et al. Coagulotoxicity of Bothrops (Lancehead Pit-Vipers) Venoms from Brazil: Differential Biochemistry and Antivenom Efficacy Resulting from Prey-Driven Venom Variation. Toxins 2018, 10, 411. [Google Scholar] [CrossRef] [PubMed]
- Aye, K.P.; Thanachartwet, V.; Soe, C.; Desakorn, V.; Thwin, K.T.; Chamnanchanunt, S.; Sahassananda, D.; Supaporn, T.; Sitprija, V. Clinical and laboratory parameters associated with acute kidney injury in patients with snakebite envenomation: A prospective observational study from Myanmar. BMC Nephrol. 2017, 18, 92. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.H.; Catharino, R.R.; Ifa, D.R.; Eberlin, M.N.; Hyslop, S. Peptide fingerprinting of snake venoms by direct infusion nano-electrospray ionization mass spectrometry: Potential use in venom identification and taxonomy. J. Mass Spectrom. 2008, 43, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Ziyadeh, F.N. The utility of the transtubular potassium gradient in the evaluation of hyperkalemia. J. Am. Soc. Nephrol. 2008, 19, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.C.; Sachett, J.A.G.; Sampaio, V.S.; Sousa, J.D.B.; Oliveira, S.S.; Nascimento, E.F.D.; Santos, A.D.S.; da Silva, I.M.; da Silva, A.M.M.; Wen, F.H.; et al. Predicting acute renal failure in Bothrops snakebite patients in a tertiary reference center, Western Brazilian Amazon. PLoS ONE 2018, 13, e0202361. [Google Scholar] [CrossRef] [PubMed]
- Boer-Lima, P.A.; Gontijo, J.A.; Cruz-Höfling, M.A. Bothrops moojeni snake venom-induced renal glomeruli changes in rat. Am. J. Trop. Med. Hyg. 2002, 67, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Panee, J. Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes. Cytokine. 2012, 60, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Haller, H.; Bertram, A.; Nadrowitz, F.; Menne, J. Monocyte chemoattractant protein-1 and the kidney. Curr. Opin. Nephrol. Hypertens 2016, 25, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Barbado, J.; Martin, D.; Vega, L.; Almansa, R.; Goncalves, L.; Nocito, M.; Jimeno, A.; Ortiz de Lejarazu, R.; Bermejo-Martin, J.F. MCP-1 in urine as biomarker of disease activity in Systemic Lupus Erythematosus. Cytokine 2012, 60, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Furuichi, K.; Sakai, N.; Iwata, Y.; Yoshimoto, K.; Shimizu, M.; Takeda, S.I.; Takasawa, K.; Yoshimura, M.; Kida, H.; et al. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int. 2000, 58, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- De Geus, H.R.; Ronco, C.; Haase, M.; Jacob, L.; Lewington, A.; Vincent, J.L. The cardiac surgery-associated neutrophil gelatinase-associated lipocalin (CSA-NGAL) score: A potential tool to monitor acute tubular damage. J. Thorac. Cardiovasc. Surg. 2016, 151, 1476–1481. [Google Scholar] [CrossRef] [PubMed]
- Martensson, J.; Bellomo, R. The rise and fall of NGAL in acute kidney injury. Blood Purif. 2014, 37, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Pepin, M.N.; Bouchard, J.; Legault, L.; Ethier, J. Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am. J. Kidney Dis. 2007, 50, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Schonermarck, U.; Kehl, K.; Samtleben, W. Diagnostic performance of fractional excretion of urea and sodium in acute kidney injury. Am. J. Kidney Dis. 2008, 51, 870–871. [Google Scholar] [CrossRef] [PubMed]
- Maciel, A.T.; Delphino Salles, L.; Vitorio, D.; Imed Research Group of Investigators. Simple blood and urinary parameters measured at ICU admission may sign for AKI development in the early postoperative period: A retrospective, exploratory study. Ren. Fail. 2016, 38, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Maciel, A.T.; Park, M.; Macedo, E. Fractional excretion of potassium in the course of acute kidney injury in critically ill patients: Potential monitoring tool? Revista Brasileira de Terapia Intensiva 2014, 26, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Isbister, G.K.; Buckley, N.A.; Page, C.B.; Scorgie, F.E.; Lincz, L.F.; Seldon, M.; Brown, S.G.; ASP Investigators. A randomized controlled trial of fresh frozen plasma for treating venom-induced consumption coagulopathy in cases of Australian snakebite (ASP-18). J. Thromb. Haemost. 2013, 11, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F. Regulation of Potassium Homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Staples, A.; LeBlond, R.; Watkins, S.; Wong, C.; Brandt, J. Validation of the revised Schwartz estimating equation in a predominantly non-CKD population. Pediatr. Nephrol. 2010, 25, 2321–2326. [Google Scholar] [CrossRef] [PubMed]
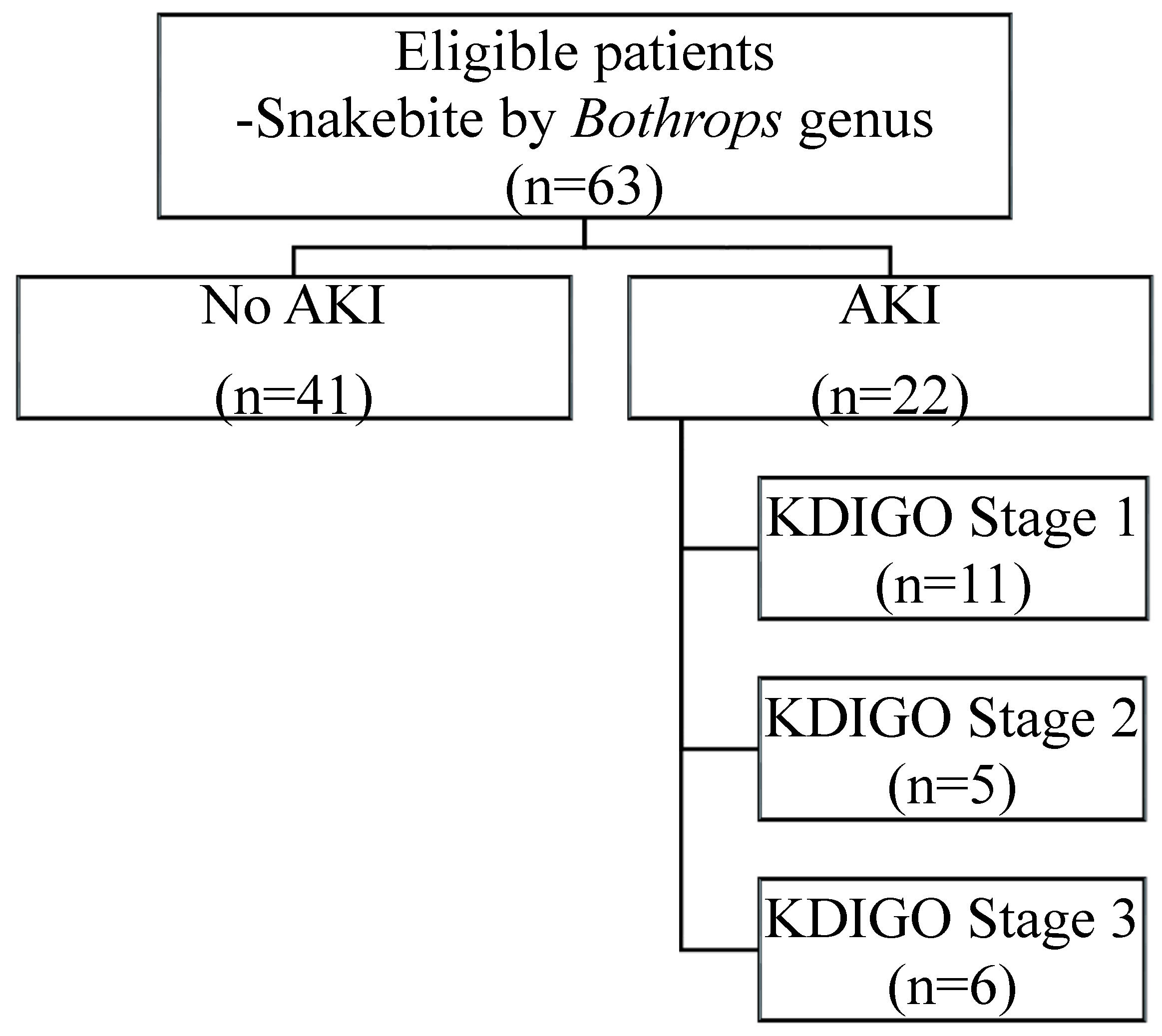
| Characteristic | No-AKI (n = 41) | AKI (n = 22) | P |
|---|---|---|---|
| Age (years) | 39 (12–86) | 42.5 (10–85) | 0.39 a |
| Time between snakebite-medical care (hours) | 8 (0.5–72) | 6.5 (1–144) | 0.85 a |
| Time between snakebite-antivenom (hours) | 10.5 (1–76.25) | 9 (4–157) | 0.64 a |
| Hospital stay (days) | 2 (0–5) | 3 (1–15) | 0.003 a |
| Vials of antivenom (n) | 6 (2–12) | 4.5 (3–12) | 0.39 a |
| Rural area n (%) | 38 (92.7) | 21 (95.5) | 1.00 b |
| Male gender n (%) | 28 (68.3) | 10 (45.5) | 0.07 c |
| Variable | No-AKI (n = 41) | AKI (n = 22) | P |
|---|---|---|---|
| Mean Hemoglobin (g/dL) | 13.4 (8.9–15.4) | 12.28 (7.27–14.55) | 0.03 a |
| Lowest Hemoglobin (g/dL) | 12.7 (8–15.4) | 11.7 (6–13.5) | 0.008 a |
| Mean Hematocrit (%) | 38.55 (25.9–44.3) | 35.1 (22.07–41.35) | 0.019 a |
| Lowest Hematocrit (%) | 37.1 (23.4–44.3) | 33.2 (17.4–38.8) | 0.005 a |
| Leukocytes on admission (per mm3) | 11,025 (5080–21,470) | 11,740 (5890–21,870) | 0.37 a |
| Mean Leukocytes (per mm3) | 10,591 (SD: 2789) | 10,595 (SD: 2529) | 0.99 b |
| Lowest Platelets (per mm3) | 177,075 (SD: 57,756) | 162,286 (SD: 93,442) | 0.51 b |
| Platelets on admission (per mm3) | 194,225 (SD: 63,305) | 187,762 (SD: 102,071) | 0.79 b |
| Lowest Serum Sodium (mEq/L) | 142 (136–150) | 139 (126–147) | 0.02 a |
| Mean Serum Sodium (mEq/L) | 143.1 (SD: 3.87) | 141.3 (SD: 4.03) | 0.09 b |
| Lowest Serum Potassium (mEq/L) | 3.75 (SD: 0.34) | 3.73 (SD: 0.26) | 0.89 b |
| Mean Serum Potassium (mEq/L) | 3.87 (SD: 0.33) | 4.04 (SD: 0.35) | 0.07 b |
| Serum Potassium on admission (mEq/L) | 3.88 (3.34–4.7) | 4.02 (3.37–5.9) | 0.11 a |
| Mean Creatine Kinase (U/L) | 300.5 (47–927) | 240.2 (49.17–1854) | 0.43 a |
| Mean Serum Glucose (mg/dL) | 99 (54–172) | 100 (85–213) | 0.59 a |
| Mean Serum Albumin (mg/dL) | 4.1 (SD: 0.4) | 4.28 (SD: 0.5) | 0.29 b |
| Renal parameters | |||
| eGFR on admission(mL/min per 1.73m2) | 99.31 (SD: 3.91) | 56.45 (SD: 6.99) | <0.0001 b |
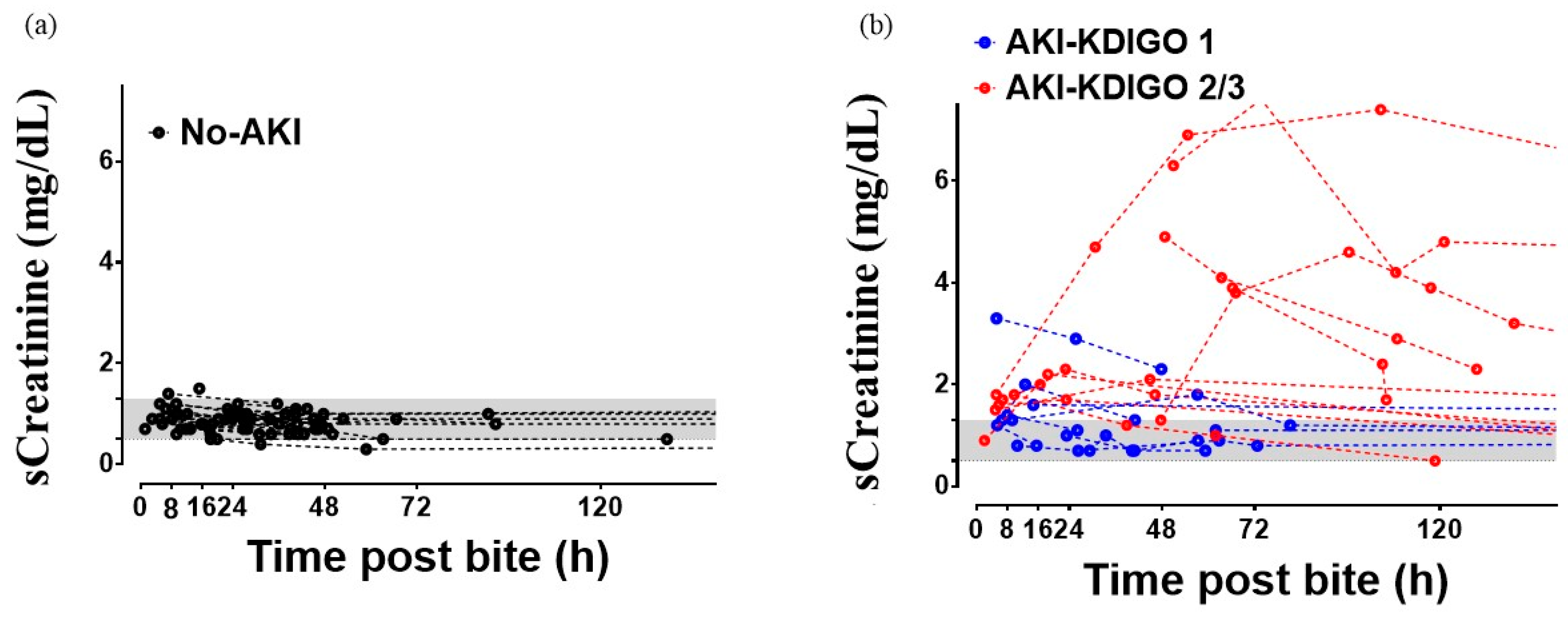
| Baseline Creatinine (mg/dL) | 0.8 (0.3–1.5) | 0.85 (0.5–2.7) | 0.23 a |
| Mean Serum Urea (mg/dL) | 34 (13.5–70) | 45.13 (20–153.1) | 0.004 a |
| Serum Creatinine on admission (mg/dL) | 0.9 (0.5–1.5) | 1.45 (0.7–6.3) | <0.0001 a |
| Proteinuria (mg/dL) | 21.8 (4.7–118.7) | 50.9 (6.9–168.9) | 0.12 a |
| Proteinuria (mg/gCr) | 260.1 (75–6303) | 643 (162–5235) | 0.03 a |
| Novel Kidney Biomarkers on admission | |||
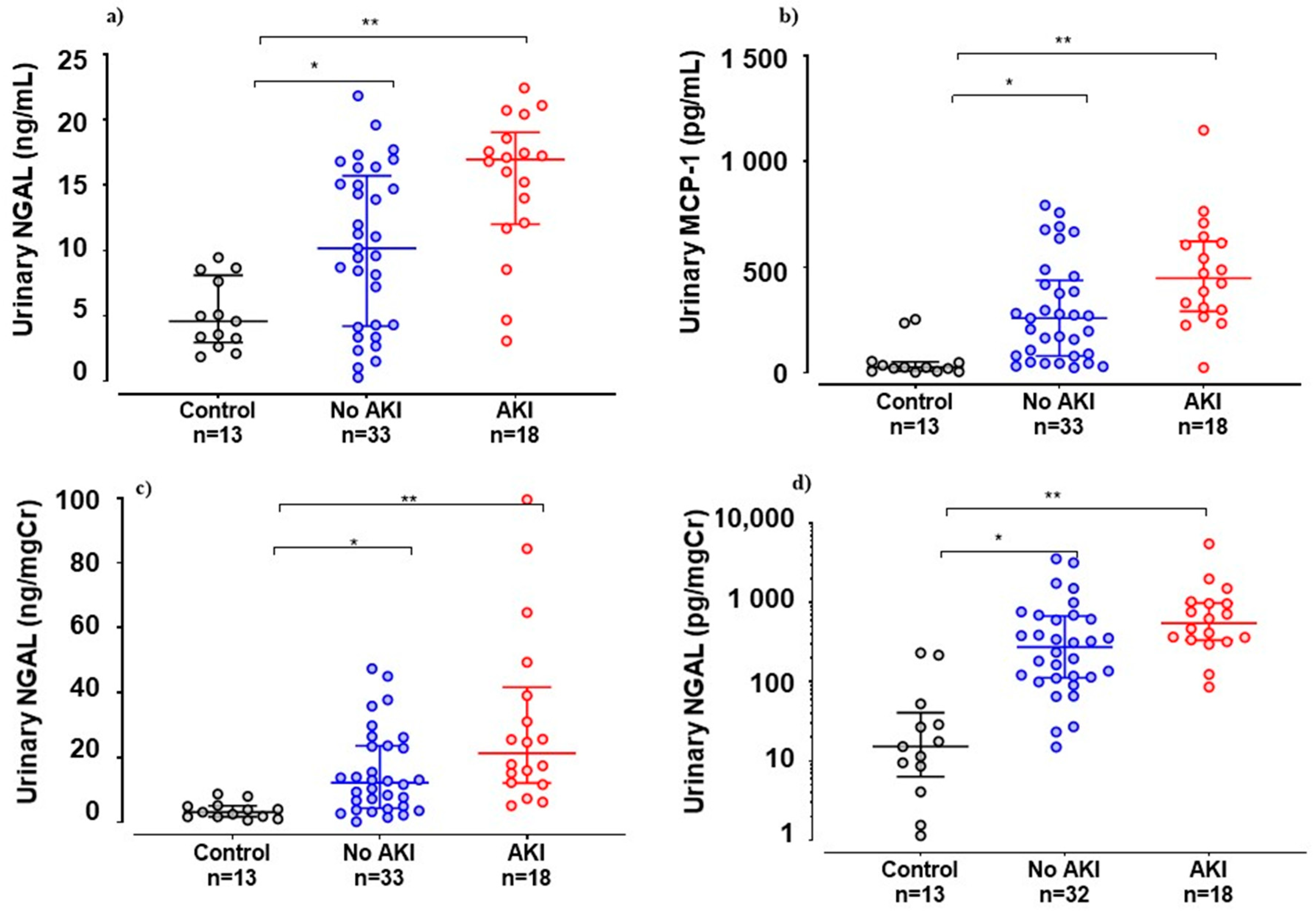
| Urinary MCP-1(pg/mgCr) | 274.1 (15.1–3562) | 547.5 (86.2–5514) | 0.02 a |
| Urinary MCP-1 (pg/mL) | 258.2 (24.7–793.1) | 447.3 (25.4–1147) | 0.01 a |
| Urinary NGAL (ng/mgCr) | 12.7 (0.2–452.5) | 21.3 (5.1–99.6) | 0.03 a |
| Urinary NGAL (ng/mL) | 10.2 (0.3–21.8) | 16.97 (3.09–22.4) | 0.004 a |
| Serum NGAL (ng/mL) | 176.5 (SD: 47.1) | 181.7 (SD: 58.3) | 0.72 b |
| Tubular function on admission | |||
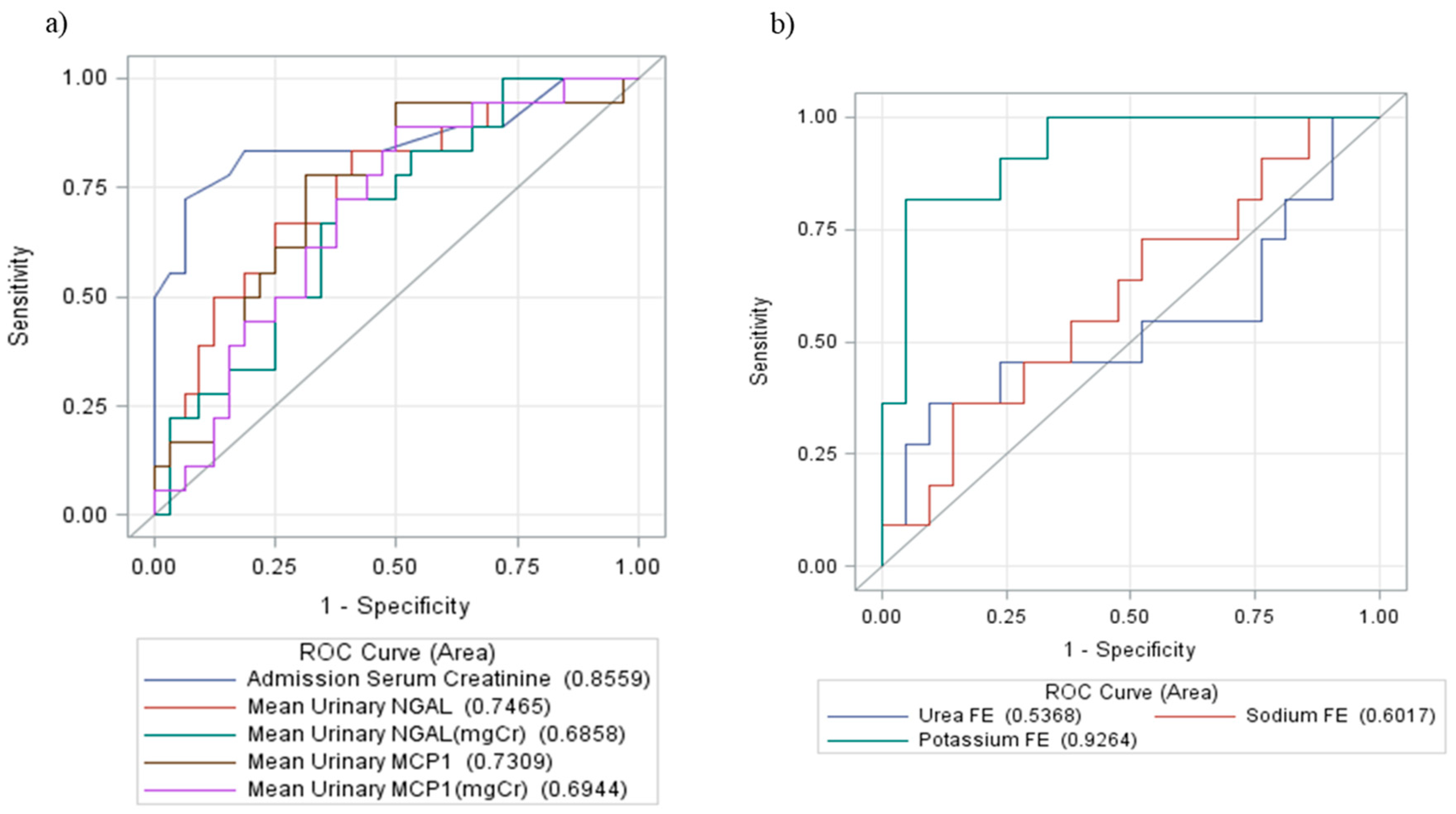
| FE Sodium (%) | 0.82 (0.01–6.8) | 1.395 (0.22–13.28) | 0.08 a |
| FE Potassium (%) | 8.6 (0.02–20.8) | 14.5 (5.4–55.5) | <0.0001 a |
| FE Chloride (%) | 1.3 (0.01–11.3) | 2.19 (0.59–16.75) | 0.04 a |
| FE Urea (%) | 38.79 (0.29–118.48) | 48.98 (2.14–66.67) | 0.33 a |
| Uosm, mOsm/kg | 516.6 (167–972) | 383.1 (131.7–852.8) | 0.08 a |
| Posm, mOsm/kg | 308.7 (271.6–321.4) | 310.5 (299.5–377) | 0.05 a |
| TTKG | 4.93 (1.39–16.99) | 5.75 (2.62–16.74) | 0.40 a |
| Uosm/Posm | 1.69 (0.54–3.3) | 1.21 (0.35–2.7) | 0.08 a |
| Urinary Sodium (mEq/L) | 116.6 (SD: 12.7) | 97.18 (SD: 13.5) | 0.32 b |
| Variables | Acute Kidney Injury | ||
|---|---|---|---|
| OR | 95% CI | P | |
| Lowest Serum Sodium (mEq/L) | 0.734 | 0.57–0.94 | 0.0160 |
| Lowest Hemoglobin (g/dL) | 1.036 | 0.664–1.616 | 0.8756 |
| Proteinuria (mg/gCr) | 1.0 | 1.00–1.001 | 0.3687 |
| aPTT on admission (normal vs. abnormal/incoagulable) | 26.272 | 1.348–512.11 | 0.031 |
| Novel Renal Biomarkers/Renal Parameters | uMCP-1 (pg/mgCr) * | uNGAL (ng/mgCr) * | ||
|---|---|---|---|---|
| Spearman’s Correlation Coefficient | P Value | Spearman’s Correlation Coefficient | P Value | |
| Proteinuria (mg/gCr) | 0.70 | <0.0001 | 0.47 | 0.001 |
| FE sodium (%) | 0.44 | 0.003 | 0.56 | <0.0001 |
| FE potassium (%) | 0.15 | 0.34 | 0.09 | 0.56 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albuquerque, P.L.M.M.; da Silva Junior, G.B.; Meneses, G.C.; Martins, A.M.C.; Lima, D.B.; Raubenheimer, J.; Fathima, S.; Buckley, N.; Daher, E.D.F. Acute Kidney Injury Induced by Bothrops Venom: Insights into the Pathogenic Mechanisms. Toxins 2019, 11, 148. https://doi.org/10.3390/toxins11030148
Albuquerque PLMM, da Silva Junior GB, Meneses GC, Martins AMC, Lima DB, Raubenheimer J, Fathima S, Buckley N, Daher EDF. Acute Kidney Injury Induced by Bothrops Venom: Insights into the Pathogenic Mechanisms. Toxins. 2019; 11(3):148. https://doi.org/10.3390/toxins11030148
Chicago/Turabian StyleAlbuquerque, Polianna Lemos Moura Moreira, Geraldo Bezerra da Silva Junior, Gdayllon Cavalcante Meneses, Alice Maria Costa Martins, Danya Bandeira Lima, Jacques Raubenheimer, Shihana Fathima, Nicholas Buckley, and Elizabeth De Francesco Daher. 2019. "Acute Kidney Injury Induced by Bothrops Venom: Insights into the Pathogenic Mechanisms" Toxins 11, no. 3: 148. https://doi.org/10.3390/toxins11030148
APA StyleAlbuquerque, P. L. M. M., da Silva Junior, G. B., Meneses, G. C., Martins, A. M. C., Lima, D. B., Raubenheimer, J., Fathima, S., Buckley, N., & Daher, E. D. F. (2019). Acute Kidney Injury Induced by Bothrops Venom: Insights into the Pathogenic Mechanisms. Toxins, 11(3), 148. https://doi.org/10.3390/toxins11030148





