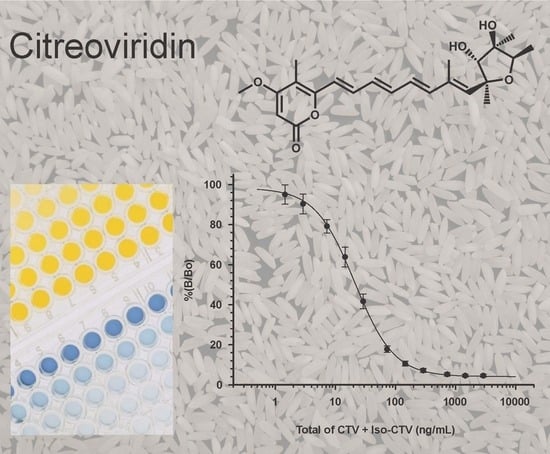
Development and Characterization of Monoclonal Antibodies for the Mycotoxin Citreoviridin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antibody Development, and Tolerance to Solvents
2.2. Application of mAb 2-4 Competitive Enzyme-Linked Immunosorbent Assay (CI-ELISA) to Spiked Rice
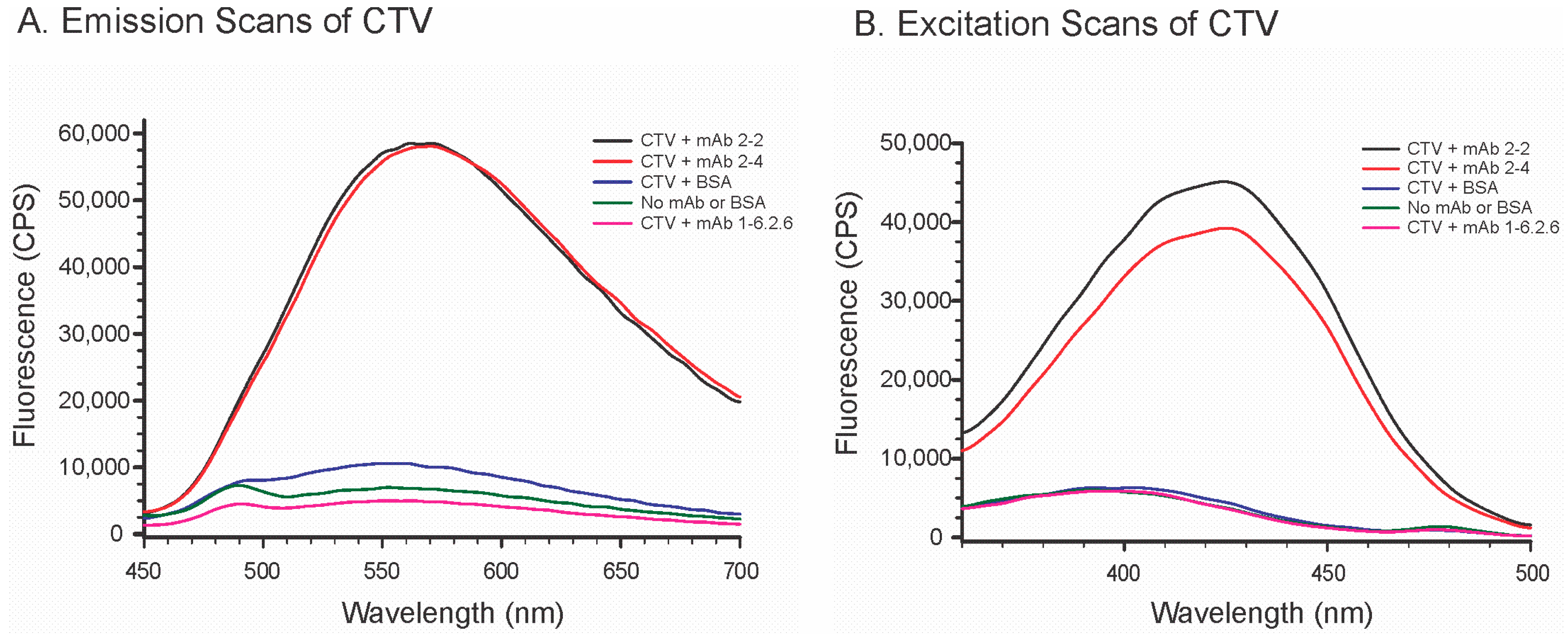
2.3. Effects of mAbs on the Fluorescence of Citreoviridin (CTV)
3. Conclusions
4. Materials and Methods
4.1. Materials
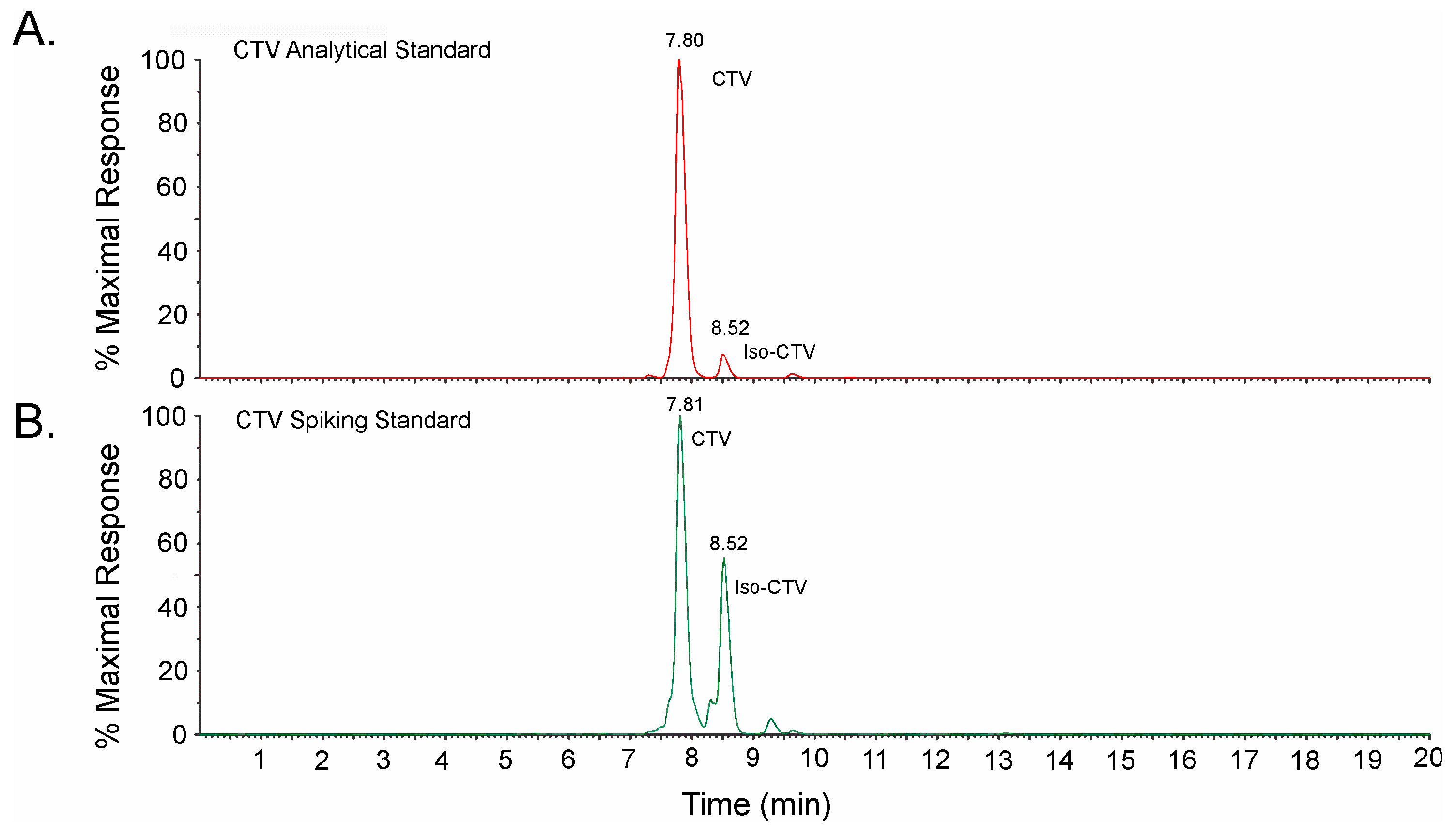
4.2. Liquid Chromatography-Photodiode Array-Mass Spectrometry (LC-PDA-MS) of CTV Stock Solutions
4.3. Preparation of CTV–Protein Conjugates
4.4. Immunization of Animals and Isolation of mAb-Producing Clones
4.5. Effects of Methanol and Acetonitrile on Two CTV mAbs
4.6. Spiking and Extraction of Rice Samples
4.7. CI-ELISA of Rice Samples
4.8. Effects of mAbs and Bovine Serum Albumin (BSA) on CTV Fluorescence
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Uraguchi, K. Mycotoxic origin of cardiac beriberi. J. Stored Prod. Res. 1969, 5, 227–236. [Google Scholar] [CrossRef]
- Kushiro, M. Historical review of researches on yellow rice and mycotoxigenic fungi adherent to rice in Japan. JSM Mycotoxins 2015, 65, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Sakabe, N.; Goto, T.; Hirata, Y. The structure of citreoviridin, a toxic compound produced by P. citreoviride molded on rice. Tetrahedron Lett. 1964, 5, 1825–1830. [Google Scholar] [CrossRef]
- Rosa, C.A.R.; Keller, K.M.; Oliveira, A.A.; Almeida, T.X.; Keller, L.A.M.; Marassi, A.C.; Kruger, C.D.; Deveza, M.V.; Monteiro, B.S.; Nunes, L.M.T.; et al. Production of citreoviridin by Penicillium citreonigrum strains associated with rice consumption and beriberi cases in the Maranhão State, Brazil. Food Add. Contam. Part A 2010, 27, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Lima, H.C.A.V.; Porto, E.A.S.; Marins, J.R.P.; Alves, R.M.; Machado, R.R.; Braga, K.N.L.; de Paiva, F.B.; Carmo, G.M.I.; e Santelli, A.C.F.S.; Sobel, J. Outbreak of beriberi in the state of Maranhão, Brazil: Revisiting the mycotoxin aetiologic hypothesis. Trop. Dr. 2010, 40, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.I.; Almeida, N.G.; Carvalho, K.L.; Gonçalves, G.A.A.; Silva, C.N.; Santos, E.A.; Garcia, J.C.; Vargas, E.A. Co-occurrence of aflatoxins B1, B2, G1 and G2, ochratoxin A, zearalenone, deoxynivalenol, and citreoviridin in rice in Brazil. Food Add. Contam. Part A 2012, 29, 694–703. [Google Scholar] [CrossRef]
- Ueno, Y.; Ueno, I. Isolation and acute toxicity of citreoviridin, a neurotoxic mycotoxin of Penicillium citreo-viride Biourge. Jpn. J. Exper. Med. 1972, 42, 91–105. [Google Scholar]
- Linnett, P.E.; Mitchell, A.D.; Osselton, M.D.; Mulheirn, L.J.; Beechey, R.B. Citreoviridin, a specific inhibitor of the mitochondiral adenosine triphosphatase. Biochem. J. 1978, 170, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Sayood, S.F.; Suh, H.; Wilcox, C.S.; Schuster, S.M. Effect of citreoviridin and isocitreoviridin on beef heart mitochondrial ATPase. Arch. Biochem. Biophys. 1989, 270, 714–721. [Google Scholar] [CrossRef]
- Datta, S.C.; Ghosh, J.J. Production and purification of Penicillium citreoviride toxin and its effect on TPP-dependent liver transketolase. Folia Microbiol. 1981, 26, 408–412. [Google Scholar] [CrossRef]
- Uchiyama, Y.; Takino, M.; Noguchi, M.; Shiratori, N.; Kobayashi, N.; Sugita-Konishi, Y. The in vivo and in vitro toxicokinetics of citreoviridin extracted from Penicillium citreonigrum. Toxins 2019, 11, 360. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y. Temperature-dependent production of citreoviridin, a neurotoxin of Penicillium citreo-viride Biourge. Jpn. J. Exper. Med. 1972, 42, 107–114. [Google Scholar]
- da Rocha, M.W.; Resck, I.S.; Caldas, E.D. Purification and full characterisation of citreoviridin produced by Penicillium citreonigrum in yeast extract sucrose (YES) medium. Food Add. Contam. Part A 2015, 32, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Wicklow, D.T.; Horn, B.W.; Cole, R.J. Cleistothecia of Eupenicillium ochrosalmoneum form naturally within corn kernels. Can. J. Bot. 1982, 60, 1050–1053. [Google Scholar] [CrossRef]
- Peterson, S.W.; Jurjević, Ž.; Frisvad, J.C. Expanding the species and chemical diversity of Penicillium section Cinnamopurpurea. PLoS ONE 2015, 10, e0121987. [Google Scholar] [CrossRef]
- Wicklow, D.T.; Stubblefield, R.D.; Horn, B.W.; Shotwell, O.L. Citreoviridin levels in Eupenicillium ochrosalmoneum-infested maize kernels at harvest. Appl. Environ. Microbiol. 1988, 54, 1096–1098. [Google Scholar]
- Cole, R.J.; Dorner, J.W.; Cox, R.H.; Hill, R.A.; Cluter, H.G.; Wells, J.M. Isolation of citreoviridin from Penicillium charlesii cultures and molded pecan fragments. Appl. Environ. Microbiol. 1981, 42, 677–681. [Google Scholar]
- Andrade, P.D.; Dantas, R.R.; Moura-Alves, T.L.d.S.d.; Caldas, E.D. Determination of multi-mycotoxins in cereals and of total fumonisins in maize products using isotope labeled internal standard and liquid chromatography/tandem mass spectrometry with positive ionization. J. Chrom. A 2017, 1490, 138–147. [Google Scholar] [CrossRef]
- Tangni, E.K.; Pussemier, L. Ergosterol and mycotoxins in grain dusts from fourteen Belgian cereal storages: A preliminary screening survey. J. Sci. Food Agric. 2007, 87, 1263–1270. [Google Scholar] [CrossRef]
- Cole, R.J.; Cox, R.H. Handbook of Toxic Fungal Metabolites; Academic Press: New York, NY, USA, 1981; p. 937. [Google Scholar]
- Stubblefield, R.D.; Greer, J.I.; Shotwell, O.L. Liquid chromatographic method for determination of citreoviridin in corn and rice. J. Assoc. Off. Anal. Chem. 1988, 71, 721–724. [Google Scholar]
- Engel, G. Untersuchungen zur bildung von mykotoxinen und deren quantitative analyse: VI. Citreoviridin. J. Chrom. A 1977, 130, 293–297. [Google Scholar] [CrossRef]
- Zhuang, Z.H.; Que, S.J.; Gao, Y.M.; Yuan, J.; Ye, Z.; Du, M.; Lin, G.M.; Liu, L.C.; Wang, S.H. Artificial antigen synthesis and the development of polyclonal antibody-based immunoassay for citreoviridin determination. World J. Microbiol. Biotechnol. 2014, 30, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Ling, S.; Yang, C.; Wang, S.H. Preparation and identification of monoclonal antibody against citreoviridin and development of detection by Ic-ELISA. Toxicon 2014, 90, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Gu, X.; Zhuang, Z.; Zhong, Y.; Yang, H.; Wang, S. Screening and molecular evolution of a single chain variable fragment antibody (scFv) against citreoviridin toxin. J. Agric. Food Chem. 2016, 64, 7640–7648. [Google Scholar] [CrossRef]
- Rebuffat, S.; Molho, D.; Dizabo, P. Mass spectra of citreoviridin A and related mycotoxins. Org. Mass Spectrom. 1984, 19, 349. [Google Scholar] [CrossRef]
- Suh, H.; Wilcox, C.S. Chemistry of F1,F0-ATPase inhibitors. Stereoselective total syntheses of (+)-citreoviral and (−)-citreoviridin. J. Am. Chem. Soc. 1988, 110, 470–481. [Google Scholar] [CrossRef]
- Nagel, D.W.; Steyn, P.S.; Ferreira, N.P. Biosynthesis of citreoviridin. Phytochemistry 1972, 11, 3215–3218. [Google Scholar] [CrossRef]
- Hermanson, G.T.; Krishna Mallia, A.; Smith, P.K. Immobilized Affinity Ligand Techniques; Academic Press, Inc.: San Diego, CA, USA, 1992; p. 454. [Google Scholar]
- ChemSpider. Available online: www.chemspider.com (accessed on 24 October 2019).
- Nakajima, K.; Masubuchi, Y.; Ito, Y.; Inohana, M.; Takino, M.; Saegusa, Y.; Yoshida, T.; Sugita-Konishi, Y.; Shibutani, M. Developmental exposure of citreoviridin transiently affects hippocampal neurogenesis targeting multiple regulatory functions in mice. Food Chem. Toxicol. 2018, 120, 590–602. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3th ed.; Springer: New York, NY, USA, 2006; p. 954. [Google Scholar]
- Hou, H.; Qu, X.; Li, Y.; Kong, Y.; Jia, B.; Yao, X.; Jiang, B. Binding of citreoviridin to human serum albumin: Multispectroscopic and molecular docking. Biomed. Res. Int. 2015. [Google Scholar] [CrossRef]
- Li, T.; Jo, E.J.; Kim, M.G. A label-free fluorescence immunoassay system for the sensitive detection of the mycotoxin, ochratoxin A. Chem. Commun. 2012, 48, 2304–2306. [Google Scholar] [CrossRef] [Green Version]
- Chu, F.S.; Lau, H.P.; Fan, T.S.; Zhang, G.S. Ethylenediamine modified bovine serum albumin as protein carrier in the production of antibody against mycotoxins. J. Immun. Meth. 1982, 55, 73–78. [Google Scholar] [CrossRef]
- Maragos, C.M. Development and evaluation of monoclonal antibodies for paxilline. Toxins 2015, 7, 3903–3915. [Google Scholar] [CrossRef] [PubMed]
- Maragos, C.M.; McCormick, S.P. Monoclonal antibodies for the mycotoxins deoxynivalenol and 3-acetyl-deoxynivalenol. Food Agric. Immun. 2000, 12, 181–192. [Google Scholar] [CrossRef]


| Solvent | Concentration a | IC50 for CTV (ng/mL) | Replicates b | |
|---|---|---|---|---|
| mAb 2-2 | mAb 2-4 | |||
| Buffer c | - | 11.1 ± 3.2 | 17.9 ± 5.6 | 8 |
| Methanol | 10% | 14.8 ± 1.2 | 20.4 ± 0.5 | 4 |
| 15% | 13.7 ± 1.2 | 21.7 ± 4.6 | 6 | |
| 20% | 15.2 ± 1.6 | 21.0 ± 1.1 | 4 | |
| 30% | 24.9 ± 14.6 | 36.9 ± 12.5 | 4 | |
| Acetonitrile | 5% | 13.3 ± 2.1 | 20.7 ± 1.1 | 4 |
| 10% | 18.7 ± 3.3 | 29.2 ± 4.6 | 4 | |
| 15% | 29.6 ± 2.5 | 43.4 ± 4.2 | 4 | |
| Type of Antibody | IC50 for CTV (ng/mL) | Dynamic Range (IC20 to IC80) (ng/mL) | Citation |
|---|---|---|---|
| pAb | 560 | Not specified | [23] |
| mAb | 161 | 11–2370 | [24] |
| scFv | 120 | 25–562 | [25] |
| mAb | 11 | 3–29 | this work, mAb 2-2 |
| mAb | 18 | 5–42 | this work, mAb 2-4 |
| CTV (mg/kg) | Iso-CTV (mg/kg) | Total (CTV + Iso-CTV) | Average Recovery (% ± 1 SD) a | Number of Replicates b |
|---|---|---|---|---|
| 0.23 | 0.13 | 0.36 | 91.9 ± 14.3 | 6 |
| 0.46 | 0.26 | 0.72 | 89.6 ± 7.8 | 6 |
| 0.93 | 0.52 | 1.45 | 97.9 ± 3.2 | 6 |
| 1.86 | 1.03 | 2.89 | 106.2 ± 8.7 | 6 |
| 4.65 | 2.58 | 7.23 | 97.7 ± 7.1 | 6 |
| Average (all): | 96.7 ± 10.2 | 30 | ||
| Volume of Stock Added (µL) | Amount of CTV Added (µg) | Amount of Iso-CTV Added (µg) | Concentration of CTV in Spiked Rice (mg/kg) | Concentration of Iso-CTV in Spiked Rice (mg/kg) | Concentration of CTV + Iso-CTV in Spiked Rice (mg/kg) |
|---|---|---|---|---|---|
| 25 | 2.33 | 1.29 | 0.233 | 0.129 | 0.362 |
| 50 | 4.65 | 2.58 | 0.465 | 0.258 | 0.723 |
| 100 | 9.30 | 5.17 | 0.930 | 0.517 | 1.447 |
| 200 | 18.60 | 10.34 | 1.861 | 1.034 | 2.894 |
| 500 | 46.50 | 25.85 | 4.652 | 2.585 | 7.235 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maragos, C.M.; Uchiyama, Y.; Kobayashi, N.; Kominato, F.; Sugita-Konishi, Y. Development and Characterization of Monoclonal Antibodies for the Mycotoxin Citreoviridin. Toxins 2019, 11, 630. https://doi.org/10.3390/toxins11110630
Maragos CM, Uchiyama Y, Kobayashi N, Kominato F, Sugita-Konishi Y. Development and Characterization of Monoclonal Antibodies for the Mycotoxin Citreoviridin. Toxins. 2019; 11(11):630. https://doi.org/10.3390/toxins11110630
Chicago/Turabian StyleMaragos, Chris M., Yosuke Uchiyama, Naoki Kobayashi, Fumichika Kominato, and Yoshiko Sugita-Konishi. 2019. "Development and Characterization of Monoclonal Antibodies for the Mycotoxin Citreoviridin" Toxins 11, no. 11: 630. https://doi.org/10.3390/toxins11110630
APA StyleMaragos, C. M., Uchiyama, Y., Kobayashi, N., Kominato, F., & Sugita-Konishi, Y. (2019). Development and Characterization of Monoclonal Antibodies for the Mycotoxin Citreoviridin. Toxins, 11(11), 630. https://doi.org/10.3390/toxins11110630