Calm Before the Storm: A Glimpse into the Secondary Metabolism of Aspergillus welwitschiae, the Etiologic Agent of the Sisal Bole Rot
Abstract
:1. Introduction
2. Results
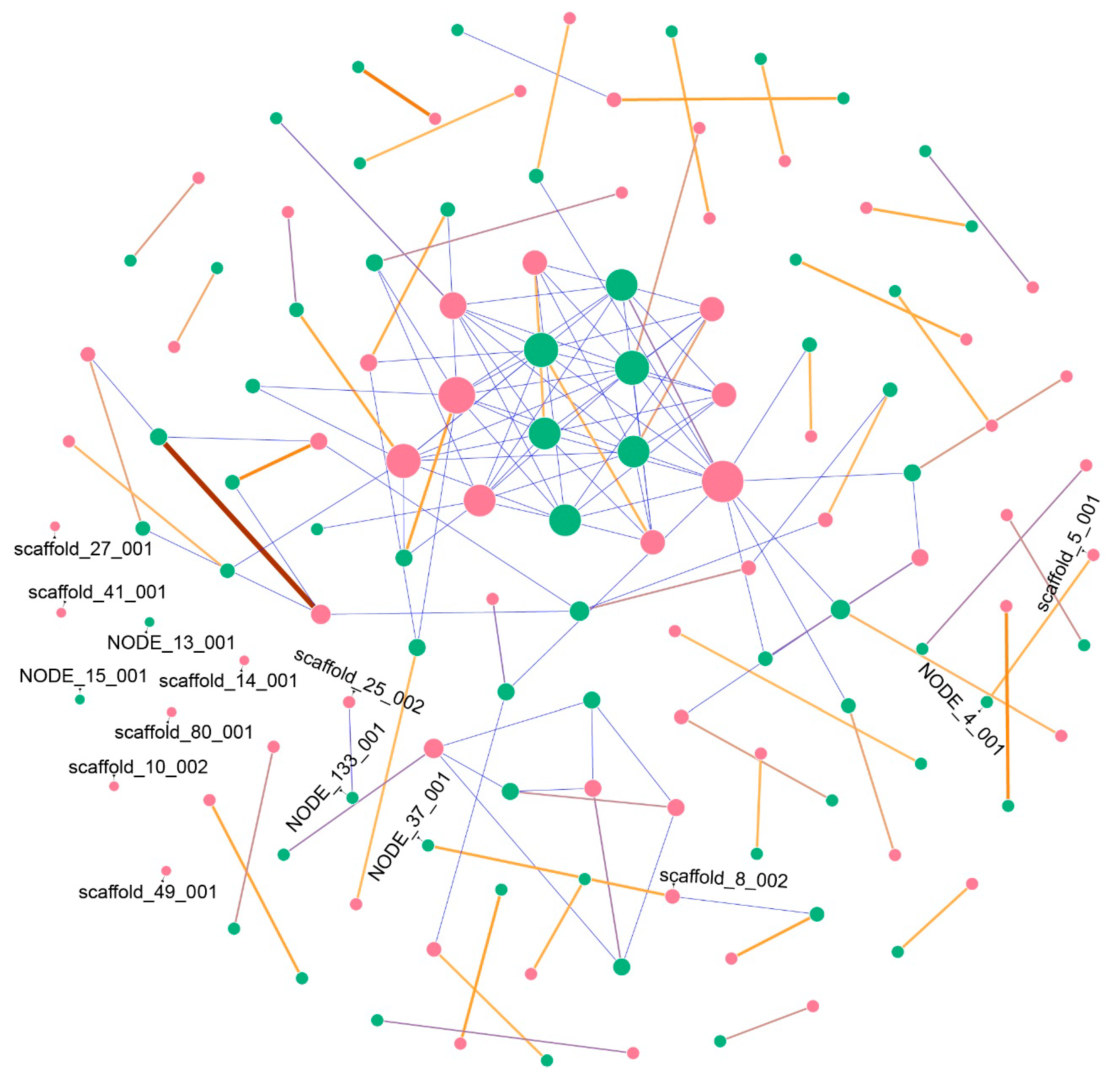
2.1. Secondary Metabolites Gene Clusters (SMGCs) Analysis
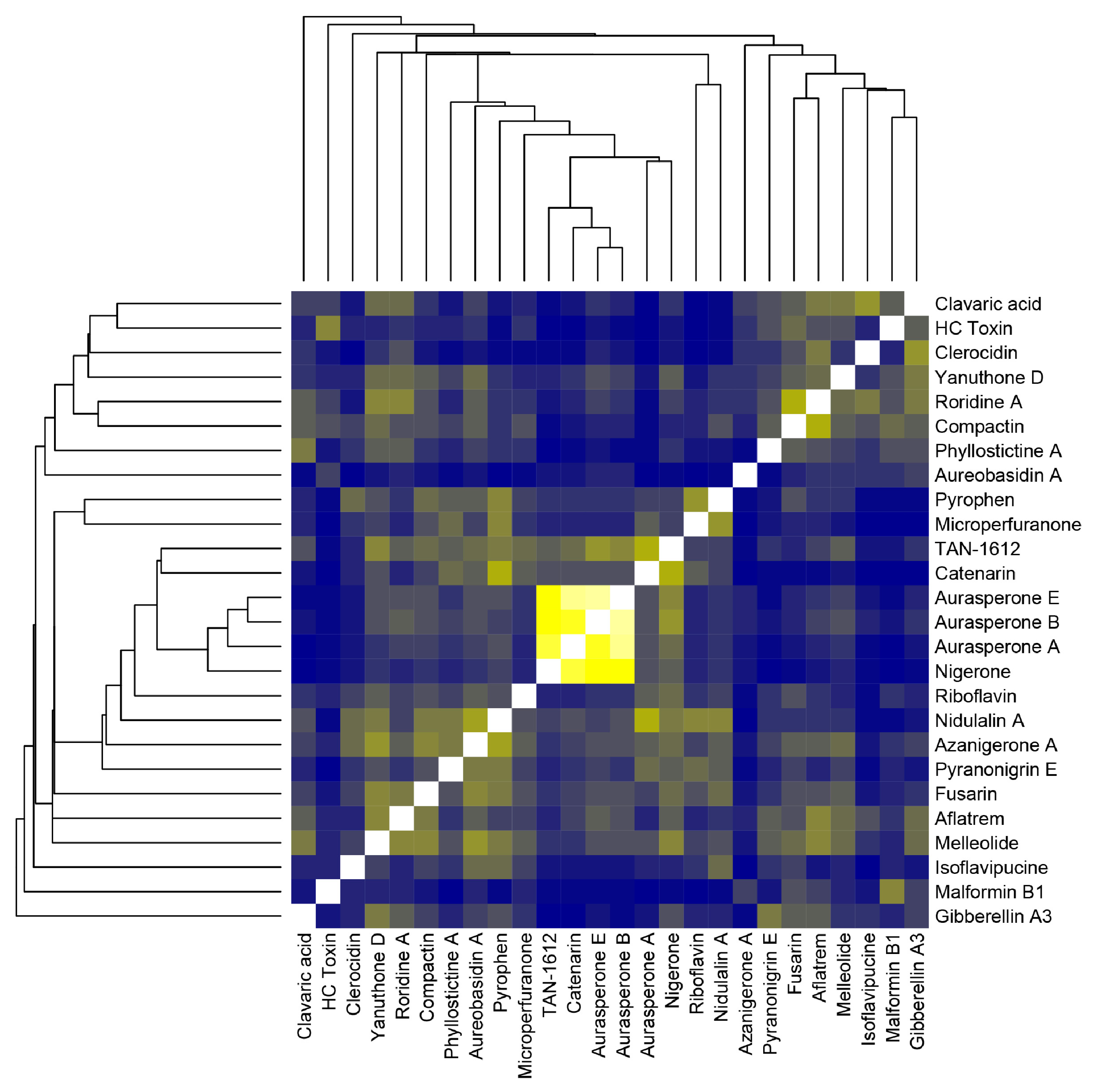
2.2. Fungal Extract HPLC-MS Analysis and Compound Similarity
2.3. Fumonisin, Ochratoxin, and Malformin C Clusters’ Characterization
3. Discussion
4. Materials and Methods
4.1. Biological Samples and Computational Resources
4.2. Genome Sequencing and Assembly
4.3. Secondary Metabolites Gene Cluster Prediction and Network Construction
4.4. HPLC Analysis
4.5. Compound Chemical Structure Comparison
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Varga, J.; Frisvad, J.C.; Kocsubé, S.; Brankovics, B.; Tóth, B.; Szigeti, G.; Samson, R.A. New and Revisited Species in Aspergillus Section Nigri. Stud. Mycol. 2011, 69, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, J.D.; O’Keeffe, T.L. Detection and Discrimination of Four Aspergillus Section Nigri Species by PCR. Lett. Appl. Microbiol. 2015, 60, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Duarte, E.A.; Damasceno, C.L.; Oliveira, T.A.; Barbosa, L.D.; Martins, F.M.; Silva, J.R.; Lima, T.E.; Silva, R.M.; Kato, R.B.; Bortoline, D.E.; et al. Putting the Mess in Order: Aspergillus Welwitschiae (and Not A. Niger) Is the Etiologic Agent of the Sisal Bole Rot Disease. Front. Microbiol. 2018, 9, 1227. [Google Scholar] [CrossRef] [PubMed]
- Abreu, K.C.L.D.M. Epidemiologia da Podridão Vermelha do Sisal No Estado da Bahia. Ph.D. Dissertation, Universidade Federal do Recôncavo da Bahia, Cruz das Almas, Brazil, 2010. [Google Scholar]
- Selin, C.; de Kievit, T.R.; Belmonte, M.F.; Fernando, W.G.D. Elucidating the Role of Effectors in Plant-Fungal Interactions: Progress and Challenges. Front. Microbiol. 2016, 7, 600. [Google Scholar] [CrossRef]
- Koeck, M.; Hardham, A.R.; Dodds, P.N. The Role of Effectors of Biotrophic and Hemibiotrophic Fungi in Infection. Cell. Microbiol. 2013, 13, 1849–1857. [Google Scholar] [CrossRef]
- Horbach, R.; Navarro-Quesada, A.R.; Knogge, W.; Deising, H.B. When and How to Kill a Plant Cell: Infection Strategies of Plant Pathogenic Fungi. J. Plant Physiol. 2011, 168, 51–62. [Google Scholar] [CrossRef]
- Bräse, S.; Encinas, A.; Keck, J.; Nising, C.F. Chemistry and Biology of Mycotoxins and Related Fungal Metabolites. Chem. Rev. 2009, 109, 3903–3990. [Google Scholar] [CrossRef]
- Reverberi, M.; Ricelli, A.; Zjalic, S.; Fabbri, A.A.; Fanelli, C. Natural Functions of Mycotoxins and Control of Their Biosynthesis in Fungi. Appl. Microbiol. Biotechnol. 2010, 87, 899–911. [Google Scholar] [CrossRef]
- Zvereva, A.S.; Pooggin, M.M. Silencing and Innate Immunity in Plant Defense against Viral and Non-Viral Pathogens. Viruses 2012, 4, 2578–2597. [Google Scholar] [CrossRef]
- Expert, D.; Patrit, O.; Shevchik, V.E.; Perino, C.; Boucher, V.; Creze, C.; Wenes, E.; Fagard, M. Dickeya Dadantii Pectic Enzymes Necessary for Virulence Are Also Responsible for Activation of the Arabidopsis Thaliana Innate Immune System. Mol. Plant Pathol. 2018, 19, 313–327. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. AntiSMASH 5.0: Updates to the Secondary Metabolite Genome Mining Pipeline. Nucleic Acids Res. 2019, 47, W81–W87. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and Applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Abu, F.; Mat Taib, C.N.; Mohd Moklas, M.A.; Mohd Akhir, S. Antioxidant Properties of Crude Extract, Partition Extract, and Fermented Medium of Dendrobium Sabin Flower. Evid. Based Complement. Altern. Med. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Reis, I.M.A.; Ribeiro, F.P.C.; Almeida, P.R.M.; Costa, L.C.B.; Kamida, H.M.; Uetanabaro, A.P.T.; Branco, A. Characterization of the Secondary Metabolites from Endophytic Fungi Nodulisporium Sp. Isolated from the Medicinal Plant Mikania Laevigata (Asteraceae) by Reversed-Phase High-Performance Liquid Chromatography Coupled with Mass Spectrometric Multistage. Pharmacogn. Mag. 2018, 14, S495–S498. [Google Scholar] [CrossRef]
- Cao, Y.; Charisi, A.; Cheng, L.C.; Jiang, T.; Girke, T. ChemmineR: A Compound Mining Framework for R. Bioinformatics 2008, 24, 1733–1734. [Google Scholar] [CrossRef]
- Susca, A.; Proctor, R.H.; Morelli, M.; Haidukowski, M.; Gallo, A.; Logrieco, A.F.; Moretti, A. Variation in Fumonisin and Ochratoxin Production Associated with Differences in Biosynthetic Gene Content in Aspergillus Niger and A. Welwitschiae Isolates from Multiple Crop and Geographic Origins. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Theobald, S.; Vesth, T.C.; Rendsvig, J.K.; Nielsen, K.F.; Riley, R.; de Abreu, L.M.; Salamov, A.; Frisvad, J.C.; Larsen, T.O.; Andersen, M.R.; et al. Uncovering Secondary Metabolite Evolution and Biosynthesis Using Gene Cluster Networks and Genetic Dereplication. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Struck, C.; Cooke, B.M.; Jones, D.G.; Kaye, B. Infection Strategies of Plant Parasitic Fungi. In The Epidemiology of Plant Diseases; Springer: Berlin, Germany, 2006; pp. 117–137. [Google Scholar] [CrossRef]
- Brown, D.W.; Butchko, R.A.E.; Proctor, R.H. Identification of Genes and Gene Clusters Involved in Mycotoxin Synthesis. In Determining Mycotoxins and Mycotoxigenic Fungi in Food and Feed; Woodhead Publishing Limited: Cambridge, UK, 2011; pp. 332–348. [Google Scholar] [CrossRef]
- Vesth, T.C.; Nybo, J.L.; Theobald, S.; Frisvad, J.C.; Larsen, T.O.; Nielsen, K.F.; Hoof, J.B.; Brandl, J.; Salamov, A.; Riley, R.; et al. Investigation of Inter- and Intraspecies Variation through Genome Sequencing of Aspergillus Section Nigri. Nat. Genet. 2018, 50, 1688–1695. [Google Scholar] [CrossRef]
- Kavanagh, K. Fungi—Biology and Applications; Kavanagh, K., Ed.; John Wiley & Sons: County Kildare, Ireland, 2011. [Google Scholar]
- Yassin, M.A.; Moslem, M.A.; El-Samawaty, A.E.-R.M.A. Mycotoxins and Non-Fungicidal Control of Corn Grain Rotting Fungi. J. Plant Sci. 2012, 7, 96–104. [Google Scholar] [CrossRef]
- Yassin, M.A.; Moslem, M.; Bahkali, A.; Abd-elsalam, K. Fungal Biota and Occurrence of Aflatoxigenic Aspergillus in Postharvest Corn grains. Fresenius Environ. Bull. 2011, 20, 903–909. [Google Scholar]
- Soares, C.; Calado, T.; Venâncio, A. Mycotoxin Production by Aspergillus Niger Aggregate Strains Isolated from Harvested Maize in Three Portuguese Regions. Rev. Iberoam. Micol. 2013, 30, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; He, F.; Zhang, X.; Bao, J.; Qi, S. New Mycotoxins from Marine-Derived Fungus Aspergillus Sp. SCSGAF0093. Food Chem. Toxicol. 2013, 53, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Kobbe, B.; Cushman, M.; Wogan, G.N.; Demain, A.L. Production and Antibacterial Activity of Malformin C, a Toxic Metabolite of Aspergillus Niger. Appl. Environ. Microbiol. 1977, 33, 996–997. [Google Scholar] [PubMed]
- Blumenthal, C.Z. Production of Toxic Metabolites in Aspergillus Niger, Aspergillus Oryzae, and Trichoderma Reesei: Justification of Mycotoxin Testing in Food Grade Enzyme Preparations Derived from the Three Fungi. Regul. Toxicol. Pharmacol. 2004, 39, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.W.; Gao, F.L.; Wang, F.R.; Chen, Q.J. Anti-TMV Activity of Malformin A1, a Cyclic Penta-Peptide Produced by an Endophytic Fungus Aspergillus Tubingensis FJBJ11. Int. J. Mol. Sci. 2015, 16, 5750–5761. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Z.; Lam, W.; Gullen, E.A.; Yu, Z.; Wei, Y.; Wang, L.; Zeiss, C.; Beck, A.; Cheng, E.C.; et al. Study of Malformin C, a Fungal Source Cyclic Pentapeptide, as an Anti-Cancer Drug. PLoS ONE 2015, 10, e0140069. [Google Scholar] [CrossRef]
- Takahashi, N.; Curtis, R.W. Isolation and Characterization of Malformin. Plant Physiol. 1960, 30–36. [Google Scholar] [CrossRef]
- Curtis, R.W. Studies on Response of Bean Seedlings & Corn Roots to Malformin. Plant Physiol. 1961, 36, 37–43. [Google Scholar] [CrossRef]
- Curtis, R.W. Phytochrome Involvement in the Induction of Resistance to Dark Abscission by Malformin. Planta 1978, 141, 311–314. [Google Scholar] [CrossRef]
- Curtis, R.W.; Stevenson, W.R.; Tuite, J. Malformin in Aspergillus Niger Infected Onion Bulbs (Allium cepa). J. Appl. Microbiol. 1974, 28, 362–365. [Google Scholar]
- Susca, A.; Proctor, R.H.; Butchko, R.A.E.; Haidukowski, M.; Stea, G.; Logrieco, A.; Moretti, A. Variation in the Fumonisin Biosynthetic Gene Cluster in Fumonisin-Producing and Nonproducing Black Aspergilli. Fungal Genet. Biol. 2014, 73, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Massi, F.P.; Sartori, D.; de Souza Ferranti, L.; Iamanaka, B.T.; Taniwaki, M.H.; Vieira, M.L.C.; Fungaro, M.H.P. Prospecting for the Incidence of Genes Involved in Ochratoxin and Fumonisin Biosynthesis in Brazilian Strains of Aspergillus Niger and Aspergillus Welwitschiae. Int. J. Food Microbiol. 2016, 221, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Gherbawy, Y.; Elhariry, H.; Kocsubé, S.; Bahobial, A.; El Deeb, B.; Altalhi, A.; Varga, J.; Vágvölgyi, C. Molecular Characterization of Black Aspergillus Species from Onion and Their Potential for Ochratoxin A and Fumonisin B2 Production. Foodborne Pathog. Dis. 2015, 12, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Kocsubé, S.; Szigeti, G.; Baranyi, N.; Vágvölgyi, C.; Despot, D.J.; Magyar, D.; Meijer, M.; Samson, R.A.; Klarić, M.Š. Occurrence of Black Aspergilli in Indoor Environments of Six Countries. Arh. Hig. Rada Toksikol. 2014, 65, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Suzuki, T.; Ono, C.; Iwamoto, K.; Hosobuchi, M.; Yoshikawa, H. Molecular Cloning and Characterization of an ML-236B (Compactin) Biosynthetic Gene Cluster in Penicillium Citrinum. Mol. Genet. Genom. 2002, 267, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.A.G.; Smale, T.C.; King, T.J.; Hasenkamp, R.; Thompson, R.H. Crystal and Molecular Structure of Compactin, a New Antifungal Metabolite from Penicillium Brevicompactum. J. Chem. Soc. Perkin Trans. 1 1976, 11, 1165–1170. [Google Scholar] [CrossRef]
- Akhtar, N.; Gupta, P.; Sangwan, N.S.; Sangwan, R.S.; Trivedi, P.K. Cloning and Functional Characterization of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Gene from Withania Somnifera: An Important Medicinal Plant. Protoplasma 2013, 250, 613–622. [Google Scholar] [CrossRef]
- Doblas, V.G.; Amorim-Silva, V.; Posé, D.; Rosado, A.; Esteban, A.; Arró, M.; Azevedo, H.; Bombarely, A.; Borsani, O.; Valpuesta, V.; et al. The SUD1 Gene Encodes a Putative E3 Ubiquitin Ligase and Is a Positive Regulator of 3-Hydroxy-3-Methylglutaryl Coenzyme a Reductase Activity in Arabidopsis. Plant Cell 2013, 25, 728–743. [Google Scholar] [CrossRef]
- McCullough, J.E.; O’sullivan, J.; Summerill, R.S.; Parker, W.L.; Wells, J.S.; Bonner, D.P.; Fernandes, P.B.; Muller, M.T.; Howells, A.J.; Maxwell, A. Clerocidin, A Terpenoid Antibiotic, Inhibits Bacterial Dna Gyrase. J. Antibiot. 1993, 46, 526–530. [Google Scholar] [CrossRef]
- Gatto, B. The Topoisomerase II Poison Clerocidin Alkylates Non-Paired Guanines of DNA: Implications for Irreversible Stimulation of DNA Cleavage. Nucleic Acids Res. 2001, 29, 4224–4230. [Google Scholar] [CrossRef]
- Vey, A.; Hoagland, R.E.; Tariq, M.B. Toxic Metabolites of Fungal Biocontrol Agents. Fungal Divers. 2001, 311–346. [Google Scholar] [CrossRef]
- Burkin, A.A.; Kononenko, G.P. Mycotoxin Contamination of Meadow Grasses in European Russia. Agric. Biol. 2015, 50, 503–512. [Google Scholar] [CrossRef]
- Brown, D.W.; McCormick, S.P.; Alexander, N.J.; Proctor, R.H.; Desjardins, A.E. A Genetic and Biochemical Approach to Study Trichothecene Diversity in Fusarium Sporotrichioides and Fusarium Graminearum. Fungal Genet. Biol. 2001, 32, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Wakuliński, W.; Kachlicki, P.; Sobiczewski, P.; Schollenberger, M.; Zamorski, C.; Łotocka, B.; Šarova, J. Catenarin Production by Isolates of Pyrenophora Tritici-Repentis (Died.) Drechsler and Its Antimicrobial Activity. J. Phytopathol. 2003, 151, 74–79. [Google Scholar] [CrossRef]
- Anke, H.; Kolthoum, I.; Zähner, H.; Laatsch, H. Metabolic Products of Microorganisms. 185. The Anthraquinones of the Aspergillus Glaucus Group. I. Occurrence, Isolation, Identification and Antimicrobial Activity. Arch. Microbiol. 1980, 126, 223–230. [Google Scholar] [CrossRef]
- Bouras, N.; Strelkov, S.E. The Anthraquinone Catenarin Is Phytotoxic and Produced in Leaves and Kernels of Wheat Infected by Pyrenophora Tritici-Repentis. Physiol. Mol. Plant Pathol. 2008, 72, 87–95. [Google Scholar] [CrossRef]
- Lu, S.; Tian, J.; Sun, W.; Meng, J.; Wang, X.; Fu, X.; Wang, A.; Lai, D.; Liu, Y.; Zhou, L. Bis-Naphtho-γ-Pyrones from Fungi and Their Bioactivities. Molecules 2014, 19, 7169–7188. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Mogensen, J.M.; Johansen, M.; Larsen, T.O.; Frisvad, J.C. Review of Secondary Metabolites and Mycotoxins from the Aspergillus Niger Group. Anal. Bioanal. Chem. 2009, 395, 1225–1242. [Google Scholar] [CrossRef]
- Reber, K.P.; Burdge, H.E. Total Synthesis of Pyrophen and Campyrones A-C. J. Nat. Prod. 2018, 81, 292–297. [Google Scholar] [CrossRef]
- Bushnell, B. BBTools: A Suite of Fast, Multithreaded Bioinformatics Tools Designed for Analysis of DNA and RNA Sequence Data; Joint Genome Institute: Walnut Creek, CA, USA, 2018. Available online: https//jgi.doe.gov/data-and-tools/bbtools (accessed on 20 September 2019).


| Peak # | RT (min) | [M + H]+ | [M+Na]+ | LC-MS m/z | Metabolite | |
|---|---|---|---|---|---|---|
| (Positive Mode) | ||||||
| Fraction A | 1 | 28.5 | 287 | 309 | [287]: 287;595 | Catenarin |
| 2 | 29.6 | 571 | - | [571]: 556 | Aurasperone A | |
| 3 | 33.3 | 530 | - | [530]: 513;417;277;175 | Malformin C | |
| 4 | 34.9 | 377 | - | [377]: 377;253;197;171 | Riboflavin | |
| 5 | 35.4 | 391 | - | [391]: 253;197;159 | Compactin | |
| Fraction B | 6 | 26.8 | 607 | - | [607]: 589;574;531;505 | Aurasperone B |
| 7 | 28.1 | 589 | - | [589]:571;531 | Aurasperone E | |
| 8 | 29.9 | 571 | - | [571]: 556;531;498 | Nigerone | |
| Fraction C | 9 | 21.1 | 288 | - | [288]: 246;575 | Pyrophen |
| 10 | 30.9 | 349 | - | [349]: 349;291;237 | Clerocidin | |
| 5 | 34.5 | 391 | 413 | [391]: 279;149 | Compactin * | |
| Fraction D | 9 | 21 | 288 | 310 | [288]: 246;597 | Pyrophen |
| 7 | 27.8 | 589 | - | [589]: 531;505 | Aurasperone E * | |
| 3 | 29.6 | 571 | 593 | [571]: 556 | Aurasperone A | |
| 11 | 30 | 533 | 555 | [533]: 267;211 | Roridin A |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintanilha-Peixoto, G.; Torres, R.O.; Reis, I.M.A.; Oliveira, T.A.S.d.; Bortolini, D.E.; Duarte, E.A.A.; Ariston de Carvalho Azevedo, V.; Brenig, B.; Aguiar, E.R.G.R.; Soares, A.C.F.; et al. Calm Before the Storm: A Glimpse into the Secondary Metabolism of Aspergillus welwitschiae, the Etiologic Agent of the Sisal Bole Rot. Toxins 2019, 11, 631. https://doi.org/10.3390/toxins11110631
Quintanilha-Peixoto G, Torres RO, Reis IMA, Oliveira TASd, Bortolini DE, Duarte EAA, Ariston de Carvalho Azevedo V, Brenig B, Aguiar ERGR, Soares ACF, et al. Calm Before the Storm: A Glimpse into the Secondary Metabolism of Aspergillus welwitschiae, the Etiologic Agent of the Sisal Bole Rot. Toxins. 2019; 11(11):631. https://doi.org/10.3390/toxins11110631
Chicago/Turabian StyleQuintanilha-Peixoto, Gabriel, Rosimére Oliveira Torres, Isabella Mary Alves Reis, Thiago Alves Santos de Oliveira, Dener Eduardo Bortolini, Elizabeth Amélia Alves Duarte, Vasco Ariston de Carvalho Azevedo, Bertram Brenig, Eric Roberto Guimarães Rocha Aguiar, Ana Cristina Fermino Soares, and et al. 2019. "Calm Before the Storm: A Glimpse into the Secondary Metabolism of Aspergillus welwitschiae, the Etiologic Agent of the Sisal Bole Rot" Toxins 11, no. 11: 631. https://doi.org/10.3390/toxins11110631
APA StyleQuintanilha-Peixoto, G., Torres, R. O., Reis, I. M. A., Oliveira, T. A. S. d., Bortolini, D. E., Duarte, E. A. A., Ariston de Carvalho Azevedo, V., Brenig, B., Aguiar, E. R. G. R., Soares, A. C. F., Góes-Neto, A., & Branco, A. (2019). Calm Before the Storm: A Glimpse into the Secondary Metabolism of Aspergillus welwitschiae, the Etiologic Agent of the Sisal Bole Rot. Toxins, 11(11), 631. https://doi.org/10.3390/toxins11110631








