The Effect of a Combined Hydrogen Peroxide-MlrA Treatment on the Phytoplankton Community and Microcystin Concentrations in a Mesocosm Experiment in Lake Ludoš
Abstract
1. Introduction
2. Results
2.1. Laboratory Experiments
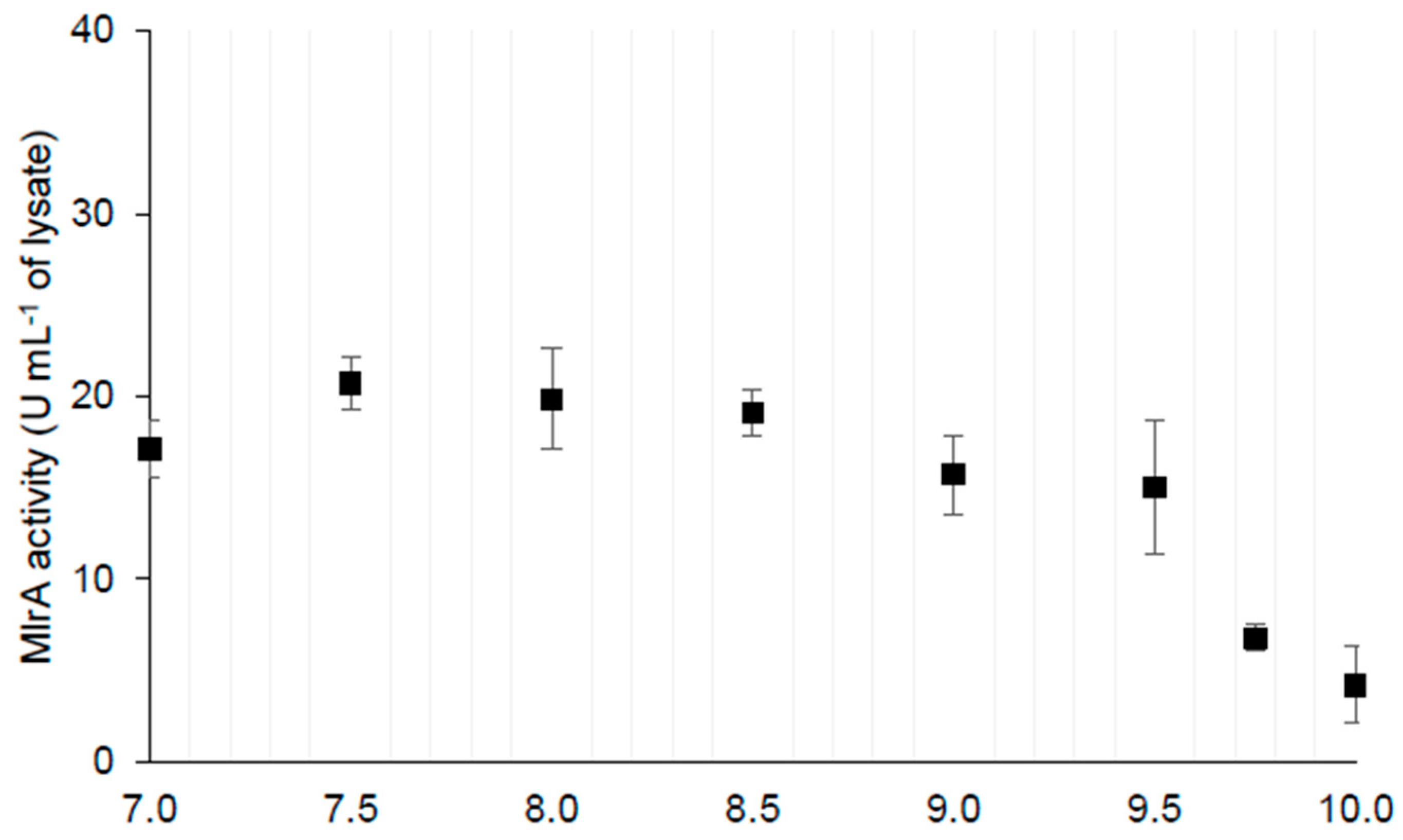
2.1.1. MlrA Activity
2.1.2. The Response of M. aeruginosa to Lower Doses of H2O2
2.2. Field Experiments
2.2.1. Phytoplankton Content in Containers
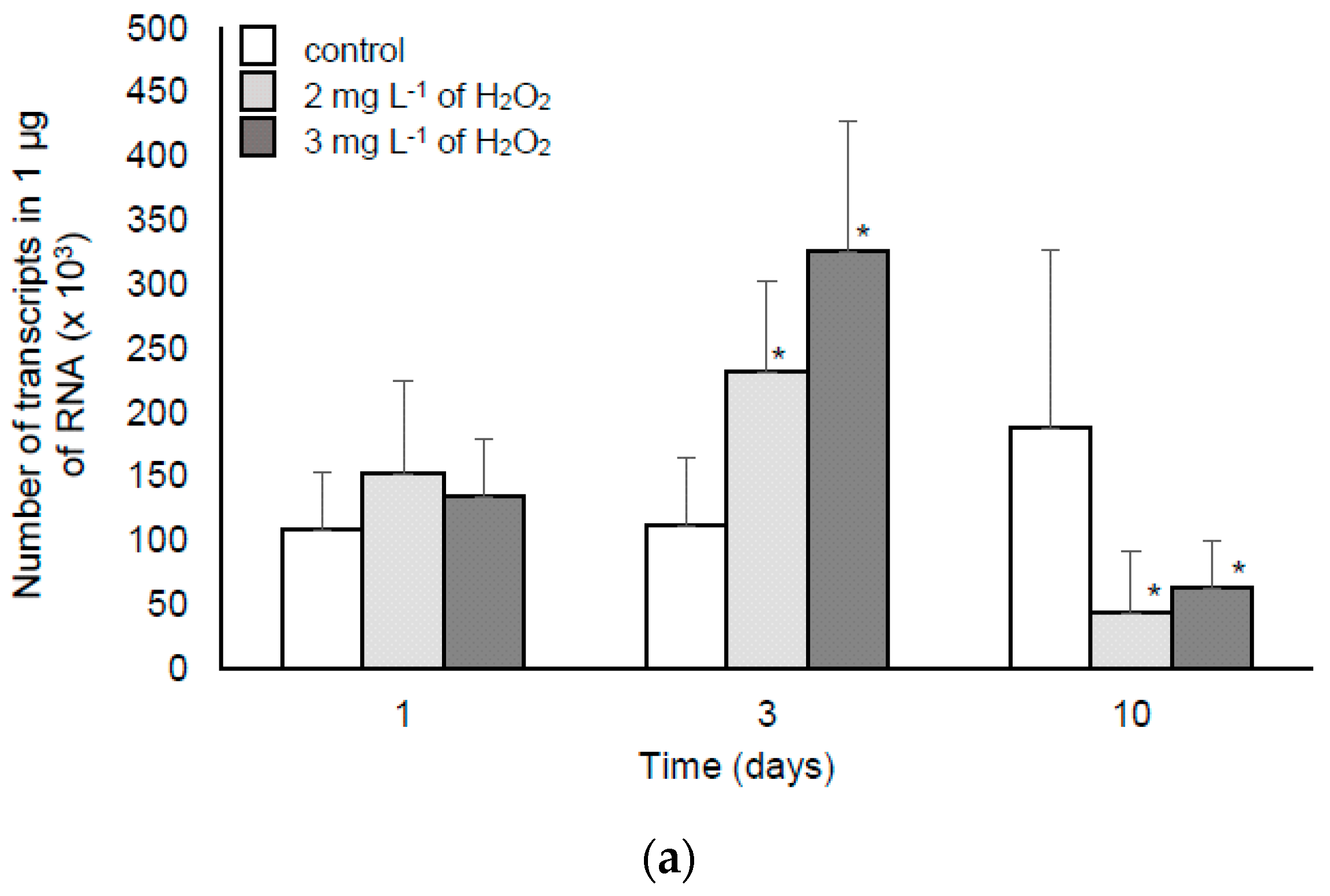
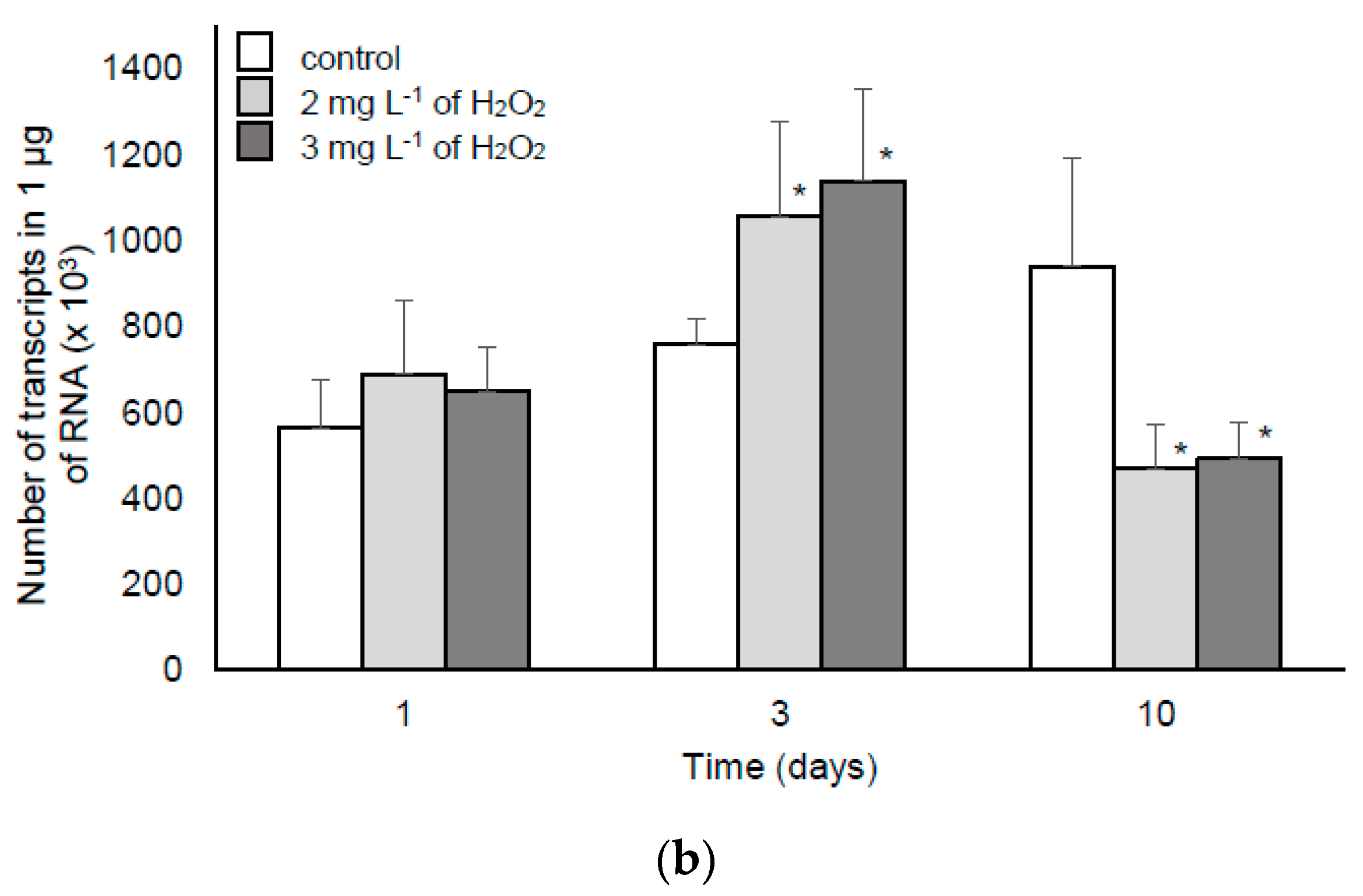
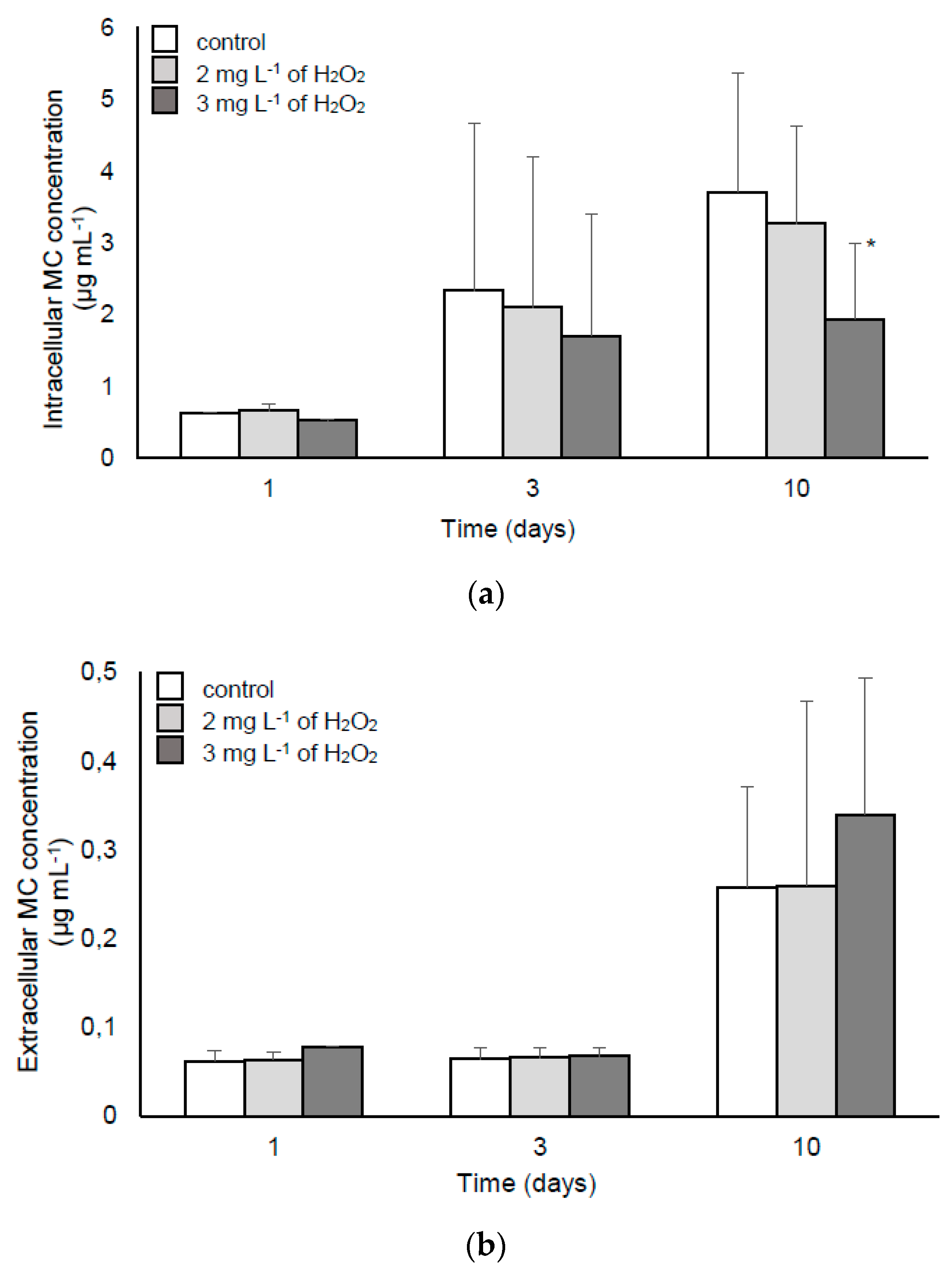
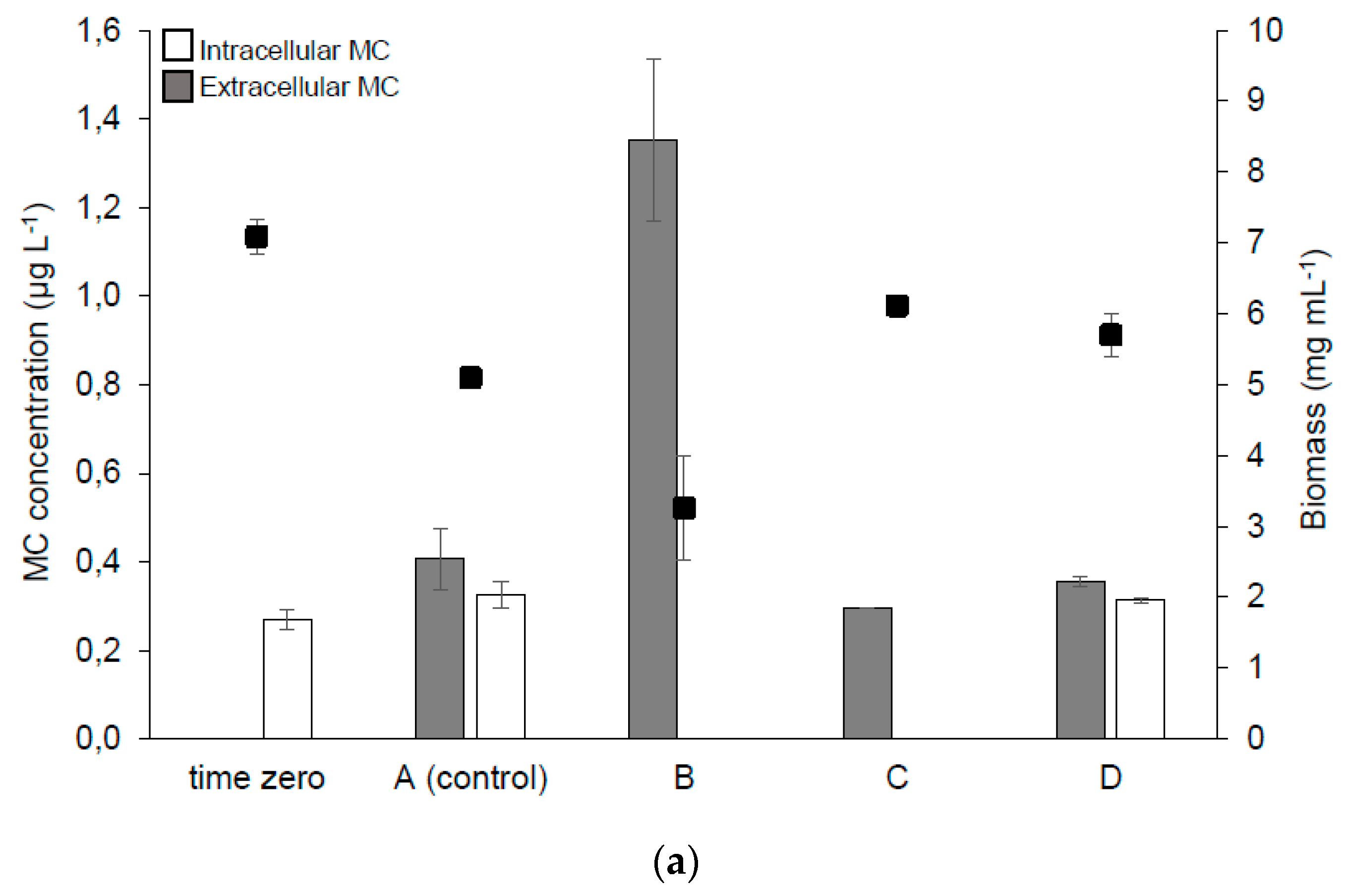
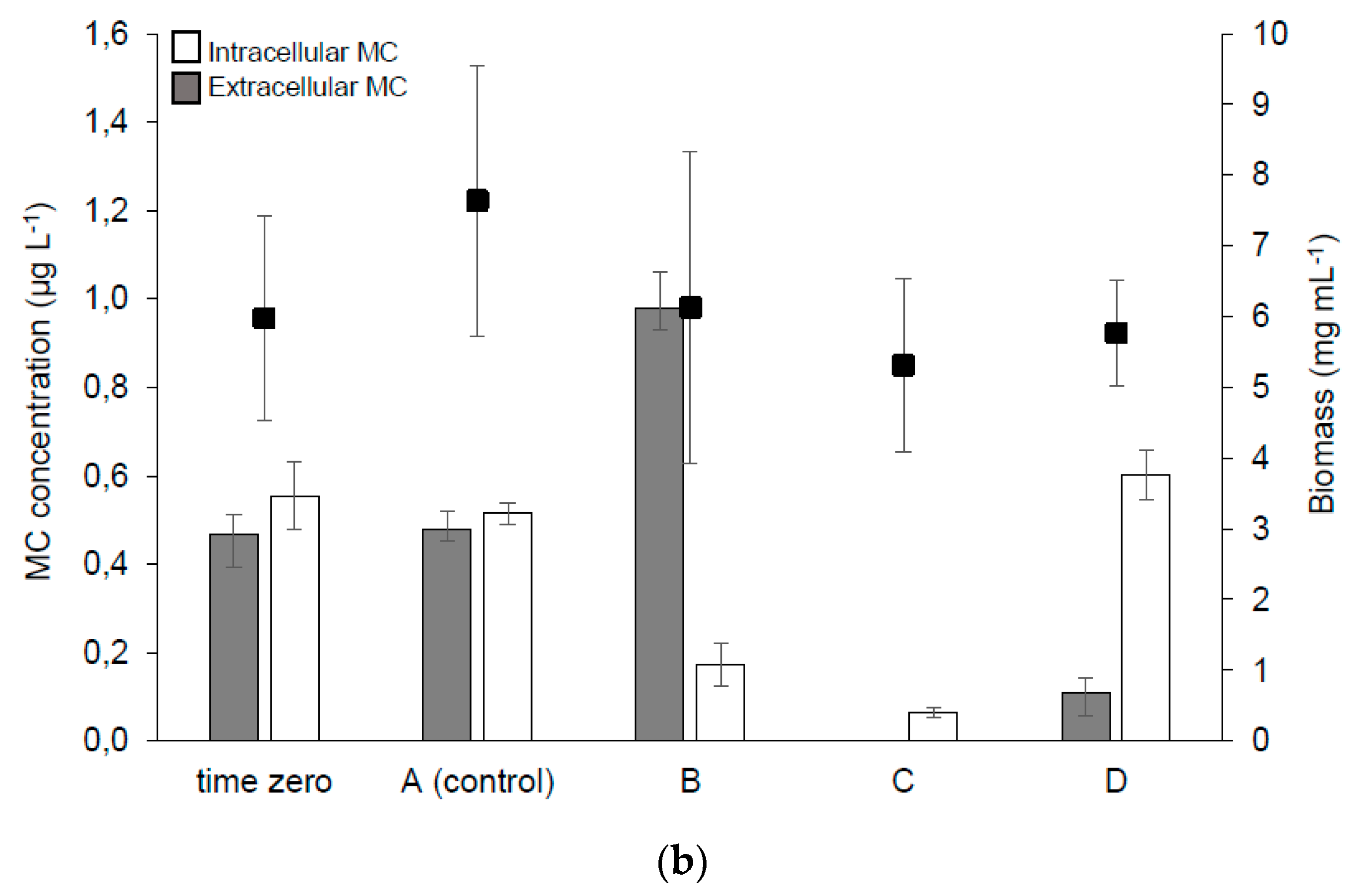
2.2.2. MC Synthesis, Extra- and Intra-cellular MC Concentration in the Samples
3. Discussion
3.1. The Impact of H2O2 on the MC Production by M. aeruginosa
3.2. The Efficiency of MlrA in Environmentally Relevant Conditions
3.3. Treatment in Lake Ludoš
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Strains
5.2. MlrA Assays
5.3. M. aeruginosa Cultivation and Treatment with H2O2
5.4. Mesocosm Experiment in Lake Ludoš
5.5. Chemo-physical and Biological Parameters of Lake Ludoš
5.6. Real Time PCR
5.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zilliges, Y.; Kehr, J.C.; Meissner, S.; Ishida, K.; Mikkat, S.; Hagemann, M.; Kaplan, A.; Börner, T.; Dittmann, E. The cyanobacterial hepatotoxin microcystin binds to proteins and increases the fitness of Microcystis under oxidative stress conditions. PLoS ONE 2011, 6, e17615. [Google Scholar] [CrossRef] [PubMed]
- Giannuzzi, L.; Krock, B.; Minaglia, M.C.C.; Rosso, L.; Houghton, C.; Sedan, D.; Malanga, G.; Espinosa, M.; Andrinolo, D.; Hernando, M. Growth, toxin production, active oxygen species and catalase activity of Microcystis aeruginosa (Cyanophyceae) exposed to temperature stress. Comp. Biochem. Physiol. Toxicol. Pharm. 2016, 189, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Schuurmans, J.M.; Brinkmann, B.W.; Makower, A.K.; Dittmann, E.; Huisman, J.; Matthijs, H.C.P. Microcystin interferes with defense against high oxidative stress in harmful cyanobacteria. Harmful Algae 2018, 78, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Matthijs, H.C.P.; Visser, P.M.; Reeze, B.; Meeuse, J.; Slot, P.C.; Wijn, G.; Talens, R.; Huisman, J. Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Res. 2012, 46, 1460–1472. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Buley, R.P.; Fernandez-Figueroa, E.G.; Barros, M.U.G.; Rajendran, S.; Wilson, A.E. Hydrogen peroxide treatment promotes chlorophytes over toxic cyanobacteria in a hyper-eutrophic aquaculture pond. Environ. Pollut. 2018, 240, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Dziga, D.; Wasylewski, M.; Wladyka, B.; Nybom, S.; Meriluoto, J. Microbial Degradation of Microcystins. Chem. Res. Toxicol. 2013, 26, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Dziga, D.; Wladyka, B.; Zielińska, G.; Meriluoto, J.; Wasylewski, M. Heterologous expression and characterisation of microcystinase. Toxicon 2012, 59, 578–586. [Google Scholar] [CrossRef]
- Dexter, J.; Dziga, D.; Lv, J.; Zhu, J.; Strzalka, W.; Maksylewicz, A.; Maroszek, M.; Marek, S.; Fu, P. Heterologous expression of mlrA in a photoautotrophic host—Engineering cyanobacteria to degrade microcystins. Environ. Pollut. 2018, 237, 926–935. [Google Scholar] [CrossRef]
- Dziga, D.; Maksylewicz, A.; Maroszek, M.; Marek, S. Combined treatment of toxic cyanobacteria Microcystis aeruginosa with hydrogen peroxide and microcystin biodegradation agents results in quick toxin elimination. Acta Biochim. Pol. 2018, 65, 133–140. [Google Scholar] [CrossRef]
- Tokodi, N.; Drobac, D.; Meriluoto, J.; Lujić, J.; Marinović, Z.; Važić, T.; Nybom, S.; Simeunović, J.; Dulić, T.; Lazić, G.; et al. Cyanobacterial effects in Lake Ludoš, Serbia—Is preservation of a degraded aquatic ecosystem justified? Sci. Total Environ. 2018, 635, 1047–1062. [Google Scholar] [CrossRef]
- Schöne, K.; Jähnichen, S.; Ihle, T.; Ludwig, F.; Benndorf, J. Arriving in better shape: Benthic Microcystis as inoculum for pelagic growth. Harmful Algae 2010, 9, 494–503. [Google Scholar] [CrossRef]
- Meissner, S.; Fastner, J.; Dittmann, E. Microcystin production revisited: Conjugate formation makes a major contribution. Environ. Microbiol. 2013, 15, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.A.; Rueckert, A.; Hamilton, D.P.; Cary, S.C.; Dietrich, D.R. Switching toxin production on and off: Intermittent microcystin synthesis in a Microcystis bloom. Environ. Microbiol. Rep. 2011, 3, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Dziga, D.; Lisznianska, M.; Wladyka, B. Bioreactor study employing bacteria with enhanced activity toward cyanobacterial toxins microcystins. Toxins 2014, 6, 2379–2392. [Google Scholar] [CrossRef]
- Silva, M.O.D.; Blom, J.F.; Yankova, Y.; Villiger, J.; Pernthaler, J. Priming of microbial microcystin degradation in biomass-fed gravity driven membrane filtration biofilms. Syst. Appl. Microbiol. 2018, 41, 221–231. [Google Scholar] [CrossRef]
- Kumar, P.; Hegde, K.; Brar, S.K.; Cledon, M.; Kermanshahi-pour, A.; Roy-Lachapelle, A.; Galvez-Cloutier, R. Novel fluidized-bed biofilm reactor for concomitant removal of microcystin-LR and organics. Chem. Eng. J. 2019, 359, 99–111. [Google Scholar] [CrossRef]
- Sharma, B.; Dangi, A.K.; Shukla, P. Contemporary enzyme based technologies for bioremediation: A review. J. Environ. Manag. 2018, 210, 10–22. [Google Scholar] [CrossRef]
- Thatoi, H.; Das, S.; Mishra, J.; Rath, B.P.; Das, N. Bacterial chromate reductase, a potential enzyme for bioremediation of hexavalent chromium: A review. J. Environ. Manag. 2014, 146, 383–399. [Google Scholar] [CrossRef]
- Barroin, G.; Feuillade, M. Hydrogen peroxide as a potential algicide for Oscillatoria rubescens D.C. Water Res. 1986, 20, 619–623. [Google Scholar] [CrossRef]
- Drábková, M.; Admiraal, W.; Maršálek, B. Combined Exposure to Hydrogen Peroxide and LightSelective Effects on Cyanobacteria, Green Algae, and Diatoms. Environ. Sci. Technol. 2007, 41, 309–314. [Google Scholar] [CrossRef]
- Kay, S.; Quimby, P.; Ouzts, J. H2O2: A potential algicide for aquaculture. In Proceedings of the 35th Annual Meeting of the Southern Weed Science, Atlanta, GA, USA, 19–21 January 1982; pp. 275–289. [Google Scholar]
- Barrington, D.J.; Reichwaldt, E.S.; Ghadouani, A. The use of hydrogen peroxide to remove cyanobacteria and microcystins from waste stabilization ponds and hypereutrophic systems. Ecol. Eng. 2013, 50, 86–94. [Google Scholar] [CrossRef]
- Bauzá, L.; Aguilera, A.; Echenique, R.; Andrinolo, D.; Giannuzzi, L. Application of hydrogen peroxide to the control of eutrophic lake systems in laboratory assays. Toxins 2014, 6, 2657–2675. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, D.; Qin, H.; Li, Y. An integrated method for removal of harmful cyanobacterial blooms in eutrophic lakes. Environ. Pollut. 2012, 160, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Weenink, E.F.J.; Luimstra, V.M.; Schuurmans, J.M.; van Herk, M.J.; Visser, P.M.; Matthijs, H.C.P. Combatting cyanobacteria with hydrogen peroxide: A laboratory study on the consequences for phytoplankton community and diversity. Front. Microbiol. 2015, 6, 714. [Google Scholar] [CrossRef]
- Fan, J.; Hobson, P.; Ho, L.; Daly, R.; Brookes, J. The effects of various control and water treatment processes on the membrane integrity and toxin fate of cyanobacteria. J. Hazard. Mater. 2014, 264, 313–322. [Google Scholar] [CrossRef]
- Miroux, B.; Walker, J.E. Over-production of Proteins in Escherichia coli: Mutant Hosts that Allow Synthesis of some Membrane Proteins and Globular Proteins at High Levels. J. Mol. Biol. 1996, 260, 289–298. [Google Scholar] [CrossRef]
- Castenholz, R.W. Culturing Methods for Cyanobacteria. In Methods in Enzymology; Packer, L., Glazer, A.N., Eds.; Academic Press: Cambridge, MA, USA, 1988; pp. 68–93. [Google Scholar]
- Meriluoto, J.; Spoof, L. Analysis of Microcystins by High-Performance Liquid Chromatography with Photodiode-Array Detection. In Toxic Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Codd, G.A., Eds.; Abo Akademi University Press: Turku, Finland, 2005; pp. 77–84. ISBN 9517652593. [Google Scholar]
- Mackinney, G. Absorption of light by chlorophyll solutions. J. Biol. Chem. 1941, 140, 315–322. [Google Scholar]
- American Public Health Association. Standard Methods for the Examination of Waste and Wastewater; APHA: Washington, DC, USA, 1992. [Google Scholar]
- Dziga, D.; Kokociński, M.; Barylski, J.; Nowicki, G.; Maksylewicz, A.; Antosiak, A.; Banaś, A.K.; Strzałka, W. Correlation between specific groups of heterotrophic bacteria and microcystin biodegradation in freshwater bodies of central Europe. FEMS Microbiol. Ecol. 2019, 95, 162. [Google Scholar] [CrossRef]
- Kurmayer, R. Quantitative PCR. In Molecular Tools for the Detection and Quantification of Toxigenic Cyanobacteria; Kurmayer, R., Sivonen, K., Wilmotte, A., Salmaso, N., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 214–221. ISBN 9781119332169. [Google Scholar]
| A. Production efficiency | average MlrA activity after isolation from E. coli C41 | mU µL−1 of lysate | U mL−1 of culture | ||
| 41.6 ± 24.0 | 1.2 ± 0.7 | ||||
| B. Dependence on pH | MlrA activity in different pH (U mL−1 of sample) | activity range in pH 7.0–9.5 | average activity in pH 7.0–9.5 17.9 ± 2.3 | average activity in pH 9.75–10.00 6.8 ± 4.2 * | |
| 15.07–20.76 | |||||
| C. Dependence on temperature | MlrA activity in different temperatures (U mL−1 of sample) percentage in comparison with 20 °C | 20 °C | 10 °C | 5 °C 2.9 21.9 | |
| 13.4 | 5.5 40.9 | ||||
| fold the reduction in comparison with 20 °C | 3.9 | 5.0 | |||
| D. Activity in the culture and sample from the lake | control | 1 h | 3 h | 24 h | |
| MlrA activity (U mL−1) in M. aeruginosa culture OD730 = 0.2 | 49.2 | 9.6 ± 1.2 | 20.8 ± 10.2 | 5.4 ± 2.6 | |
| MlrA activity (U mL−1) in M. aeruginosa culture OD730 = 0.5 | 49.2 | 8.5 ± 2.2 | 15.3 ± 5.4 | 7.4 ± 4.4 | |
| MlrA activity (U mL−1) in the sample from Lake Ludoš (collected in 16.03.2018) | 49.2 | 3.0 ± 0.8 | 2.5 ± 0.2 | 2.8 ± 0.7 | |
| E. Amount of MlrA (Units) required for complete MC degradation in 100 l (initial MC concentration 2 µg L−1) | Optimal conditions 0.16 | L. Ludoš, stage I 12.6 | L. Ludoš, stage II 2.8 | ||
| Parameters | 16 March 2018 | |
|---|---|---|
| Pier | Center of the Lake | |
| (46.103207 N, 19.821360 E) | (46.102159 N, 19.821149 E) | |
| temperature (°C), in situ | 10 | 10.1 |
| pH, in situ | 8.3 | 8.3 |
| concentration O2, in situ (μg mL−1) | 13.78 | 18.56 |
| saturation O2, in situ (%) | 129.8 | 166.6 |
| conductivity, in situ (µS cm−1) | 875 | 872 |
| total suspended solids (TSS) (mg dm−3) | 47.0 | 39.0 |
| total organic carbon (TOC) (mg dm−3) | 8.5 | 8.4 |
| NO3 (mg dm−3) | ≤0.5 | ≤0.5 |
| detergents (mg dm−3) | 2.1 | 2.0 |
| chemical oxygen demand (COD) (mgO2 dm−3) | 24.6 | 23.1 |
| biological oxygen demand (BOD) (mgO2 dm−3) | 12.1 | 11.9 |
| MC content (μg L−1) | ||
| intracellular MC content | 1.67 | 1.84 |
| extracellular content | 1.31 | 1.22 |
| total | 1.55 | 1.57 |
| unprepared | 1.49 | 1.53 |
| Phytoplankton | Time Zero | A (Control) | B | C | D |
|---|---|---|---|---|---|
| Cyanobacteria (cells mL−1) | 10,620 | 17013 * | 380 ** | 5400 ** | 15100 |
| percentage in comparison with group A | 2 | 32 | 89 | ||
| percentage of the whole phytoplankton | 23 | 44 | 2 | 18 | 39 |
| MC-producers (cells mL−1) | 100 | 893 * | 60 ** | 120 ** | 220 ** |
| percentage in comparison with group A | 7 | 13 | 25 | ||
| % MC-producers within cyanobacteria | 1 | 5 | 16 | 2 | 1 |
| Eukaryotic phytoplankton (cells mL−1) | 36,180 | 21,520 * | 16,560 ** | 23,840 | 23,620 |
| percentage in comparison with group A | 77 | 111 | 110 |
| Phytoplankton | Time Zero | A (control) | B | C | D |
|---|---|---|---|---|---|
| Cyanobacteria (cells mL−1) | 2054 | 2277 | 2677 | 569 ** | 3610 ** |
| percentage in comparison with group A | 118 | 25 | 159 | ||
| percentage of the whole phytoplankton | 19 | 14 | 15 | 6 | 22 |
| Cyanobacteria, excluding Jaaginema sp. | 1985 | 1554 | 846 ** | 354 ** | 3487 ** |
| percentage in comparison with group A | 55 | 23 | 224 | ||
| MC-producers (cells mL−1) | 1954 | 1338 | 723 ** | 200 ** | 3364 ** |
| percentage in comparison with group A | 54 | 15 | 251 | ||
| % of MC-producers within cyanobacteria | 95 | 59 | 27 | 35 | 93 |
| Eukaryotic phytoplankton (cells mL−1) | 8592 | 14,092 * | 14,985 | 9262 | 12,779 |
| percentage in comparison with group A | 106 | 66 | 91 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziga, D.; Tokodi, N.; Drobac, D.; Kokociński, M.; Antosiak, A.; Puchalski, J.; Strzałka, W.; Madej, M.; Svirčev, Z.; Meriluoto, J. The Effect of a Combined Hydrogen Peroxide-MlrA Treatment on the Phytoplankton Community and Microcystin Concentrations in a Mesocosm Experiment in Lake Ludoš. Toxins 2019, 11, 725. https://doi.org/10.3390/toxins11120725
Dziga D, Tokodi N, Drobac D, Kokociński M, Antosiak A, Puchalski J, Strzałka W, Madej M, Svirčev Z, Meriluoto J. The Effect of a Combined Hydrogen Peroxide-MlrA Treatment on the Phytoplankton Community and Microcystin Concentrations in a Mesocosm Experiment in Lake Ludoš. Toxins. 2019; 11(12):725. https://doi.org/10.3390/toxins11120725
Chicago/Turabian StyleDziga, Dariusz, Nada Tokodi, Damjana Drobac, Mikołaj Kokociński, Adam Antosiak, Jakub Puchalski, Wojciech Strzałka, Mariusz Madej, Zorica Svirčev, and Jussi Meriluoto. 2019. "The Effect of a Combined Hydrogen Peroxide-MlrA Treatment on the Phytoplankton Community and Microcystin Concentrations in a Mesocosm Experiment in Lake Ludoš" Toxins 11, no. 12: 725. https://doi.org/10.3390/toxins11120725
APA StyleDziga, D., Tokodi, N., Drobac, D., Kokociński, M., Antosiak, A., Puchalski, J., Strzałka, W., Madej, M., Svirčev, Z., & Meriluoto, J. (2019). The Effect of a Combined Hydrogen Peroxide-MlrA Treatment on the Phytoplankton Community and Microcystin Concentrations in a Mesocosm Experiment in Lake Ludoš. Toxins, 11(12), 725. https://doi.org/10.3390/toxins11120725







