Spider Knottin Pharmacology at Voltage-Gated Sodium Channels and Their Potential to Modulate Pain Pathways
Abstract
:1. Introduction
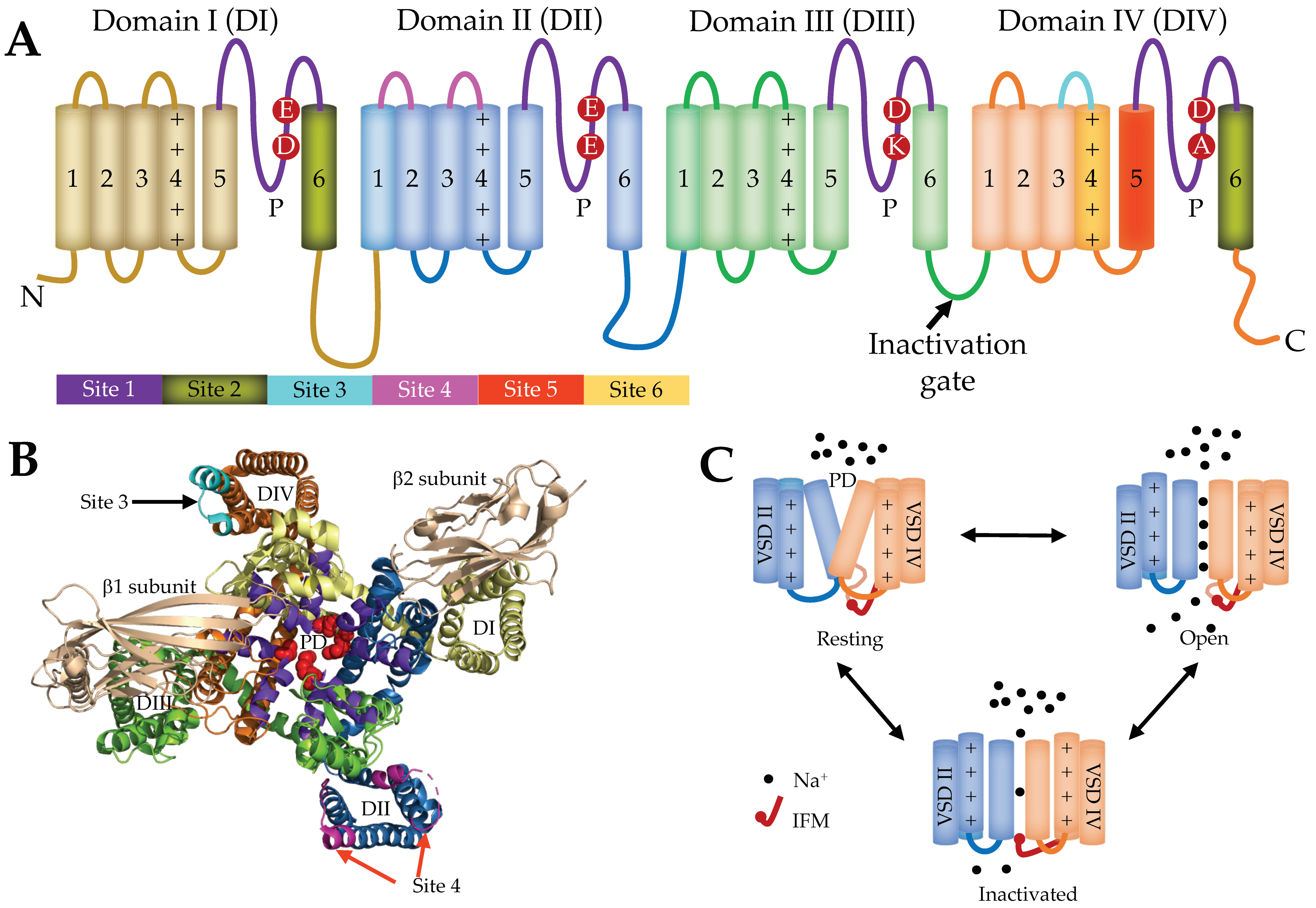
2. Voltage-Gated Sodium Channel Function and Structure
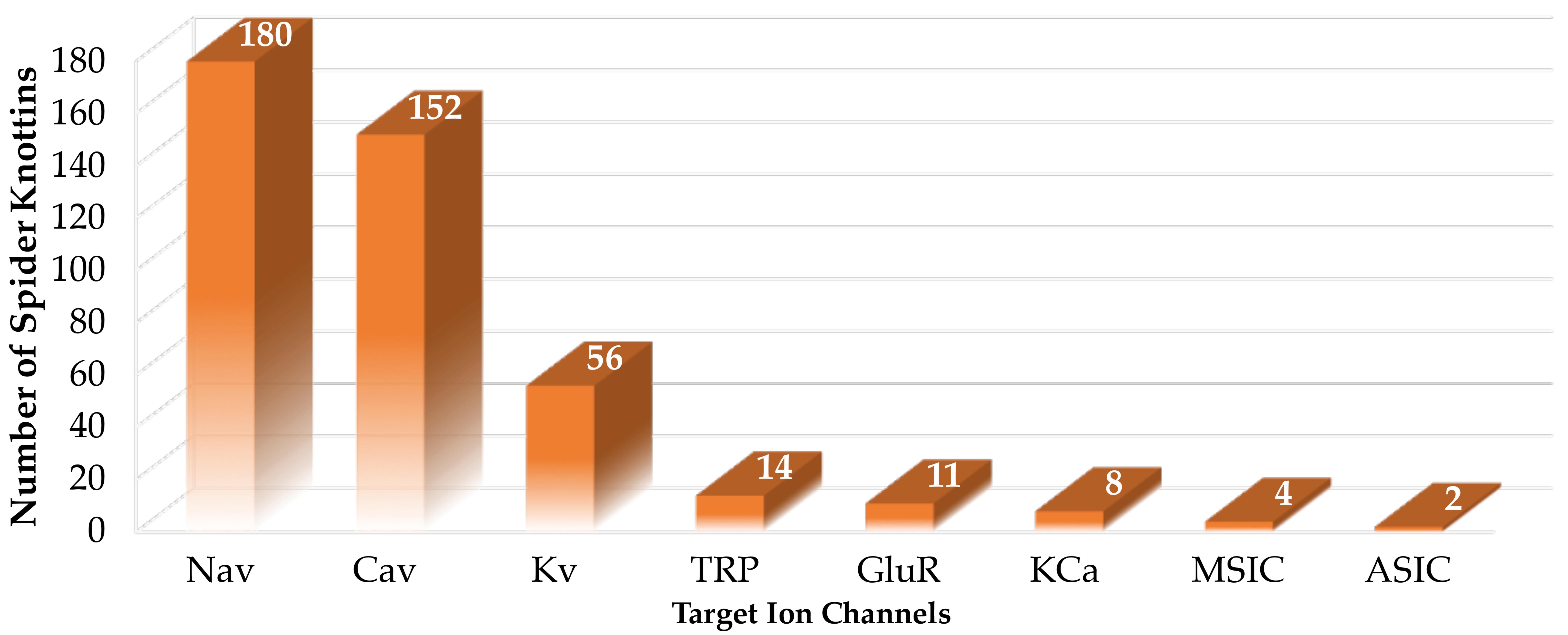
3. Spider-Venom ICK Peptides
3.1. Pharmacology of NaSpTx
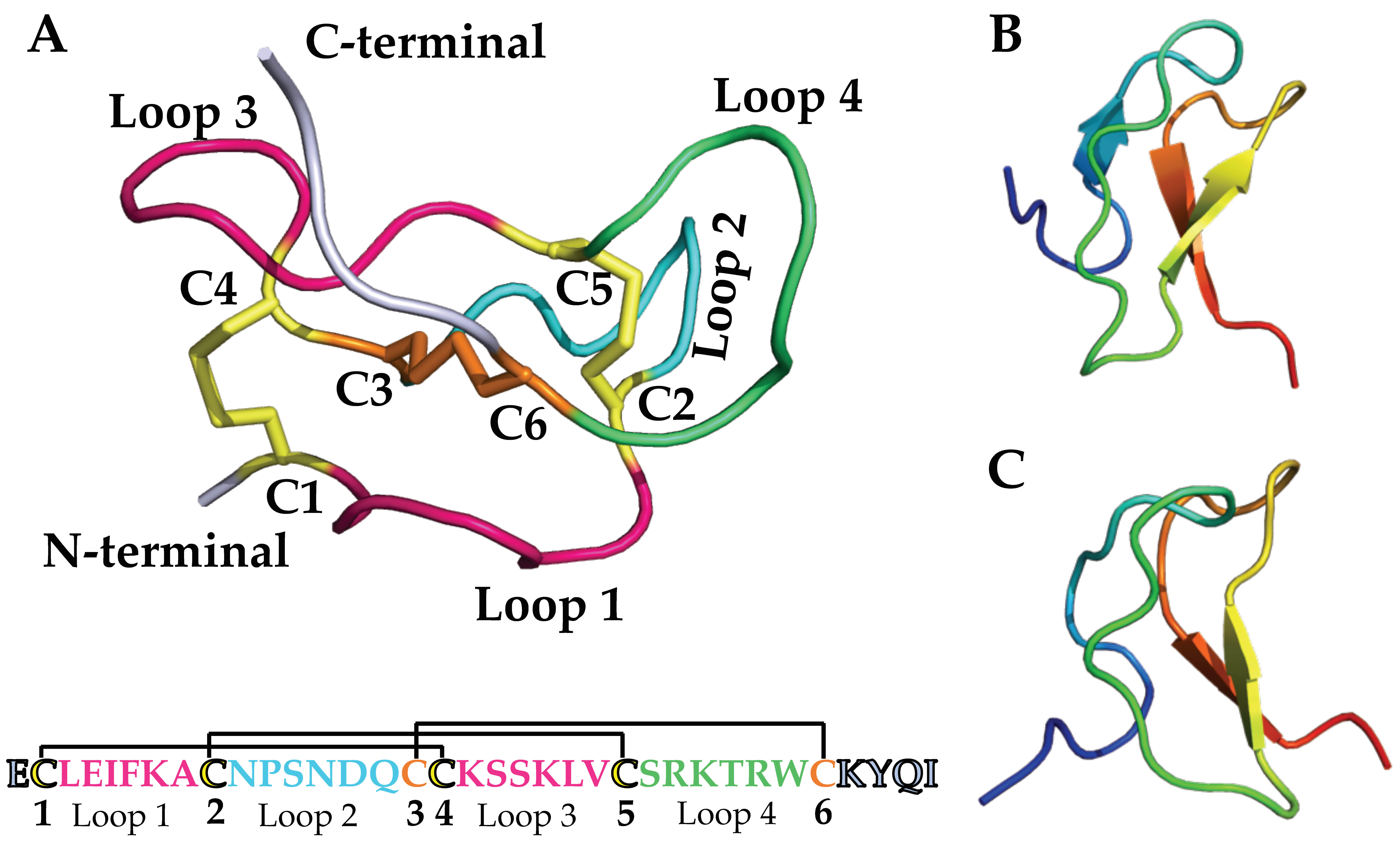
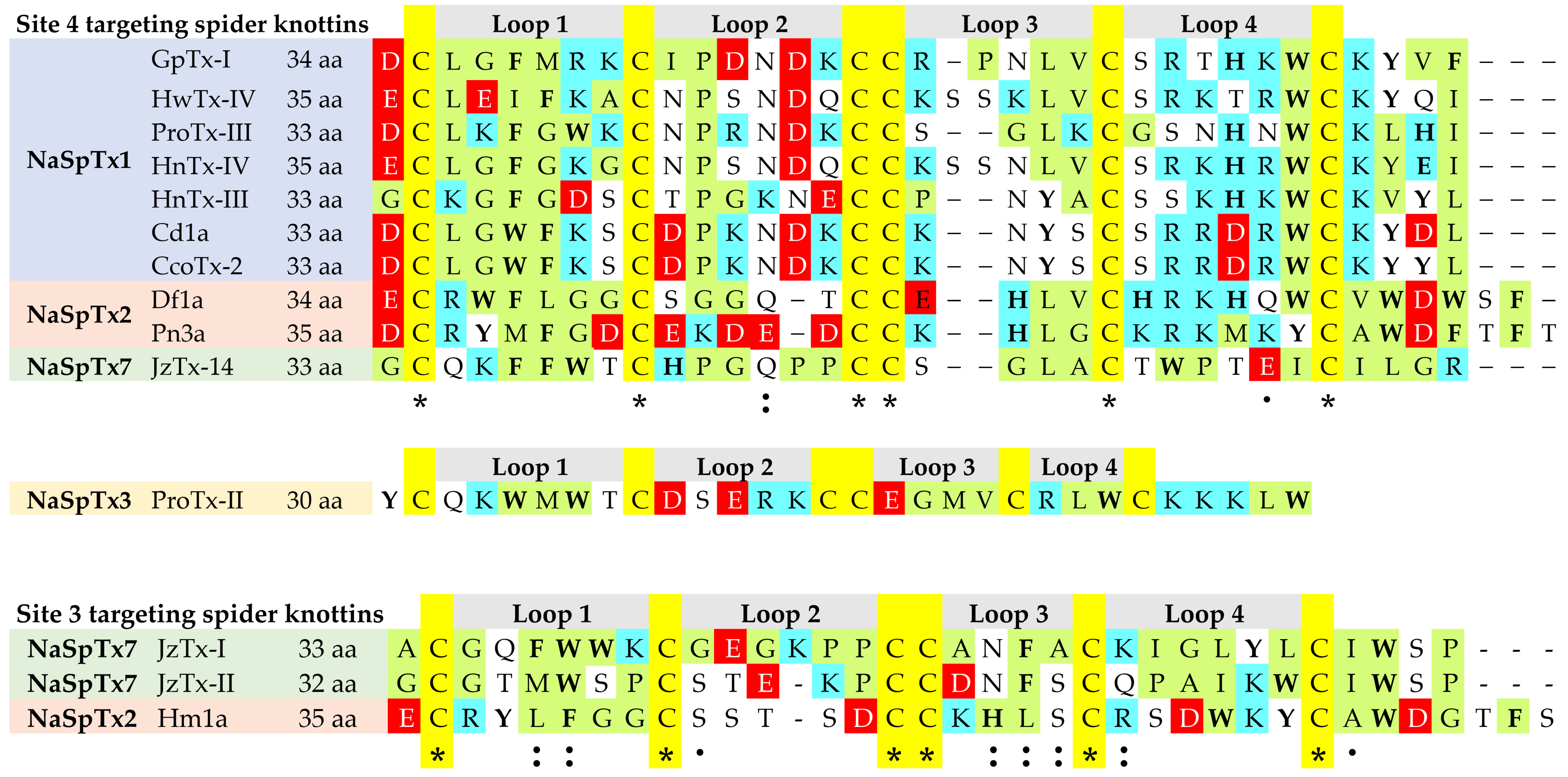
3.2. Structure–Function of NaSpTx
4. Knottins for NaVs in Pain Pathways
4.1. NaV1.1
4.2. NaV1.3
4.3. NaV1.6
4.4. NaV1.7
4.5. NaV1.8
4.6. NaV1.9
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Toxin | NaSpTx Family | NaV1.1 | NaV1.2 | NaV1.3 | NaV1.4 | NaV1.5 | NaV1.6 | NaV1.7 | NaV1.8 | NaV1.9 | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ProTx-I | 2 | 51 nM (n, X, h); 72 nM (s, X, h); [103] | 27 nM (n, X, r) [103] | ||||||||
| ProTx-II | 3 | 15.8 nM (s, C, h) [141] | 52.9 nM (n, H, rNav1.2a) [104] 41 nM (n, H, h) [132] 79.4 nM (s, H, h) [141] | 109.9 nM (n, H, r) [104] 102 nM (n, C, h) [132] 25 nM (s, h) [141] | 107.6 nM (n, H, r) [104] 39 nM (n, H, h) [132] 79.4 nM (s, H, h) [141] | 29 nM (s, H, h) [103] 79.4 nM (n, H, h) [104] 79 nM (n, H, h) [132] 398 nM (s, H, h) [141] | 26 nM (n, H, h) [132] 31.6 nM (s, C, h) [141] | 0.7 nM (n, H, h) [104] 0.3 nM (n, H, h) [132] 0.8 nM (s, H, h) [141] | 19 nM (n, X, r) [103] 146 nM (n, H, h) [132] | ||
| GP-W7Q-W30L ProTx-II | 3 | 6310 nM (s, C, h) [141] | 794 nM (s, H, h) [141] | 15,849 nM (s, H, h) [141] | >3162 nM (s, H, h) [141] | 2512 nM (s, C, h) [141] | 10 nM (s, H, h) [141] | ||||
| ProTx-III | 1 | 60 nM, rec-G; 101 nM, s-OH; 11.3 nM, s-NH2 (H, h) [80] | 21.9 nM, rec-G; 41.3 nM, s-OH; 11.5 nM, s-NH2 (H, h) [80] | >500 nM, rec-G and s-OH; >50 nM, s-NH2 (H, h) [80] | 9.5 nM, rec-G; 11.5 nM, s-OH; 2.5 nM, s-NH2 (H, h) [80] | ||||||
| HwTX-IV | 1 | 41 nM (n, H, h) [237] | 150 nM (n, H, r) [114] 10 nM, s-NH2, 54 nM, rec (H, h) [83] 44 nM (n, H, h) [237] | 338 nM (n, H, r) [114] 190 nM (n, H, h) [237] | 400 nM (n, H, r) [114] >10,000 nM (n, H, h) [237] | >10,000 nM (n, H, h) [114] >10,000 nM (n, H, h) [237] | 52 nM (n, H, h) [237] 83.3 nM (n, H, h) [237] | 22.7 nM (n, H, h) [104] 26 nM (n, H, r) [114] 16 nM, s-NH2, 55 nM, rec (H, h) [83] 17 nM (s, H, h) [142] 32.4 nM (s, S, h, FLIPR) [137] 100 nM (n, H, h) [237] 32.6 nM (n, H, h, Manual ephys) [237] | >10,000 nM (n, H, h) [237] | 30 nM (n, TTX-S currents, rat DRG) [113] | |
| E1G-E4G-Y33W HwTx-IV | 1 | 8.4 nM (rec, H, h) [143] 1100 nM (rec, H, h, FLIPR) [143] | 11.9 nM (rec, H, h) [143] 540 nM (rec, H, h, FLIPR) [143] | 7.2 nM (rec, H, h) [143] 1400 nM (rec, H, h, FLIPR) [143] | 369 nM (rec, H, h) [143] >30,000 nM (rec, H, h, FLIPR) [143] | Insensitive up to 1000 nM (rec, H, h) [143] >30,000 nM (rec, H, h, FLIPR) [143] | 6.8 nM (rec, C, h) [143] 600 nM (rec, H, h, FLIPR) [143] | 0.4 nM (s, H, h) [142] 3.3 nM (rec, C, h) [143] 5100 nM (rec, H, h, FLIPR) [143] | Insensitive up to 1000 nM (rec, C, h) [143] >30,000 nM (rec, H, h, FLIPR) [143] | ||
| E1G-E4G-F6W-Y33W HwTx-IV | 1 | 7.55 nM (s, S, h, FLIPR) [137] | |||||||||
| HnTx-I | 1 | 68,000 nM (n, X, r) [145] | >10,000 nM (s, H, h) [238] | No inhibition of TTX-S and TTX-R currents up to 100,000 nM [145] | |||||||
| G7W-N24S HnTx-I | 1 | 440 nM (rec, C, h) [81] | |||||||||
| E1G-N23S-D26H-L32W HnTx-I | 1 | 3.6 nM (s, H, h) [238] | |||||||||
| HnTx-III | 1 | 1270 nM (n, H, h) [117] | 275 nM (n, H, h) [117] | 491 nM (n, H, h) [117] | No activity [117] | No activity [117] | 232 nM (n, H, h) [117] | 1.1 nM (TTX-S rat DRG); No inhibition in TTX-R currents [116] | |||
| HnTx-IV | 1 | 36.1 nM (n, H, r) [129] | 375 nM (n, H, r) [129] | >10,000 nM (n, H, r) [129] | No inhibition up to 1000 nM [129] | 21 nM (s, n, H, h) [127] | 44.6 nM (TTX-S rat DRG) [116] 34 nm (TTX-S rat DRG) [85] | ||||
| JzTx-III | 7 | No effect [106] | No effect [106] | No effect [106] | 348 nM (rec, H, h) [106] | No effect [106] | No effect [106] | 380 nM (n, rat cardiac myocytes) [105] | |||
| JzTx-V | 3 | 292 nM (s, H, h) [79] | 5.12 nM (s, H, r) [79] | 2700 nM (s, H, h) [79] | 61 nM (s, H, h) [79] | 27.6 nM (n, TTX-R currents, rat DRG); 30.2 nM (n, TTX-S currents, rat DRG) [107] | |||||
| JzTx-IX | 2 | 5420 nM (n, HT) [108] | 450 nM (n, HT) [108] | 650 nM (n, TTX-R currents, rat DRG); 360 nM (n, TTX-S currents, rat DRG) [108] | |||||||
| JzTx-XI | 2 | 124 nM (n, C) [91] | |||||||||
| JzTx-14 | 3 | 194 nM (n, HT, M) [125] | 426.3 nM (n, HT, M) [125] | 290.1 nM (n, HT, M) [125] | 478 nM (n, HT, M) [125] | 158.6 nM (n, HT, M) [125] | 188.9 nM (n, HT, M) [125] | 824 nM (n, HT, M) [125] | |||
| JzTx-34 | 2 | No inhibition (s, HT, r) [119] | No inhibition (s, HT, r) [119] | 7950 nM (s, HT, r) [119] | No inhibition (s, HT, r) [119] | No inhibition (s, HT, r) [119] | No inhibition (s, HT) [119] | 610 nM (s, HT, h) [119] | No inhibition (s, ND cells, h) [119] | 85 nM (rec, TTX-S currents, rat DRG); No inhibition in TTX-R currents [118] 91 nM (s, TTX-S currents, rat DRG); No inhibition in TTX-R currents [119] | |
| JzTx-35 | 2 | 1070 nM (n, H, h) [112] | |||||||||
| CcoTx-1 | 1 | 523 nM (n, X) [73] 1060 nM (n, H, h, FLIPR) [110] | 3 nM (n, X) [73] 70 nM (n, H, h, FLIPR) [110] | No activity [73] >10,000 nM (n, H, h, FLIPR) [110] | 888 nM (n, X) [73] >10,000 nM (n, H, h, FLIPR) [110] | 323 nM (n, X) [73] >10,000 nM (n, H, h, FLIPR) [110] | >10,000 nM (n, H, h, FLIPR) [110] | 5120 nM (n, H, h, FLIPR) [110] | 55% block (n, X) [73] >10,000 nM (n, H, h, FLIPR) [110] | ||
| CcoTx-2 | 1 | 407 nM (n, X) [73] 170 nM (n, H, h, FLIPR) [110] | 8 nM (n, X) [73] 80 nM (n, H, h, FLIPR) [110] | 88 nM (n, X) [73] 5570 nM (n, H, h, FLIPR) [110] | 400 nM (n, X) [73] >10,000 nM (n, H, h, FLIPR) [110] | 1634 nM (n, X) [73] >10,000 nM (n, H, h, FLIPR) [110] | 3990 nM (n, H, h, FLIPR) [110] | 230 nM (n, H, h, FLIPR) [110] | 40% block (n, X) [73] >10,000 nM (n, H, h, FLIPR) [110] | ||
| CcoTx-3 | 2 | No activity [73] | No activity [73] | No activity [73] | No activity [73] | 447 nM (n, X) [73] | 45% block (n, X) [73] | ||||
| PaurTx3 | 1 | 610 nM (n, X) [73] | 0.6 nM (n, X) [73] | 42 nM (n, X) [73] | 288 nM (n, X) [73] | 72 nM (n, X) [73] | 65% block (n, X) [73] | ||||
| Hm-1 | 9 | 32.4% block at 200 nM (n, X, r) [120] | 336.4 nM (n, X, r) [120] 40% block at 200 nM (n, X, r) [120] | 36.5% block at 200 nM (n, X, h) [120] | 38.7% block at 200 nM (n, X, m) [120] | ||||||
| Hm-2 | 9 | 64.6% block at 200 nM (n, X, r) [120] | 154.8 nM (n, X, r) [120] 61.9% block at 200 nM (n, X, r) [120] | 17.8% block at 200 nM (n, X, h) [120] | 38.7% block at 200 nM (n, X, m) [120] | ||||||
| GTx1-15 | 1 | 120 nM (s, H, h) [239] | No effect up to 2000 nM (s, H, h) [239] | 7 nM (s, H, h) [239] | No effect up to 930 nM (s, H, h) [239] | ||||||
| VSTx-3 | 1 | 190 nM (s, H, h) [239] | No effect up to 1000 nM (s, H, h) [239] | 430 nM (s, H, h) [239] | 770 nM (s, H, h) [239] | ||||||
| Hd1a | 1 | 87% block (rec, X, h) [121] | 55% block (rec, X, h) [121] | 23%–31% block (rec, X, h) [121] | 23%–31% block (rec, X, h) [121] | No inhibition up to 1000 nM (rec, X, h) [121] | 23%–31% block (rec, X, h) [121] | 111 nM (rec, X, h) [121] 87% block (rec, X, h) [121] | No inhibition up to 1000 nM (rec, X, h) [121] | ||
| Cd1a | 1 | 2180 μM (s, H, h, FLIPR) [110] | 130 μM (s, H, h, FLIPR) [110] | >30,000 nM (s, H, h, FLIPR) [110] | >30,000 nM (s, H, h, FLIPR) [110] | >30,000 nM (s, H, h, FLIPR) [110] | >30,000 nM (s, H, h, FLIPR) [110] | 3340 nM (s, H, h, FLIPR) [110] 16 nM (s, H, h) [110] | 6920 nM (s, H, h, FLIPR) [110] | ||
| Pre1a | 1 | 57.1 nM (s, H, h) [111] | 189.6 nM (s, X, r) [111] | 8000 nM (s, X, r) [111] | 16.5% block at 1000 nM (s, X, r) [111] | 8.6% block at 1000 nM (s, X, r) [111] | 221.6 nM (s, H, h) [111] | 114 nM (s, X, r) [111] 15 nM (s, H, h) [111] | |||
| Pn3a | 2 | 37 nM (s, H, h) [61] | 124 nM (s, H, h) [61] | 210 nM (s, H, h) [61] ( | 144 nM (s, H, h) [61] | 800 nM (s, H, h) [61] | 129 nM (s, H, h) [61]) | 0.9 nM (s, H, h) [61] 1.5 nM (s, H, r) [61] 4.4 nM (s, H, m) [61] | 50,000 nM (s, H, h) [61] | 2427 nM (s, H, h) [61] | |
| Hl1a | 7 | No inhibition (s, HT, h) [123] | No inhibition (s, HT, h) [123] | No inhibition (s, HT, h) [123] | No inhibition (s, HT, h) [123] | No inhibition (s, HT, h) [123] | No inhibition (s, HT, h) [123] | No inhibition (s, HT, h) [123] | 2190 nM (s, ND7/23 cell, h) [123] | 3760 nM (s, rat DRG) [123] | |
| GpTx-1 | 1 | 6000 nM (s, H, h, FLIPR) [122] | 5000 nM (s, H, h, FLIPR) [122] | 20 nM (s, C, h) [76] 22,000 nM (s, H, h, FLIPR) [122] | 301 nM (s, H, h, Manual ephys) [76] 200 nM (HEK293, h, synthetic, PatchXpress) [76] 326,000 nM (s, H, h, FLIPR) [122] | 4200 nM (s, H, h, Manual ephys) [76] >10,000 nM (s, H, h, PatchXpress) [76] 140,000 nM (s, H, h, FLIPR) [122] | 17,000 nM (s, H, h, FLIPR) [122] | 4.4 nM (s, H, h, Manual ephys) [76] 10 nM (s, H, h, PatchXpress) [76] 580 nM (s, H, h, FLIPR) [122] 8 nM, open/inactivated state; 13 nM, closed/resting state (s, C, h,) [122] | 12,200 nM (s, C, h, Manual ephys) [76] 68,000 nM (s, H, h, FLIPR) [122] | ||
| F5A-M6F-T26L-K28R-GpTx-1 | 1 | 1900 nM (s, H, h, PatchXpress) [76] | >10,000 nM (s, H, h, PatchXpress) [76] | 1.6 nM (s, H, h, PatchXpress) [76] | |||||||
| Df1a | 2 | 14.3 nM, s-NH2; 30.7 nM, s-OH; (H, h) [92] | 1.9 nM, s-NH2; 3 nM, s-OH; (H, h) [92] | 3 nM, s-NH2; 10 nM, s-OH; (H, h) [92] | 24 nM, s-NH2; 53.6 nM, s-OH; (H, h) [92] | 45.3 nM, s-NH2; 125.6 nM, s-OH; (H, h) [92] | 7.6 nM, s-NH2; 23 nM, s-OH; (C, h) [92] | 1.9 nM, s-NH2; 60.5 nM, s-OH; (H, h) [92] | |||
| Phlo1a | 2 | 459 nM (n, X, h) [240] | |||||||||
| Phlo1b | 2 | 360 nM (n, X, h) [240] | |||||||||
| Phlo2a | 3 | 404 nM (n, X, h) [240] | 218 nM (n, X, h) [240] | 333 nM (n, X, h) [240] | |||||||
| GrTx-1 | 3 | 630 nM (n, H, h) [241] | 230 nM (n, H, h) [241] | 770 nM (n, H, h) [241] | 1290 nM (n, HT, h) [241] | ~22,000 nM (n, H, h) [241] | 630 nM (n, H, h) [241] | 370 nM (n, HT, h) [241] | |||
| GsAFII | 3 | 5700 nM (n, H, h) [241] | 12,000 nM (n, H, h) [241] | 24,000 nM (n, H, h) [241] | 4000 nM (n, HT, h) [241] | ~42,000 nM (n, H, h) [241] | 6600 nM (n, H, h) [241] | 1030 nM (n, HT, h) [241] |
| Toxin | NaSpTx Family | NaV1.1 | NaV1.2 | NaV1.3 | NaV1.4 | NaV1.5 | NaV1.6 | NaV1.7 | NaV1.8 | NaV1.9 | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|
| JzTx-I | 7 | 870 nM (n, HT, r) [100] | 845 nM (n, HT, r) [100] | 339 nM (n, HT, r) [100] | 335 nM (n, HT, h) [100] | 348 nM (n, HT, h) [100] | 130 nM (n, TTX-S currents, rat DRG); No inhibition in rat DRG TTX-R currents; 31 nM in rat cardiac myocytes) [101] | ||||
| JzTx-II | 7 | 1650 nM (n, HT, r) [98] | 125 nM (n, HT, h) [98] | ||||||||
| Hm1a | 2 | 38 nM (s, X, h) [20] | 236 nM (s, X, h) [20] | 220 nM (s, X, h) [20] | No inhibition up to 1000 nM (s, X, h) [20] | No inhibition up to 1000 nM (s, X, h) [20] | No inhibition up to 1000 nM (s, X, h) [20] | No inhibition up to 1000 nM (s, X, h) [20] | No inhibition up to 1000 nM (s, X, h) [20] | ||
| PnTx2-1 | 6 | 122 nM (n, X, r) [147] | No inhibition up to 1000 nM (n, X, r) [147] | No inhibition up to 1000 nM (n, X, r) [147] | No inhibition up to 1000 nM (n, X, r) [147] | 87 nM (n, X, h) [147] | No inhibition up to 1000 nM (n, X, m) [147] | 101.1 nM (n, X, r) [147] |
References
- Nicholson, G.M. Spider Peptides. In Handbook of Biologically Active Peptides, 2nd ed.; Kastin, A.J., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 461–472. [Google Scholar] [CrossRef]
- Catalog, W.S. World Spider Catalog. Available online: https://wsc.nmbe.ch/statistics/ (accessed on 25 June 2019).
- Caddington, J.; Levi, H. Systematic and evolution of spiders (Araenea). Annu. Rev. Ecol. Syst. 1991, 22, 565–592. [Google Scholar] [CrossRef]
- King, G.F.; Hardy, M.C. Spider-venom peptides: Structure, pharmacology, and potential for control of insect pests. Annu. Rev. Entomol. 2013, 58, 475–496. [Google Scholar] [CrossRef] [PubMed]
- Samiayyan, K. Spiders—The generalist super predators in agro-ecosystems. In Integrated Pest Management; Elsevier: San Diego, CA, USA, 2014; pp. 283–310. [Google Scholar] [CrossRef]
- Cooper, A.M.; Nelsen, D.R.; Hayes, W.K. The Strategic Use of Venom by Spiders. In Evolution of Venomous Animals and Their Toxins; Malhotra, A., Ed.; Springer: Dordrecht, The Netherlands, 2017; pp. 145–166. [Google Scholar] [CrossRef]
- Dongol, Y.; Dhananjaya, B.L.; Shrestha, R.K.; Aryal, G. Animal Venoms and Nephrotoxic Effects. In Clinical Toxinology in Australia, Europe, and Americas. Toxinology; Gopalakrishnakone, P., Vogel, C.-W., Seifert, S.A., Tambourgi, D.V., Eds.; Springer: Dordrecht, The Netherlands, 2018; pp. 539–556. [Google Scholar] [CrossRef]
- Zlotkin, E. The insect voltage-gated sodium channel as target of insecticides. Annu. Rev. Entomol. 1999, 44, 429–455. [Google Scholar] [CrossRef] [PubMed]
- Billen, B.; Vassilevski, A.; Nikolsky, A.; Debaveye, S.; Tytgat, J.; Grishin, E. Unique bell-shaped voltage-dependent modulation of Na+ channel gating by novel insect-selective toxins from the spider Agelena orientalis. J. Biol. Chem. 2010, 285, 18545–18554. [Google Scholar] [CrossRef] [PubMed]
- Escoubas, P.; Sollod, B.; King, G.F. Venom landscapes: Mining the complexity of spider venoms via a combined cDNA and mass spectrometric approach. Toxicon 2006, 47, 650–663. [Google Scholar] [CrossRef]
- Sannaningaiah, D.; Subbaiah, G.K.; Kempaiah, K. Pharmacology of spider venom toxins. Toxin Rev. 2014, 33, 206–220. [Google Scholar] [CrossRef]
- Pineda, S.S.; Undheim, E.A.; Rupasinghe, D.B.; Ikonomopoulou, M.P.; King, G.F. Spider venomics: Implications for drug discovery. Future Med. Chem. 2014, 6, 1699–1714. [Google Scholar] [CrossRef]
- Escoubas, P.; Diochot, S.; Corzo, G. Structure and pharmacology of spider venom neurotoxins. Biochimie 2000, 82, 893–907. [Google Scholar] [CrossRef]
- Cardoso, F.C.; Lewis, R.J. Structure–Function and therapeutic potential of spider venom-derived cysteine knot peptides targeting sodium channels. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Klint, J.K.; Senff, S.; Rupasinghe, D.B.; Er, S.Y.; Herzig, V.; Nicholson, G.M.; King, G.F. Spider-venom peptides that target voltage-gated sodium channels: Pharmacological tools and potential therapeutic leads. Toxicon 2012, 60, 478–491. [Google Scholar] [CrossRef] [Green Version]
- Stevens, M.; Peigneur, S.; Tytgat, J. Neurotoxins and their binding areas on voltage-gated sodium channels. Front. Pharmacol. 2011, 2, 71. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ma, H.; Zhang, F.; Zhang, C.; Zou, X.; Cao, Z. Selective voltage-gated sodium channel peptide toxins from animal venom: Pharmacological probes and analgesic drug development. ACS Chem. Neurosci. 2018, 9, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Kalia, J.; Milescu, M.; Salvatierra, J.; Wagner, J.; Klint, J.K.; King, G.F.; Olivera, B.M.; Bosmans, F. From foe to friend: Using animal toxins to investigate ion channel function. J. Mol. Biol. 2015, 427, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Salvatierra, J.; Castro, J.; Erickson, A.; Li, Q.; Braz, J.; Gilchrist, J.; Grundy, L.; Rychkov, G.Y.; Deiteren, A.; Rais, R. NaV1.1 inhibition can reduce visceral hypersensitivity. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Osteen, J.D.; Herzig, V.; Gilchrist, J.; Emrick, J.J.; Zhang, C.; Wang, X.; Castro, J.; Garcia-Caraballo, S.; Grundy, L.; Rychkov, G.Y.; et al. Selective spider toxins reveal a role for the NaV1.1 channel in mechanical pain. Nature 2016, 534, 494–499. [Google Scholar] [CrossRef]
- Cardoso, F.C.; Lewis, R.J. Sodium channels and pain: From toxins to therapies. Br. J. Pharmacol. 2018, 175, 2138–2157. [Google Scholar] [CrossRef]
- Lukowski, A.L.; Narayan, A.R.H. Natural voltage-gated sodium channel ligands: Biosynthesis and Biology. ChemBioChem 2019, 20, 1231–1241. [Google Scholar] [CrossRef]
- Black, J.A.; Waxman, S.G. Noncanonical roles of voltage-gated sodium channels. Neuron 2013, 80, 280–291. [Google Scholar] [CrossRef]
- Yamamoto, R.; Yanagita, T.; Kobayashi, H.; Yokoo, H.; Wada, A. Up-regulation of sodium channel subunit mRNAs and their cell surface expression by antiepileptic valproic acid: Activation of calcium channel and catecholamine secretion in adrenal chromaffin cells. J. Neurochem. 1997, 68, 1655–1662. [Google Scholar] [CrossRef]
- Andrikopoulos, P.; Fraser, S.P.; Patterson, L.; Ahmad, Z.; Burcu, H.; Ottaviani, D.; Diss, J.K.; Box, C.; Eccles, S.A.; Djamgoz, M.B. Angiogenic functions of voltage-gated Na+ Channels in human endothelial cells: Modulation of vascular endothelial growth factor (VEGF) signaling. J. Biol. Chem. 2011, 286, 16846–16860. [Google Scholar] [CrossRef]
- Carrithers, M.D.; Dib-Hajj, S.; Carrithers, L.M.; Tokmoulina, G.; Pypaert, M.; Jonas, E.A.; Waxman, S.G. Expression of the voltage-gated sodium channel NaV1.5 in the macrophage late endosome regulates endosomal acidification. J. Immunol. 2007, 178, 7822–7832. [Google Scholar] [CrossRef] [PubMed]
- Carrithers, M.D.; Chatterjee, G.; Carrithers, L.M.; Offoha, R.; Iheagwara, U.; Rahner, C.; Graham, M.; Waxman, S.G. Regulation of podosome formation in macrophages by a splice variant of the sodium channel SCN8A. J. Biol. Chem. 2009, 284, 8114–8126. [Google Scholar] [CrossRef] [PubMed]
- Pucca, M.B.; Peigneur, S.; Cologna, C.T.; Cerni, F.A.; Zoccal, K.F.; Bordon, K.D.C.F.; Faccioli, L.H.; Tytgat, J.; Arantes, E.C. Electrophysiological characterization of the first Tityus serrulatus α-like toxin, Ts5: Evidence of a pro-inflammatory toxin on macrophages. Biochimie 2015, 115, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.P.; Ozerlat-Gunduz, I.; Brackenbury, W.J.; Fitzgerald, E.M.; Campbell, T.M.; Coombes, R.C.; Djamgoz, M.B.A. Regulation of voltage-gated sodium channel expression in cancer: Hormones, growth factors and auto-regulation. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369. [Google Scholar] [CrossRef] [PubMed]
- Waxman, S.G. Axonal conduction and injury in multiple sclerosis: The role of sodium channels. Nat. Rev. Neurosci. 2006, 7, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.I.; Isom, L.; Petrou, S. 17. Role of Sodium Channels in Epilepsy. Cold Spring Harb. Perspect. Med. 2016, 6. [Google Scholar] [CrossRef]
- Raouf, R.; Quick, K.; Wood, J.N. Pain as a channelopathy. J. Clin. Investig. 2010, 120, 3745–3752. [Google Scholar] [CrossRef]
- Dib-Hajj, S.D.; Waxman, S.G. Sodium channels in human pain disorders: Genetics and pharmacogenomics. Annu. Rev. Neurosci. 2019, 42. [Google Scholar] [CrossRef]
- Savio Galimberti, E.; Gollob, M.; Darbar, D. Voltage-gated sodium channels: Biophysics, pharmacology, and related channelopathies. Front. Pharmacol. 2012, 3. [Google Scholar] [CrossRef]
- Probst, V.; Kyndt, F.; Potet, F.; Trochu, J.N.; Mialet, G.; Demolombe, S.; Schott, J.J.; Baro, I.; Escande, D.; Le Marec, H. Haploinsufficiency in combination with aging causes SCN5A-linked hereditary Lenegre disease. J. Am. Coll. Cardiol. 2003, 41, 643–652. [Google Scholar] [CrossRef]
- Cox, J.J.; Reimann, F.; Nicholas, A.K.; Thornton, G.; Roberts, E.; Springell, K.; Karbani, G.; Jafri, H.; Mannan, J.; Raashid, Y. An SCN9A channelopathy causes congenital inability to experience pain. Nature 2006, 444, 894. [Google Scholar] [CrossRef]
- Claes, L.; Del-Favero, J.; Ceulemans, B.; Lagae, L.; van Broeckhoven, C.; de Jonghe, P. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am. J. Hum. Genet. 2001, 68, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Mantegazza, M.; Gambardella, A.; Rusconi, R.; Schiavon, E.; Annesi, F.; Cassulini, R.R.; Labate, A.; Carrideo, S.; Chifari, R.; Canevini, M.P.; et al. Identification of an NaV1.1 sodium channel (SCN1A) loss-of-function mutation associated with familial simple febrile seizures. Proc. Natl. Acad. Sci. USA 2005, 102, 18177–18182. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhou, Q.; Pan, X.; Li, Z.; Wu, J.; Yan, N. Structure of a eukaryotic voltage-gated sodium channel at near-atomic resolution. Science 2017, 355, eaal4326. [Google Scholar] [CrossRef] [PubMed]
- Patino, G.A.; Brackenbury, W.J.; Bao, Y.; Lopez-Santiago, L.F.; O’Malley, H.A.; Chen, C.; Calhoun, J.D.; Lafrenière, R.G.; Cossette, P.; Rouleau, G.A. Voltage-gated Na+ channel β1B: A secreted cell adhesion molecule involved in human epilepsy. J. Neurosci. 2011, 31, 14577–14591. [Google Scholar] [CrossRef]
- O’Malley, H.A.; Isom, L.L. Sodium channel β subunits: Emerging targets in channelopathies. Annu. Rev. Physiol. 2015, 77, 481–504. [Google Scholar] [CrossRef]
- Patino, G.A.; Isom, L.L. Electrophysiology and beyond: Multiple roles of Na+ channel β subunits in development and disease. Neurosci. Lett. 2010, 486, 53–59. [Google Scholar] [CrossRef]
- Sato, C.; Ueno, Y.; Asai, K.; Takahashi, K.; Sato, M.; Engel, A.; Fujiyoshi, Y. The voltage-sensitive sodium channel is a bell-shaped molecule with several cavities. Nature 2001, 409, 1047. [Google Scholar] [CrossRef]
- De Lera Ruiz, M.; Kraus, R.L. Voltage-Gated Sodium Channels: Structure, function, pharmacology, and clinical indications. J. Med. Chem. 2015, 58, 7093–7118. [Google Scholar] [CrossRef]
- Yu, F.H.; Catterall, W.A. Overview of the voltage-gated sodium channel family. Genome Biol. 2003, 4, 207. [Google Scholar] [CrossRef]
- Bezanilla, F. Gating currents. J. Gen. Physiol. 2018, 150, 911–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catterall, W.A. Molecular properties of voltage-sensitive sodium channels. Annu. Rev. Biochem. 1986, 55, 953–985. [Google Scholar] [CrossRef] [PubMed]
- Guy, H.R.; Seetharamulu, P. Molecular model of the action potential sodium channel. Proc. Natl. Acad. Sci. USA 1986, 83, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Forty Years of sodium channels: Structure, function, pharmacology, and epilepsy. Neurochem. Res. 2017, 42, 2495–2504. [Google Scholar] [CrossRef]
- Catterall, W.A. Ion channel voltage sensors: Structure, function, and pathophysiology. Neuron 2010, 67, 915–928. [Google Scholar] [CrossRef]
- Catterall, W.A. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron 2000, 26, 13–25. [Google Scholar] [CrossRef]
- Clairfeuille, T.; Xu, H.; Koth, C.M.; Payandeh, J. Voltage-gated sodium channels viewed through a structural biology lens. Curr. Opin. Struct. Biol. 2017, 45, 74–84. [Google Scholar] [CrossRef]
- West, J.W.; Patton, D.E.; Scheuer, T.; Wang, Y.; Goldin, A.L.; Catterall, W.A. A cluster of hydrophobic amino acid residues required for fast Na+-channel inactivation. Proc. Natl. Acad. Sci. USA 1992, 89, 10910–10914. [Google Scholar] [CrossRef]
- Goldin, A.L. Mechanisms of sodium channel inactivation. Curr. Opin. Neurobiol. 2003, 13, 284–290. [Google Scholar] [CrossRef]
- Shen, H.; Liu, D.; Wu, K.; Lei, J.; Yan, N. Structures of human NaV1.7 channel in complex with auxiliary subunits and animal toxins. Science 2019, 363, 1303–1308. [Google Scholar] [CrossRef]
- Deuis, J.R.; Mueller, A.; Israel, M.R.; Vetter, I. The pharmacology of voltage-gated sodium channel activators. Neuropharmacology 2017, 127, 87–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, A.H.; Sharma, G.; Undheim, E.A.B.; Jia, X.; Mobli, M. A complicated complex: Ion channels, voltage sensing, cell membranes and peptide inhibitors. Neurosci. Lett. 2018. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, T.; Rohou, A.; Arthur, C.P.; Tzakoniati, F.; Wong, E.; Estevez, A.; Kugel, C.; Franke, Y.; Chen, J. Structural basis of NaV1.7 inhibition by a gating-modifier spider toxin. Cell 2019, 176, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Hieber, C.; Bischofberger, J. Fast sodium channel gating supports localized and efficient axonal action potential initiation. J. Neurosci. 2010, 30, 10233–10242. [Google Scholar] [CrossRef] [PubMed]
- Capes, D.L.; Goldschen-Ohm, M.P.; Arcisio-Miranda, M.; Bezanilla, F.; Chanda, B. Domain IV voltage-sensor movement is both sufficient and rate limiting for fast inactivation in sodium channels. J. Gen. Physiol. 2013, 142, 101–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deuis, J.R.; Dekan, Z.; Wingerd, J.S.; Smith, J.J.; Munasinghe, N.R.; Bhola, R.F.; Imlach, W.L.; Herzig, V.; Armstrong, D.A.; Rosengren, K.J. Pharmacological characterisation of the highly NaV1.7 selective spider venom peptide Pn3a. Sci. Rep. 2017, 7, 40883. [Google Scholar] [CrossRef]
- Kozlov, S. Animal toxins for channelopathy treatment. Neuropharmacology 2018, 132, 83–97. [Google Scholar] [CrossRef]
- King, G.F. Tying pest insects in knots: The deployment of spider-venom-derived knottins as bioinsecticides. Pest Manage. Sci. 2019, 75, 2437–2445. [Google Scholar] [CrossRef]
- Moore, S.J.; Leung, C.L.; Cochran, J.R. Knottins: Disulfide-bonded therapeutic and diagnostic peptides. Drug Discov. Today Technol. 2012, 9, e3–e11. [Google Scholar] [CrossRef]
- Le Nguyen, D.; Heitz, A.; Chiche, L.; Castro, B.; Boigegrain, R.A.; Favel, A.; Coletti-Previero, M.A. Molecular recognition between serine proteases and new bioactive microproteins with a knotted structure. Biochimie 1990, 72, 431–435. [Google Scholar] [CrossRef]
- McDonald, N.Q.; Lapatto, R.; Murray-Rust, J.; Gunning, J.; Wlodawer, A.; Blundell, T.L. New protein fold revealed by a 2.3-Å resolution crystal structure of nerve growth factor. Nature 1991, 354, 411–414. [Google Scholar] [CrossRef] [PubMed]
- McDonald, N.Q.; Hendrickson, W.A. A structural superfamily of growth factors containing a cystine knot motif. Cell 1993, 73, 421–424. [Google Scholar] [CrossRef]
- Pallaghy, P.K.; Nielsen, K.J.; Craik, D.J.; Norton, R.S. A common structural motif incorporating a cystine knot and a triple-stranded β-sheet in toxic and inhibitory polypeptides. Protein Sci. 1994, 3, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Postic, G.; Gracy, J.; Périn, C.; Chiche, L.; Gelly, J.-C. KNOTTIN: The database of inhibitor cystine knot scaffold after 10 years, toward a systematic structure modeling. Nucleic Acids Res. 2017, 46, D454–D458. [Google Scholar] [CrossRef] [PubMed]
- Craik, D.J.; Daly, N.L.; Waine, C. The cystine knot motif in toxins and implications for drug design. Toxicon 2001, 39, 43–60. [Google Scholar] [CrossRef]
- Saez, N.J.; Senff, S.; Jensen, J.E.; Er, S.Y.; Herzig, V.; Rash, L.D.; King, G.F. Spider-venom peptides as therapeutics. Toxins 2010, 2, 2851–2871. [Google Scholar] [CrossRef] [PubMed]
- Agwa, A.J.; Huang, Y.H.; Craik, D.J.; Henriques, S.T.; Schroeder, C.I. Lengths of the C-terminus and interconnecting loops impact stability of spider-derived gating modifier toxins. Toxins 2017, 9, 248. [Google Scholar] [CrossRef]
- Bosmans, F.; Rash, L.; Zhu, S.; Diochot, S.; Lazdunski, M.; Escoubas, P.; Tytgat, J. Four novel tarantula toxins as selective modulators of voltage-gated sodium channel subtypes. Mol. Pharmacol. 2006, 69, 419–429. [Google Scholar] [CrossRef]
- Wang, J.M.; Roh, S.H.; Kim, S.; Lee, C.W.; Kim, J.I.; Swartz, K.J. Molecular surface of tarantula toxins interacting with voltage sensors in Kv channels. J. Gen. Physiol. 2004, 123, 455–467. [Google Scholar] [CrossRef]
- Lawrence, N.; Wu, B.; Ligutti, J.; Cheneval, O.; Agwa, A.J.; Benfield, A.H.; Biswas, K.; Craik, D.J.; Miranda, L.P.; Henriques, S.T. Peptide-membrane interactions affect the inhibitory potency and selectivity of spider toxins ProTx-II and GpTx-1. ACS Chem. Biol. 2018, 14, 118–130. [Google Scholar] [CrossRef]
- Murray, J.K.; Ligutti, J.; Liu, D.; Zou, A.; Poppe, L.; Li, H.; Andrews, K.L.; Moyer, B.D.; McDonough, S.I.; Favreau, P. Engineering potent and selective analogues of GpTx-1, a tarantula venom peptide antagonist of the NaV1.7 sodium channel. J. Med. Chem. 2015, 58, 2299–2314. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.J.; Cummins, T.R.; Alphy, S.; Blumenthal, K.M. Molecular interactions of the gating modifier toxin, ProTx-II, with NaV1.5: Implied existence of a novel toxin binding site coupled to activation. J. Biol. Chem. 2007. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, D.; Wu, Z.; Li, J.; Nie, D.; Xiang, Y.; Liu, Z. A positively charged surface patch is important for hainantoxin-IV binding to voltage-gated sodium channels. J. Pept. Sci. 2012, 18, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, Y.; Gong, M.; Lu, S.; Ma, Y.; Zeng, X.; Liang, S. Molecular surface of JZTX-V (β-Theraphotoxin-Cj2a) interacting with voltage-gated sodium channel subtype NaV1.4. Toxins 2014, 6, 2177–2193. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.C.; Dekan, Z.; Rosengren, K.J.; Erickson, A.; Vetter, I.; Deuis, J.; Herzig, V.; Alewood, P.; King, G.F.; Lewis, R.J. Identification and characterization of ProTx-III [μ-TRTX-Tp1a], a new voltage-gated sodium channel inhibitor from venom of the tarantula Thrixopelma pruriens. Mol. Pharmacol. 2015, 88, 291–303. [Google Scholar] [CrossRef]
- Klint, J.K.; Chin, Y.K.; Mobli, M. Rational engineering defines a molecular switch that is essential for activity of spider-venom peptides against the analgesics target NaV1.7. Mol. Pharmacol. 2015, 88, 1002–1010. [Google Scholar] [CrossRef]
- Henriques, S.T.; Deplazes, E.; Lawrence, N.; Cheneval, O.; Chaousis, S.; Inserra, M.; Thongyoo, P.; King, G.F.; Mark, A.E.; Vetter, I.; et al. Interaction of tarantula venom peptide ProTx-II with lipid membranes is a prerequisite for its inhibition of human voltage-gated sodium channel NaV1.7. J. Biol. Chem. 2016, 291, 17049–17065. [Google Scholar] [CrossRef]
- Minassian, N.A.; Gibbs, A.; Shih, A.Y.; Liu, Y.; Neff, R.A.; Sutton, S.W.; Mirzadegan, T.; Connor, J.; Fellows, R.; Husovsky, M. Analysis of the structural and molecular basis of voltage-sensitive sodium channel inhibition by the spider toxin huwentoxin-IV (μ-TRTX-Hh2a). J. Biol. Chem. 2013, 288, 22707–22720. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2018, 47, D506–D515. [Google Scholar] [CrossRef]
- Li, D.; Xiao, Y.; Xu, X.; Xiong, X.; Lu, S.; Liu, Z.; Zhu, Q.; Wang, M.; Gu, X.; Liang, S. Structure-activity relationships of hainantoxin-IV and structure determination of active and inactive sodium channel blockers. J. Biol. Chem. 2004, 279, 37734–37740. [Google Scholar] [CrossRef]
- Agwa, A.J.; Peigneur, S.; Chow, C.Y.; Lawrence, N.; Craik, D.J.; Tytgat, J.; King, G.F.; Henriques, S.T.; Schroeder, C.I. Gating modifier toxins isolated from spider venom: Modulation of voltage-gated sodium channels and the role of lipid membranes. J. Biol. Chem. 2018, 293, 9041–9052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontana, M.; Vital-Brazil, O. Mode of action of Phoneutria nigriventer spider venom at the isolated phrenic nerve-diaphragm of the rat. Braz. J. Med. Biol. Res. 1985, 18, 557–565. [Google Scholar] [PubMed]
- Rezende, L., Jr.; Cordeiro, M.N.; Oliveira, E.B.; Diniz, C.R. Isolation of neurotoxic peptides from the venom of the ‘armed’spider Phoneutria nigriventer. Toxicon 1991, 29, 1225–1233. [Google Scholar] [CrossRef]
- Arújo, D.A.; Cordeiro, M.N.; Diniz, C.R.; Beirão, P.S. Effects of a toxic fraction, PhTx 2, from the spider Phoneutria nigriventer on the sodium current. Naunyn Schmiedeberg’s Arch. Pharmacol. 1993, 347, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.E.; Herold, E.E.; Venema, V.J. Two classes of channel-specific toxins from funnel web spider venom. J. Comp. Physiol. A Neuroethol. Sen. Neural. Behav. Physiol. 1989, 164, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhou, X.; Huang, Y.; Zhang, Y.; Hu, Z.; Wang, M.; Chen, P.; Liu, Z.; Liang, S. The tarantula toxin jingzhaotoxin-XI (κ-theraphotoxin-Cj1a) regulates the activation and inactivation of the voltage-gated sodium channel NaV1.5. Toxicon 2014, 92, 6–13. [Google Scholar] [CrossRef]
- Cardoso, F.C.; Dekan, Z.; Smith, J.J.; Deuis, J.R.; Vetter, I.; Herzig, V.; Alewood, P.F.; King, G.F.; Lewis, R.J. Modulatory features of the novel spider toxin μ-TRTX-Df1a isolated from the venom of the spider Davus fasciatus. Br. J. Pharmacol. 2017, 174, 2528–2544. [Google Scholar] [CrossRef]
- Matavel, A.; Cruz, J.S.; Penaforte, C.L.; Araújo, D.A.; Kalapothakis, E.; Prado, V.F.; Diniz, C.R.; Cordeiro, M.N.; Beirão, P.S. Electrophysiological characterization and molecular identification of the Phoneutria nigriventer peptide toxin PnTx2-6 1. FEBS Lett. 2002, 523, 219–223. [Google Scholar] [CrossRef]
- Nicholson, G.M.; Little, M.J.; Tyler, M.; Narahashi, T. Selective alteration of sodium channel gating by Australian funnel-web spider toxins. Toxicon 1996, 34, 1443–1453. [Google Scholar] [CrossRef]
- Little, M.J.; Zappia, C.; Gilles, N.; Connor, M.; Tyler, M.I.; Martin-Eauclaire, M.-F.; Gordon, D.; Nicholson, G.M. δ-Atracotoxins from Australian funnel-web spiders compete with scorpion α-toxin binding but differentially modulate alkaloid toxin activation of voltage-gated sodium channels. J. Biol. Chem. 1998, 273, 27076–27083. [Google Scholar] [CrossRef]
- Nicholson, G.M.; Walsh, R.; Little, M.J.; Tyler, M.I. Characterisation of the effects of robustoxin, the lethal neurotoxin from the Sydney funnel-web spider Atrax robustus, on sodium channel activation and inactivation. Pflügers Archiv 1998, 436, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Szeto, T.H.; Birinyi-Strachan, L.C.; Smith, R.; Connor, M.; Christie, M.J.; King, G.F.; Nicholson, G.M. Isolation and pharmacological characterisation of δ-atracotoxin-Hv1b, a vertebrate-selective sodium channel toxin. FEBS Lett. 2000, 470, 293–299. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, X.; Tang, C.; Zhang, Y.; Tao, H.; Chen, P.; Liu, Z. Molecular basis of the inhibition of the fast inactivation of voltage-gated sodium channel NaV1.5 by tarantula toxin Jingzhaotoxin-II. Peptides 2015, 68, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, G.M.; Willow, M.; Howden, M.E.; Narahashi, T. Modification of sodium channel gating and kinetics by versutoxin from the Australian funnel-web spider Hadronyche versuta. Pflügers Archiv 1994, 428, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Chen, X.; Lu, M.; Wu, Y.; Deng, M.; Zeng, X.; Liu, Z.; Liang, S. Molecular determinant for the tarantula toxin Jingzhaotoxin-I slowing the fast inactivation of voltage-gated sodium channels. Toxicon 2016, 111, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Tang, J.; Hu, W.; Xie, J.; Maertens, C.; Tytgat, J.; Liang, S. Jingzhaotoxin-I, a novel spider neurotoxin preferentially inhibiting cardiac sodium channel inactivation. J. Biol. Chem. 2005, 280, 12069–12076. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, J.; Deng, M.; Dai, C.; Liang, S. Characterization of the excitatory mechanism induced by Jingzhaotoxin-I inhibiting sodium channel inactivation. Toxicon 2007, 50, 507–517. [Google Scholar] [CrossRef]
- Middleton, R.E.; Warren, V.A.; Kraus, R.L.; Hwang, J.C.; Liu, C.J.; Dai, G.; Brochu, R.M.; Kohler, M.G.; Gao, Y.-D.; Garsky, V.M. Two tarantula peptides inhibit activation of multiple sodium channels. Biochemistry 2002, 41, 14734–14747. [Google Scholar] [CrossRef]
- Xiao, Y.; Blumenthal, K.M.; Jackson, J.O.; Liang, S.; Cummins, T.R. The tarantula toxins ProTx-II and HwTX-IV differentially interact with human NaV1.7 voltage-sensors to inhibit channel activation and inactivation. Mol. Pharmacol. 2010, 78, 1124–1134. [Google Scholar] [CrossRef]
- Xiao, Y.; Tang, J.; Yang, Y.; Wang, M.; Hu, W.; Xie, J.; Zeng, X.; Liang, S. Jingzhaotoxin-III, a novel spider toxin inhibiting activation of voltage-gated sodium channel in rat cardiac myocytes. J. Biol. Chem. 2004, 279, 26220–26226. [Google Scholar] [CrossRef]
- Rong, M.; Chen, J.; Tao, H.; Wu, Y.; Jiang, P.; Lu, M.; Su, H.; Chi, Y.; Cai, T.; Zhao, L. Molecular basis of the tarantula toxin jingzhaotoxin-III (β-TRTX-Cj1α) interacting with voltage sensors in sodium channel subtype NaV1.5. FASEB J. 2011, 25, 3177–3185. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Deng, M.; Lin, Y.; Yuan, C.; Pi, J.; Liang, S. Isolation and characterization of Jingzhaotoxin-V, a novel neurotoxin from the venom of the spider Chilobrachys jingzhao. Toxicon 2007, 49, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Kuang, F.; Sun, Z.; Tao, H.; Cai, T.; Zhong, L.; Chen, Z.; Xiao, Y.; Liang, S. Jingzhaotoxin-IX, a novel gating modifier of both sodium and potassium channels from Chinese tarantula Chilobrachys jingzhao. Neuropharmacology 2009, 57, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Nikolsky, A.; Billen, B.; Vassilevski, A.; Filkin, S.Y.; Tytgat, J.; Grishin, E. Voltage-gated sodium channels are targets for toxins from the venom of the spider Heriaeus melloteei. Biochem. Moscow Suppl. Ser. A 2009, 3, 245–253. [Google Scholar] [CrossRef]
- Sousa, S.R.; Wingerd, J.S.; Brust, A.; Bladen, C.; Ragnarsson, L.; Herzig, V.; Deuis, J.R.; Dutertre, S.; Vetter, I.; Zamponi, G.W. Discovery and mode of action of a novel analgesic β-toxin from the African spider Ceratogyrus darlingi. PLoS ONE 2017, 12, e0182848. [Google Scholar] [CrossRef] [PubMed]
- Wingerd, J.S.; Mozar, C.A.; Ussing, C.A.; Murali, S.S.; Chin, Y.K.; Cristofori-Armstrong, B.; Durek, T.; Gilchrist, J.; Vaughan, C.W.; Bosmans, F.; et al. The tarantula toxin β/δ-TRTX-Pre1a highlights the importance of the S1-S2 voltage-sensor region for sodium channel subtype selectivity. Sci. Rep. 2017, 7, 974. [Google Scholar] [CrossRef]
- Wei, P.; Xu, C.; Wu, Q.; Huang, L.; Liang, S.; Yuan, C. Jingzhaotoxin-35, a novel gating-modifier toxin targeting both NaV1.5 and KV2.1 channels. Toxicon 2014, 92, 90–96. [Google Scholar] [CrossRef]
- Peng, K.; Shu, Q.; Liu, Z.; Liang, S. Function and solution structure of huwentoxin-IV, a potent neuronal tetrodotoxin (TTX)-sensitive sodium channel antagonist from Chinese bird spider Selenocosmia huwena. J. Biol. Chem. 2002, 277, 47564–47571. [Google Scholar] [CrossRef]
- Xiao, Y.; Bingham, J.-P.; Zhu, W.; Moczydlowski, E.; Liang, S.; Cummins, T.R. Tarantula huwentoxin-IV inhibits neuronal sodium channels by binding to receptor site 4 and trapping the domain II voltage sensor in the closed configuration. J. Biol. Chem. 2008, 283, 27300–27313. [Google Scholar] [CrossRef]
- Deng, M.; Luo, X.; Jiang, L.; Chen, H.; Wang, J.; He, H.; Liang, S. Synthesis and biological characterization of synthetic analogs of Huwentoxin-IV (µ-theraphotoxin-Hh2a), a neuronal tetrodotoxin-sensitive sodium channel inhibitor. Toxicon 2013, 71, 57–65. [Google Scholar] [CrossRef]
- Xiao, Y.; Liang, S. Inhibition of neuronal tetrodotoxin-sensitive Na+ channels by two spider toxins: Hainantoxin-III and hainantoxin-IV. Eur. J. Pharmacol. 2003, 477, 1–7. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, T.; Zhu, Q.; Deng, M.; Li, J.; Zhou, X.; Zhang, F.; Li, D.; Li, J.; Liu, Y. Structure and function of Hainantoxin-III-a selective antagonist of neuronal tetrodotoxin-sensitive voltage-gated sodium channels isolated from the Chinese bird spider Ornithoctonus hainana. J. Biol. Chem. 2013, 288, 20392–20403. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Y.; Rong, M.; Zhao, L.; Jiang, L.; Zhang, D.; Wang, M.; Xiao, Y.; Liang, S. Expression and characterization of jingzhaotoxin-34, a novel neurotoxin from the venom of the tarantula Chilobrachys jingzhao. Peptides 2009, 30, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, P.; Chen, B.; Huang, J.; Lai, R.; Liu, J.; Rong, M. Selective closed-state NaV1.7 blocker JZTX-34 exhibits analgesic effects against pain. Toxins 2018, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Billen, B.; Vassilevski, A.; Nikolsky, A.; Tytgat, J.; Grishin, E. Two novel sodium channel inhibitors from Heriaeus melloteei spider venom differentially interacting with mammalian channel’s isoforms. Toxicon 2008, 52, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Klint, J.K.; Smith, J.J.; Vetter, I.; Rupasinghe, D.B.; Er, S.Y.; Senff, S.; Herzig, V.; Mobli, M.; Lewis, R.J.; Bosmans, F. Seven novel modulators of the analgesic target NaV1.7 uncovered using a high-throughput venom-based discovery approach. Br. J. Pharmacol. 2015, 172, 2445–2458. [Google Scholar] [CrossRef] [PubMed]
- Deuis, J.R.; Wingerd, J.S.; Winter, Z.; Durek, T.; Dekan, Z.; Sousa, S.R.; Zimmermann, K.; Hoffmann, T.; Weidner, C.; Nassar, M.A. Analgesic effects of GpTx-1, PF-04856264 and CNV1014802 in a mouse model of NaV1.7-mediated pain. Toxins 2016, 8, 78. [Google Scholar] [CrossRef]
- Meng, P.; Huang, H.; Wang, G.; Yang, S.; Lu, Q.; Liu, J.; Lai, R.; Rong, M. A novel toxin from Haplopelma lividum selectively inhibits the NaV1.8 channel and possesses potent analgesic efficacy. Toxins 2017, 9, 7. [Google Scholar] [CrossRef]
- Martin-Moutot, N.; Mansuelle, P.; Alcaraz, G.; Gouvea dos Santos, R.; Cordeiro, M.N.; De Lima, M.E.; Seagar, M.; van Renterghem, C. Phoneutria nigriventer toxin 1: A novel, state-dependent inhibitor of neuronal sodium channels which interacts with micro conotoxin binding sites. Mol. Pharmacol. 2006, 69, 1931–1937. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, D.; Liu, S.; Hu, H.; Liang, S.; Tang, C.; Liu, Z. Purification and characterization of JZTx-14, a potent antagonist of mammalian and prokaryotic voltage-gated sodium channels. Toxins 2018, 10, 408. [Google Scholar] [CrossRef]
- Paiva, A.L.; Matavel, A.; Peigneur, S.; Cordeiro, M.N.; Tytgat, J.; Diniz, M.R.; de Lima, M.E. Differential effects of the recombinant toxin PnTx4(5-5) from the spider Phoneutria nigriventer on mammalian and insect sodium channels. Biochimie 2016, 121, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, J.; Zhang, Y.; Xun, X.; Tang, D.; Peng, D.; Yi, J.; Liu, Z.; Shi, X. Synthesis and analgesic effects of μ-TRTX-Hhn1b on models of inflammatory and neuropathic pain. Toxins 2014, 6, 2363–2378. [Google Scholar] [CrossRef] [PubMed]
- King, G.F.; Escoubas, P.; Nicholson, G.M. Peptide toxins that selectively target insect NaV and CaV channels. Channels 2008, 2, 100–116. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Luo, J.; Meng, E.; Ding, J.; Liang, S.; Wang, S.; Liu, Z. Mapping the interaction site for the tarantula toxin hainantoxin-IV (β-TRTX-Hn2a) in the voltage sensor module of domain II of voltage-gated sodium channels. Peptides 2015, 68, 148–156. [Google Scholar] [CrossRef]
- Cestele, S.; Qu, Y.; Rogers, J.C.; Rochat, H.; Scheuer, T.; Catterall, W.A. Voltage sensor-trapping: Enhanced activation of sodium channels by β-scorpion toxin bound to the S3-S4 loop in domain II. Neuron 1998, 21, 919–931. [Google Scholar] [CrossRef]
- Swartz, K.J.; MacKinnon, R. Mapping the receptor site for hanatoxin, a gating modifier of voltage-dependent K+ channels. Neuron 1997, 18, 675–682. [Google Scholar] [CrossRef]
- Schmalhofer, W.; Calhoun, J.; Burrows, R.; Bailey, T.; Kohler, M.G.; Weinglass, A.B.; Kaczorowski, G.J.; Garcia, M.L.; Koltzenburg, M.; Priest, B.T. ProTx-II, a selective inhibitor of NaV1.7 sodium channels, blocks action potential propagation in nociceptors. Mol. Pharmacol. 2008, 74, 1476–1484. [Google Scholar] [CrossRef]
- Corzo, G.; Gilles, N.; Satake, H.; Villegas, E.; Dai, L.; Nakajima, T.; Haupt, J. Distinct primary structures of the major peptide toxins from the venom of the spider Macrothele gigas that bind to sites 3 and 4 in the sodium channel. FEBS Lett. 2003, 547, 43–50. [Google Scholar] [CrossRef]
- Winterfield, J.R.; Swartz, K.J. A hot spot for the interaction of gating modifier toxins with voltage-dependent ion channels. J. Gen. Physiol. 2000, 116, 637–644. [Google Scholar] [CrossRef]
- Gonçalves, T.C.; Benoit, E.; Partiseti, M.; Servent, D. The NaV1.7 Channel subtype as an antinociceptive target for spider toxins in adult dorsal root ganglia neurons. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Shcherbatko, A.; Ono, F.; Mandel, G.; Brehm, P. Voltage-dependent sodium channel function is regulated through membrane mechanics. Biophys. J. 1999, 77, 1945–1959. [Google Scholar] [CrossRef]
- Agwa, A.J.; Lawrence, N.; Deplazes, E.; Cheneval, O.; Chen, R.M.; Craik, D.J.; Schroeder, C.I.; Henriques, S.T. Spider peptide toxin HwTx-IV engineered to bind to lipid membranes has an increased inhibitory potency at human voltage-gated sodium channel hNaV1.7. Biochim. Biophys. Acta 2017, 1859, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.; Kuyucak, S.; Schroeder, C.I.; Kaas, Q. Modelling the interactions between animal venom peptides and membrane proteins. Neuropharmacology 2017, 127, 20–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agwa, A.J.; Henriques, S.T.; Schroeder, C.I. Gating modifier toxin interactions with ion channels and lipid bilayers: Is the trimolecular complex real? Neuropharmacology 2017, 127, 32–45. [Google Scholar] [CrossRef] [Green Version]
- Deplazes, E.; Henriques, S.T.; Smith, J.J.; King, G.F.; Craik, D.J.; Mark, A.E.; Schroeder, C.I. Membrane-binding properties of gating modifier and pore-blocking toxins: Membrane interaction is not a prerequisite for modification of channel gating. Biochim. Biophys. Acta 2016, 1858, 872–882. [Google Scholar] [CrossRef]
- Flinspach, M.; Xu, Q.; Piekarz, A.; Fellows, R.; Hagan, R.; Gibbs, A.; Liu, Y.; Neff, R.; Freedman, J.; Eckert, W. Insensitivity to pain induced by a potent selective closed-state NaV1.7 inhibitor. Sci. Rep. 2017, 7, 39662. [Google Scholar] [CrossRef]
- Revell, J.D.; Lund, P.-E.; Linley, J.E.; Metcalfe, J.; Burmeister, N.; Sridharan, S.; Jones, C.; Jermutus, L.; Bednarek, M.A. Potency optimization of Huwentoxin-IV on hNaV1.7: A neurotoxin TTX-S sodium-channel antagonist from the venom of the Chinese bird-eating spider Selenocosmia huwena. Peptides 2013, 44, 40–46. [Google Scholar] [CrossRef]
- Rahnama, S.; Deuis, J.R.; Cardoso, F.C.; Ramanujam, V.; Lewis, R.J.; Rash, L.D.; King, G.F.; Vetter, I.; Mobli, M. The structure, dynamics and selectivity profile of a NaV1.7 potency-optimised huwentoxin-IV variant. PLoS ONE 2017, 12, e0173551. [Google Scholar] [CrossRef]
- Shcherbatko, A.; Rossi, A.; Foletti, D.; Zhu, G.; Bogin, O.; Galindo Casas, M.; Rickert, M.; Hasa-Moreno, A.; Bartsevich, V.; Crameri, A.; et al. Engineering highly potent and selective microproteins against NaV1.7 sodium channel for treatment of Pain. J. Biol. Chem. 2016, 291, 13974–13986. [Google Scholar] [CrossRef]
- Li, D.; Xiao, Y.; Hu, W.; Xie, J.; Bosmans, F.; Tytgat, J.; Liang, S. Function and solution structure of hainantoxin-I, a novel insect sodium channel inhibitor from the Chinese bird spider Selenocosmia hainana. FEBS Lett. 2003, 555, 616–622. [Google Scholar] [CrossRef]
- Mueller, A.; Starobova, H.; Morgan, M.; Dekan, Z.; Cheneval, O.; Schroeder, C.I.; Alewood, P.F.; Deuis, J.R.; Vetter, I. Anti-allodynic effects of the selective NaV1.7 inhibitor Pn3a in a mouse model of acute post-surgical pain: Evidence for analgesic synergy with opioids and baclofen. Pain 2019, 160, 1766–1780. [Google Scholar] [CrossRef] [PubMed]
- Peigneur, S.; Paiva, A.; Cordeiro, M.; Borges, M.; Diniz, M.; de Lima, M.; Tytgat, J. Phoneutria nigriventer Spider Toxin PnTx2-1 (δ-Ctenitoxin-Pn1a) Is a Modulator of Sodium Channel Gating. Toxins 2018, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Bosmans, F.; Martin-Eauclaire, M.-F.; Swartz, K.J. Deconstructing voltage sensor function and pharmacology in sodium channels. Nature 2008, 456, 202–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Jackson, J.O.; Liang, S.; Cummins, T.R. Common molecular determinants of tarantula huwentoxin-IV inhibition of Na+ channel voltage-sensors in domains II and IV. J. Biol. Chem. 2011, 286, 27301–27310. [Google Scholar] [CrossRef]
- Bosmans, F.; Milescu, M.; Swartz, K.J. Palmitoylation influences the function and pharmacology of sodium channels. Proc. Natl. Acad. Sci. USA 2011, 108, 20213–20218. [Google Scholar] [CrossRef] [Green Version]
- Kanellopoulos, A.H.; Matsuyama, A. Voltage-gated sodium channels and pain-related disorders. Clin. Sci. (Lond.) 2016, 130, 2257–2265. [Google Scholar] [CrossRef]
- Dib-Hajj, S.D.; Geha, P.; Waxman, S.G. Sodium channels in pain disorders: Pathophysiology and prospects for treatment. Pain 2017, 158 (Suppl. 1), s97–s107. [Google Scholar] [CrossRef]
- Vetter, I.; Deuis, J.R.; Mueller, A.; Israel, M.R.; Starobova, H.; Zhang, A.; Rash, L.D.; Mobli, M. NaV1.7 as a pain target–from gene to pharmacology. Pharmacol. Ther. 2017, 172, 73–100. [Google Scholar] [CrossRef]
- Dib-Hajj, S.D.; Cummins, T.R.; Black, J.A.; Waxman, S.G. Sodium channels in normal and pathological pain. Annu. Rev. Neurosci. 2010, 33, 325–347. [Google Scholar] [CrossRef]
- Leipold, E.; Liebmann, L.; Korenke, G.C.; Heinrich, T.; Giesselmann, S.; Baets, J.; Ebbinghaus, M.; Goral, R.O.; Stodberg, T.; Hennings, J.C.; et al. A de novo gain-of-function mutation in SCN11A causes loss of pain perception. Nat. Genet. 2013, 45, 1399–1404. [Google Scholar] [CrossRef]
- Chen, H.P.; Zhou, W.; Kang, L.M.; Yan, H.; Zhang, L.; Xu, B.H.; Cai, W.H. Intrathecal miR-96 inhibits NaV1.3 expression and alleviates neuropathic pain in rat following chronic construction injury. Neurochem. Res. 2014, 39, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Shao, J.; Zhao, Q.; Ren, X.; Cai, W.; Li, L.; Bai, Q.; Chen, X.; Xu, B.; Wang, J.; et al. MiR-30b Attenuates neuropathic pPain by regulating voltage-gated sodium channel NaV1.3 in Rats. Front. Mol. Neurosci. 2017, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Faber, C.G.; Hoeijmakers, J.G.; Ahn, H.S.; Cheng, X.; Han, C.; Choi, J.S.; Estacion, M.; Lauria, G.; Vanhoutte, E.K.; Gerrits, M.M.; et al. Gain of function NaV1.7 mutations in idiopathic small fiber neuropathy. Ann. Neurol. 2012, 71, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Dib-Hajj, S.D.; Black, J.A.; Waxman, S.G. Voltage-gated sodium channels: Therapeutic targets for pain. Pain Med. 2009, 10, 1260–1269. [Google Scholar] [CrossRef]
- Deuis, J.R.; Zimmermann, K.; Romanovsky, A.A.; Possani, L.D.; Cabot, P.J.; Lewis, R.J.; Vetter, I. An animal model of oxaliplatin-induced cold allodynia reveals a crucial role for NaV1.6 in peripheral pain pathways. Pain 2013, 154, 1749–1757. [Google Scholar] [CrossRef]
- Qin, S.; Jiang, F.; Zhou, Y.; Zhou, G.; Ye, P.; Ji, Y. Local knockdown of NaV1.6 relieves pain behaviors induced by BmK I. Acta Biochim. Biophys. Sin. 2017, 49, 713–721. [Google Scholar] [CrossRef]
- Zhao, P.; Barr, T.P.; Hou, Q.; Dib-Hajj, S.D.; Black, J.A.; Albrecht, P.J.; Petersen, K.; Eisenberg, E.; Wymer, J.P.; Rice, F.L.; et al. Voltage-gated sodium channel expression in rat and human epidermal keratinocytes: Evidence for a role in pain. Pain 2008, 139, 90–105. [Google Scholar] [CrossRef]
- Catterall, W.A.; Goldin, A.L.; Waxman, S.G. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 2005, 57, 397–409. [Google Scholar] [CrossRef]
- Erickson, A.; Deiteren, A.; Harrington, A.M.; Garcia-Caraballo, S.; Castro, J.; Caldwell, A.; Grundy, L.; Brierley, S.M. Voltage-gated sodium channels: (NaV)igating the field to determine their contribution to visceral nociception. J. Physiol. 2018, 596, 785–807. [Google Scholar] [CrossRef]
- Black, J.A.; Dib-Hajj, S.; McNabola, K.; Jeste, S.; Rizzo, M.A.; Kocsis, J.D.; Waxman, S.G. Spinal sensory neurons express multiple sodium channel α-subunit mRNAs. Brain Res. Mol. Brain Res. 1996, 43, 117–131. [Google Scholar] [CrossRef]
- Wang, W.; Gu, J.; Li, Y.Q.; Tao, Y.X. Are voltage-gated sodium channels on the dorsal root ganglion involved in the development of neuropathic pain? Mol. Pain 2011, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, T.; Kobayashi, K.; Yamanaka, H.; Obata, K.; Dai, Y.; Noguchi, K. Comparative study of the distribution of the α-subunits of voltage-gated sodium channels in normal and axotomized rat dorsal root ganglion neurons. J. Comp. Neurol. 2008, 510, 188–206. [Google Scholar] [CrossRef] [PubMed]
- Hockley, J.R.; González-Cano, R.; McMurray, S.; Tejada-Giraldez, M.A.; McGuire, C.; Torres, A.; Wilbrey, A.L.; Cibert-Goton, V.; Nieto, F.R.; Pitcher, T. Visceral and somatic pain modalities reveal NaV1.7-independent visceral nociceptive pathways. J. Physiol. 2017, 595, 2661–2679. [Google Scholar] [CrossRef] [PubMed]
- Jay Gargus, J.; Tournay, A. Novel mutation confirms seizure locus SCN1A is also FHM3 migraine locus. Pediatr. Neurol. 2007, 37, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, J.; Dutton, S.; Diaz-Bustamante, M.; McPherson, A.; Olivares, N.; Kalia, J.; Escayg, A.; Bosmans, F. NaV1.1 modulation by a novel triazole compound attenuates epileptic seizures in rodents. ACS Chem. Biol. 2014, 9, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Bosmans, F.; Kalia, J. Derivatives of rufinamide and their use in inhibtion of the activation of human voltage-gated sodium channels. US Patent 9,771,335, 26 September 2017. [Google Scholar]
- Suter, M.R.; Kirschmann, G.; Laedermann, C.J.; Abriel, H.; Decosterd, I. Rufinamide attenuates mechanical allodynia in a model of neuropathic pain in the mouse and stabilizes voltage-gated sodium channel inactivated state. Anesthesiology 2013, 118, 160–172. [Google Scholar] [CrossRef]
- Cummins, T.R.; Aglieco, F.; Renganathan, M.; Herzog, R.I.; Dib-Hajj, S.D.; Waxman, S.G. NaV1.3 sodium channels: Rapid repriming and slow closed-state inactivation display quantitative differences after expression in a mammalian cell line and in spinal sensory neurons. J. Neurosci. 2001, 21, 5952–5961. [Google Scholar] [CrossRef]
- Waxman, S.G.; Kocsis, J.D.; Black, J.A. Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is reexpressed following axotomy. J. Neurophysiol. 1994, 72, 466–470. [Google Scholar] [CrossRef]
- Black, J.A.; Nikolajsen, L.; Kroner, K.; Jensen, T.S.; Waxman, S.G. Multiple sodium channel isoforms and mitogen-activated protein kinases are present in painful human neuromas. Ann. Neurol. 2008, 64, 644–653. [Google Scholar] [CrossRef]
- Strege, P.R.; Knutson, K.; Eggers, S.J.; Li, J.H.; Wang, F.; Linden, D.; Szurszewski, J.H.; Milescu, L.; Leiter, A.B.; Farrugia, G.; et al. Sodium channel NaV1.3 is important for enterochromaffin cell excitability and serotonin release. Sci. Rep. 2017, 7, 15650. [Google Scholar] [CrossRef]
- Bellono, N.W.; Bayrer, J.R.; Leitch, D.B.; Castro, J.; Zhang, C.; O’Donnell, T.A.; Brierley, S.M.; Ingraham, H.A.; Julius, D. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 2017, 170, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Black, J.A.; Cummins, T.R.; Plumpton, C.; Chen, Y.H.; Hormuzdiar, W.; Clare, J.J.; Waxman, S.G. Upregulation of a silent sodium channel after peripheral, but not central, nerve injury in DRG neurons. J. Neurophysiol. 1999, 82, 2776–2785. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Oh, Y.; Chung, J.M.; Chung, K. The changes in expression of three subtypes of TTX sensitive sodium channels in sensory neurons after spinal nerve ligation. Mol. Brain Res. 2001, 95, 153–161. [Google Scholar] [CrossRef]
- Lindia, J.A.; Kohler, M.G.; Martin, W.J.; Abbadie, C. Relationship between sodium channel NaV1.3 expression and neuropathic pain behavior in rats. Pain 2005, 117, 145–153. [Google Scholar] [CrossRef]
- Hains, B.C.; Saab, C.Y.; Klein, J.P.; Craner, M.J.; Waxman, S.G. Altered sodium channel expression in second-order spinal sensory neurons contributes to pain after peripheral nerve injury. J. Neurosci. 2004, 24, 4832–4839. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, J.; Wang, Y.; Wang, L.; Wang, X. Changes in the expression of voltage-gated sodium channels NaV1.3, NaV1.7, NaV1.8, and NaV1.9 in rat trigeminal ganglia following chronic constriction injury. Neuroreport 2016, 27, 929–934. [Google Scholar] [CrossRef]
- Black, J.A.; Liu, S.; Tanaka, M.; Cummins, T.R.; Waxman, S.G. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain 2004, 108, 237–247. [Google Scholar] [CrossRef]
- Hong, S.; Morrow, T.J.; Paulson, P.E.; Isom, L.L.; Wiley, J.W. Early painful diabetic neuropathy is associated with differential changes in tetrodotoxin-sensitive and -resistant sodium channels in dorsal root ganglion neurons in the rat. J. Biol. Chem. 2004, 279, 29341–29350. [Google Scholar] [CrossRef]
- Tan, A.M.; Samad, O.A.; Dib-Hajj, S.D.; Waxman, S.G. Virus-mediated knockdown of NaV1.3 in dorsal root ganglia of STZ-induced diabetic rats alleviates tactile allodynia. Mol. Med. 2015, 21, 544–552. [Google Scholar] [CrossRef]
- Garry, E.M.; Delaney, A.; Anderson, H.A.; Sirinathsinghji, E.C.; Clapp, R.H.; Martin, W.J.; Kinchington, P.R.; Krah, D.L.; Abbadie, C.; Fleetwood-Walker, S.M. Varicella zoster virus induces neuropathic changes in rat dorsal root ganglia and behavioral reflex sensitisation that is attenuated by gabapentin or sodium channel blocking drugs. Pain 2005, 118, 97–111. [Google Scholar] [CrossRef]
- Nassar, M.A.; Baker, M.D.; Levato, A.; Ingram, R.; Mallucci, G.; McMahon, S.B.; Wood, J.N. Nerve injury induces robust allodynia and ectopic discharges in NaV1.3 null mutant mice. Mol. Pain 2006, 2, 33. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.E.; Meisler, M.H. Sodium channel SCN8A (NaV1.6): Properties and de novo mutations in epileptic encephalopathy and intellectual disability. Front. Genet. 2013, 4, 213. [Google Scholar] [CrossRef] [PubMed]
- Black, J.A.; Renganathan, M.; Waxman, S.G. Sodium channel NaV1.6 is expressed along nonmyelinated axons and it contributes to conduction. Brain Res. Mol. Brain Res. 2002, 105, 19–28. [Google Scholar] [CrossRef]
- Ramachandra, R.; Elmslie, K.S. EXPRESS: Voltage-dependent sodium (NaV) channels in group IV sensory afferents. Mol. Pain 2016, 12. [Google Scholar] [CrossRef]
- Persson, A.K.; Black, J.A.; Gasser, A.; Cheng, X.; Fischer, T.Z.; Waxman, S.G. Sodium-calcium exchanger and multiple sodium channel isoforms in intra-epidermal nerve terminals. Mol. Pain 2010, 6, 84. [Google Scholar] [CrossRef]
- Kennedy, P.G.; Montague, P.; Scott, F.; Grinfeld, E.; Ashrafi, G.H.; Breuer, J.; Rowan, E.G. Varicella-zoster viruses associated with post-herpetic neuralgia induce sodium current density increases in the ND7-23 NaV-1.8 neuroblastoma cell line. PLoS ONE 2013, 8, e51570. [Google Scholar] [CrossRef]
- Ren, Y.-S.; Qian, N.-S.; Tang, Y.; Liao, Y.-H.; Yang, Y.-L.; Dou, K.-F.; Toi, M. Sodium channel NaV1.6 is up-regulated in the dorsal root ganglia in a mouse model of type 2 diabetes. Brain Res. Bull. 2012, 87, 244–249. [Google Scholar] [CrossRef]
- Grasso, G.; Landi, A.; Alafaci, C. A novel pathophysiological mechanism contributing to trigeminal neuralgia. Mol. Med. 2016, 22, 452–454. [Google Scholar] [CrossRef]
- Tanaka, B.S.; Zhao, P.; Dib-Hajj, F.B.; Morisset, V.; Tate, S.; Waxman, S.G.; Dib-Hajj, S.D. A gain-of-function mutation in NaV1.6 in a case of trigeminal neuralgia. Mol. Med. 2016, 22, 338–348. [Google Scholar] [CrossRef]
- Israel, M.R.; Thongyoo, P.; Deuis, J.R.; Craik, D.J.; Vetter, I.; Durek, T. The E15R point mutation in scorpion toxin Cn2 uncouples its depressant and excitatory activities on human NaV1.6. J. Med. Chem. 2018, 61, 1730–1736. [Google Scholar] [CrossRef]
- Xie, W.; Strong, J.A.; Zhang, J.M. Local knockdown of the NaV1.6 sodium channel reduces pain behaviors, sensory neuron excitability, and sympathetic sprouting in rat models of neuropathic pain. Neuroscience 2015, 291, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Strong, J.A.; Ye, L.; Mao, J.X.; Zhang, J.M. Knockdown of sodium channel NaV1.6 blocks mechanical pain and abnormal bursting activity of afferent neurons in inflamed sensory ganglia. Pain 2013, 154, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Dib-Hajj, S.D.; Yang, Y.; Black, J.A.; Waxman, S.G. The NaV1.7 sodium channel: From molecule to man. Nat. Rev. Neurosci. 2013, 14, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Black, J.A.; Frézel, N.; Dib-Hajj, S.D.; Waxman, S.G. Expression of NaV1.7 in DRG neurons extends from peripheral terminals in the skin to central preterminal branches and terminals in the dorsal horn. Mol. Pain 2012, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.M.; Cummins, T.R.; Waxman, S.G. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J. Physiol. 2007, 579, 1–14. [Google Scholar] [CrossRef]
- Herzog, R.I.; Cummins, T.R.; Ghassemi, F.; Dib-Hajj, S.D.; Waxman, S.G. Distinct repriming and closed-state inactivation kinetics of NaV1.6 and NaV1.7 sodium channels in mouse spinal sensory neurons. J. Physiol. 2003, 551, 741–750. [Google Scholar] [CrossRef]
- Fertleman, C.R.; Baker, M.D.; Parker, K.A.; Moffatt, S.; Elmslie, F.V.; Abrahamsen, B.; Ostman, J.; Klugbauer, N.; Wood, J.N.; Gardiner, R.M.; et al. SCN9A mutations in paroxysmal extreme pain disorder: Allelic variants underlie distinct channel defects and phenotypes. Neuron 2006, 52, 767–774. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Li, S.; Xu, Z.; Li, H.; Ma, L.; Fan, J.; Bu, D.; Liu, B.; Fan, Z.; et al. Mutations in SCN9A, encoding a sodium channel α subunit, in patients with primary erythermalgia. J. Med. Genet. 2004, 41, 171–174. [Google Scholar] [CrossRef]
- Blesneac, I.; Themistocleous, A.C.; Fratter, C.; Conrad, L.J.; Ramirez, J.D.; Cox, J.J.; Tesfaye, S.; Shillo, P.R.; Rice, A.S.; Tucker, S.J. Rare NaV1.7 variants associated with painful diabetic peripheral neuropathy. Pain 2018, 159, 469–480. [Google Scholar] [CrossRef]
- Shields, S.D.; Ahn, H.S.; Yang, Y.; Han, C.; Seal, R.P.; Wood, J.N.; Waxman, S.G.; Dib-Hajj, S.D. NaV1.8 expression is not restricted to nociceptors in mouse peripheral nervous system. Pain 2012, 153, 2017–2030. [Google Scholar] [CrossRef]
- Akopian, A.N.; Sivilotti, L.; Wood, J.N. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 1996, 379, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Black, J.A.; Waxman, S.G. Molecular identities of two tetrodotoxin-resistant sodium channels in corneal axons. Exp. Eye Res. 2002, 75, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Dib-Hajj, S.D.; Tyrrell, L.; Cummins, T.R.; Black, J.A.; Wood, P.M.; Waxman, S.G. Two tetrodotoxin-resistant sodium channels in human dorsal root ganglion neurons. FEBS Lett. 1999, 462, 117–120. [Google Scholar] [CrossRef] [Green Version]
- Akopian, A.N.; Souslova, V.; England, S.; Okuse, K.; Ogata, N.; Ure, J.; Smith, A.; Kerr, B.J.; McMahon, S.B.; Boyce, S.; et al. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat. Neurosci. 1999, 2, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.-Y.; Piekarz, A.D.; Priest, B.T.; Knopp, K.L.; Krajewski, J.L.; McDermott, J.S.; Nisenbaum, E.S.; Cummins, T.R. Tetrodotoxin-resistant sodium channels in sensory neurons generate slow resurgent currents that are enhanced by inflammatory mediators. J. Neurosci. 2014, 34, 7190–7197. [Google Scholar] [CrossRef] [PubMed]
- Blair, N.T.; Bean, B.P. Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons. J. Neurosci. 2002, 22, 10277–10290. [Google Scholar] [CrossRef]
- Tanaka, M.; Cummins, T.R.; Ishikawa, K.; Dib-Hajj, S.D.; Black, J.A.; Waxman, S.G. SNS Na+ channel expression increases in dorsal root ganglion neurons in the carrageenan inflammatory pain model. Neuroreport 1998, 9, 967–972. [Google Scholar] [CrossRef]
- Binshtok, A.M.; Wang, H.; Zimmermann, K.; Amaya, F.; Vardeh, D.; Shi, L.; Brenner, G.J.; Ji, R.-R.; Bean, B.P.; Woolf, C.J. Nociceptors are interleukin-1β sensors. J. Neurosci. 2008, 28, 14062–14073. [Google Scholar] [CrossRef]
- Gold, M.S.; Reichling, D.B.; Shuster, M.J.; Levine, J.D. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc. Natl. Acad. Sci. USA 1996, 93, 1108–1112. [Google Scholar] [CrossRef]
- Beyak, M.J.; Ramji, N.; Krol, K.M.; Kawaja, M.D.; Vanner, S.J. Two TTX-resistant Na+ currents in mouse colonic dorsal root ganglia neurons and their role in colitis-induced hyperexcitability. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G845–G855. [Google Scholar] [CrossRef]
- Yu, Y.-Q.; Zhao, F.; Guan, S.-M.; Chen, J. Antisense-mediated knockdown of NaV1.8, but not NaV1.9, generates inhibitory effects on complete Freund’s adjuvant-induced inflammatory pain in rat. PLoS ONE 2011, 6, e19865. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-M.; Fu, Y.; Winston, J.; Radhakrishnan, R.; Sarna, S.K.; Huang, L.-Y.M.; Shi, X.-Z. Pathogenesis of abdominal pain in bowel obstruction: Role of mechanical stress-induced upregulation of nerve growth factor in gut smooth muscle cells. Pain 2017, 158, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Dib-Hajj, S.; Black, J.; Felts, P.; Waxman, S. Down-regulation of transcripts for Na channel α-SNS in spinal sensory neurons following axotomy. Proc. Natl. Acad. Sci. USA 1996, 93, 14950–14954. [Google Scholar] [CrossRef] [PubMed]
- Cummins, T.R.; Waxman, S.G. Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin-sensitive sodium current in small spinal sensory neurons after nerve injury. J. Neurosci. 1997, 17, 3503–3514. [Google Scholar] [CrossRef]
- Decosterd, I.; Ji, R.-R.; Abdi, S.; Tate, S.; Woolf, C.J. The pattern of expression of the voltage-gated sodium channels NaV1.8 and NaV1.9 does not change in uninjured primary sensory neurons in experimental neuropathic pain models. Pain 2002, 96, 269–277. [Google Scholar] [CrossRef]
- Gold, M.S.; Weinreich, D.; Kim, C.-S.; Wang, R.; Treanor, J.; Porreca, F.; Lai, J. Redistribution of NaV1.8 in uninjured axons enables neuropathic pain. J. Neurosci. 2003, 23, 158–166. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Zhu, C.Z.; Thimmapaya, R.; Choi, W.S.; Honore, P.; Scott, V.E.; Kroeger, P.E.; Sullivan, J.P.; Faltynek, C.R.; Gopalakrishnan, M. Differential action potentials and firing patterns in injured and uninjured small dorsal root ganglion neurons after nerve injury. Brain Res. 2004, 1009, 147–158. [Google Scholar] [CrossRef]
- Faber, C.G.; Lauria, G.; Merkies, I.S.; Cheng, X.; Han, C.; Ahn, H.S.; Persson, A.K.; Hoeijmakers, J.G.; Gerrits, M.M.; Pierro, T.; et al. Gain-of-function NaV1.8 mutations in painful neuropathy. Proc. Natl. Acad. Sci. USA 2012, 109, 19444–19449. [Google Scholar] [CrossRef]
- Dib-Hajj, S.D.; Tyrrell, L.; Black, J.A.; Waxman, S.G. NaN, a novel voltage-gated Na channel, is expressed preferentially in peripheral sensory neurons and down-regulated after axotomy. Proc. Natl. Acad. Sci. USA 1998, 95, 8963–8968. [Google Scholar] [CrossRef] [Green Version]
- Rugiero, F.; Mistry, M.; Sage, D.; Black, J.A.; Waxman, S.G.; Crest, M.; Clerc, N.; Delmas, P.; Gola, M. Selective expression of a persistent tetrodotoxin-resistant Na+ current and NaV1.9 subunit in myenteric sensory neurons. J. Neurosci. 2003, 23, 2715–2725. [Google Scholar] [CrossRef]
- Cummins, T.R.; Dib-Hajj, S.D.; Black, J.A.; Akopian, A.N.; Wood, J.N.; Waxman, S.G. A novel persistent tetrodotoxin-resistant sodium current in SNS-null and wild-type small primary sensory neurons. J. Neurosci. 1999, 19, RC43. [Google Scholar] [CrossRef] [PubMed]
- Herzog, R.; Cummins, T.; Waxman, S. Persistent TTX-resistant Na+ current affects resting potential and response to depolarization in simulated spinal sensory neurons. J. Neurophysiol. 2001, 86, 1351–1364. [Google Scholar] [CrossRef] [PubMed]
- Maingret, F.; Coste, B.; Padilla, F.; Clerc, N.; Crest, M.; Korogod, S.M.; Delmas, P. Inflammatory mediators increase NaV1.9 current and excitability in nociceptors through a coincident detection mechanism. J. Gen. Physiol. 2008, 131, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Priest, B.T.; Murphy, B.A.; Lindia, J.A.; Diaz, C.; Abbadie, C.; Ritter, A.M.; Liberator, P.; Iyer, L.M.; Kash, S.F.; Kohler, M.G.; et al. Contribution of the tetrodotoxin-resistant voltage-gated sodium channel NaV1.9 to sensory transmission and nociceptive behavior. Proc. Natl. Acad. Sci. USA 2005, 102, 9382–9387. [Google Scholar] [CrossRef] [PubMed]
- Amaya, F.; Wang, H.; Costigan, M.; Allchorne, A.J.; Hatcher, J.P.; Egerton, J.; Stean, T.; Morisset, V.; Grose, D.; Gunthorpe, M.J.; et al. The voltage-gated sodium channel NaV1.9 is an effector of peripheral inflammatory pain hypersensitivity. J. Neurosci. 2006, 26, 12852–12860. [Google Scholar] [CrossRef] [PubMed]
- Lolignier, S.; Amsalem, M.; Maingret, F.; Padilla, F.; Gabriac, M.; Chapuy, E.; Eschalier, A.; Delmas, P.; Busserolles, J. NaV1.9 channel contributes to mechanical and heat pain hypersensitivity induced by subacute and chronic inflammation. PLoS ONE 2011, 6, e23083. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wen, J.; Yang, W.; Wang, C.; Gao, L.; Zheng, L.H.; Wang, T.; Ran, K.; Li, Y.; Li, X.; et al. Gain-of-function mutations in SCN11A cause familial episodic pain. Am. J. Hum. Genet. 2013, 93, 957–966. [Google Scholar] [CrossRef]
- Han, C.; Yang, Y.; de Greef, B.T.A.; Hoeijmakers, J.G.J.; Gerrits, M.M.; Verhamme, C.; Qu, J.; Lauria, G.; Merkies, I.S.J.; Faber, C.G.; et al. The domain II S4-S5 linker in NaV1.9: A missense mutation enhances activation, impairs fast inactivation, and produces human painful neuropathy. Neuromol. Med. 2015, 17, 158–169. [Google Scholar] [CrossRef]
- Huang, J.; Han, C.; Estacion, M.; Vasylyev, D.; Hoeijmakers, J.G.; Gerrits, M.M.; Tyrrell, L.; Lauria, G.; Faber, C.G.; Dib-Hajj, S.D.; et al. Gain-of-function mutations in sodium channel NaV1.9 in painful neuropathy. Brain 2014, 137, 1627–1642. [Google Scholar] [CrossRef]
- Bosmans, F.; Puopolo, M.; Martin-Eauclaire, M.F.; Bean, B.P.; Swartz, K.J. Functional properties and toxin pharmacology of a dorsal root ganglion sodium channel viewed through its voltage sensors. J. Gen. Physiol. 2011, 138, 59–72. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, T.C.; Boukaiba, R.; Molgó, J.; Amar, M.; Partiseti, M.; Servent, D.; Benoit, E. Direct evidence for high affinity blockade of NaV1.6 channel subtype by huwentoxin-IV spider peptide, using multiscale functional approaches. Neuropharmacology 2018, 133, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Q.; Zhang, Q.; Peng, D.; Chen, M.; Liang, S.; Zhou, X.; Liu, Z. Engineering gain-of-function analogues of the spider venom peptide HNTX-I, a potent blocker of the hNaV1.7 sodium channel. Toxins 2018, 10, 358. [Google Scholar] [CrossRef] [PubMed]
- Cherki, R.S.; Kolb, E.; Langut, Y.; Tsveyer, L.; Bajayo, N.; Meir, A. Two tarantula venom peptides as potent and differential NaV channels blockers. Toxicon 2014, 77, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.Y.; Cristofori-Armstrong, B.; Undheim, E.A.; King, G.F.; Rash, L.D. Three peptide modulators of the human voltage-gated sodium channel 1.7, an important analgesic target, from the venom of an Australian tarantula. Toxins 2015, 7, 2494–2513. [Google Scholar] [CrossRef]
- Redaelli, E.; Cassulini, R.R.; Silva, D.F.; Clement, H.; Schiavon, E.; Zamudio, F.Z.; Odell, G.; Arcangeli, A.; Clare, J.J.; Alagón, A. Target promiscuity and heterogeneous effects of tarantula venom peptides affecting Na+ and K+ ion channels. J. Biol. Chem. 2010, 285, 4130–4142. [Google Scholar] [CrossRef]
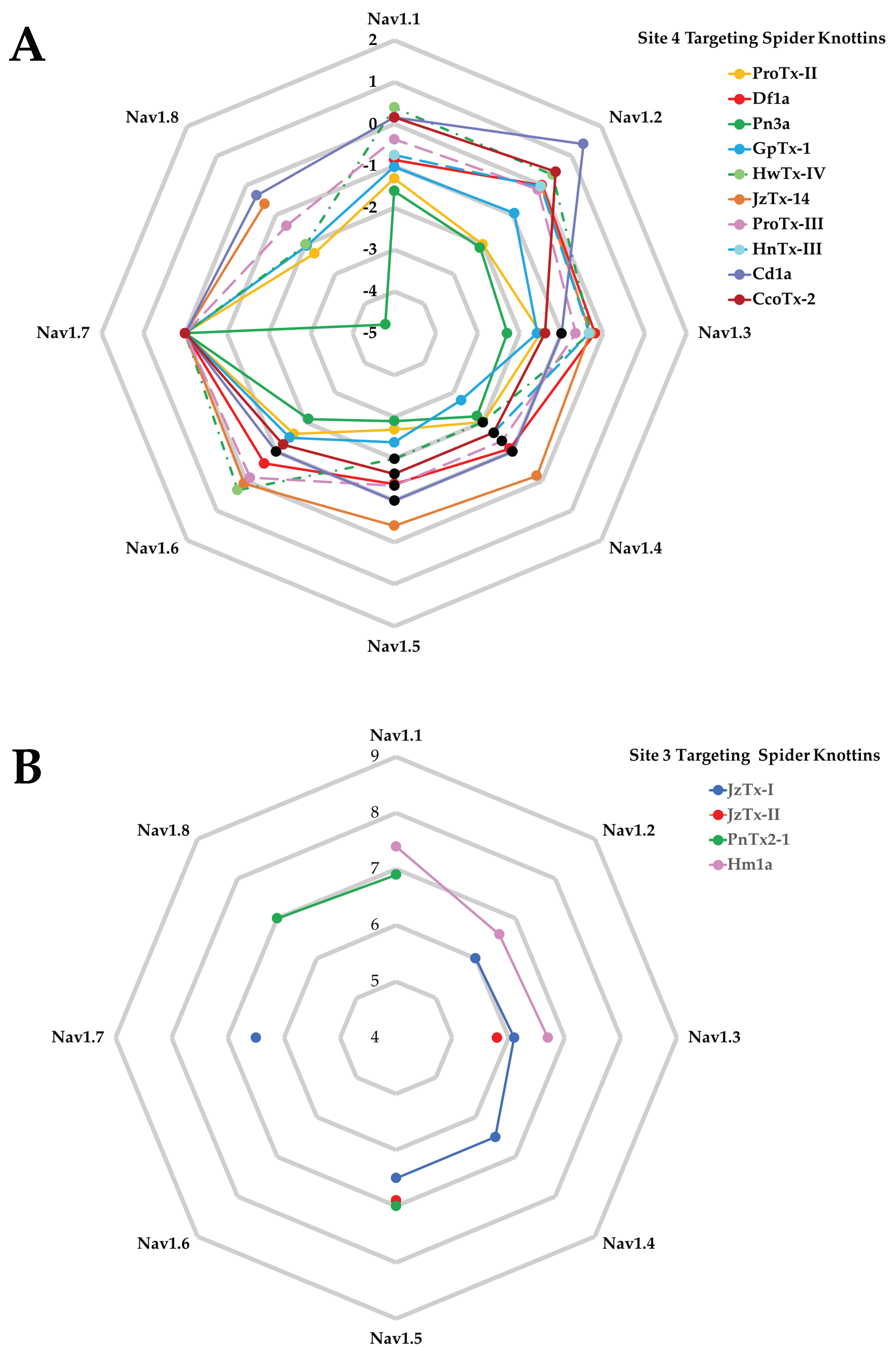
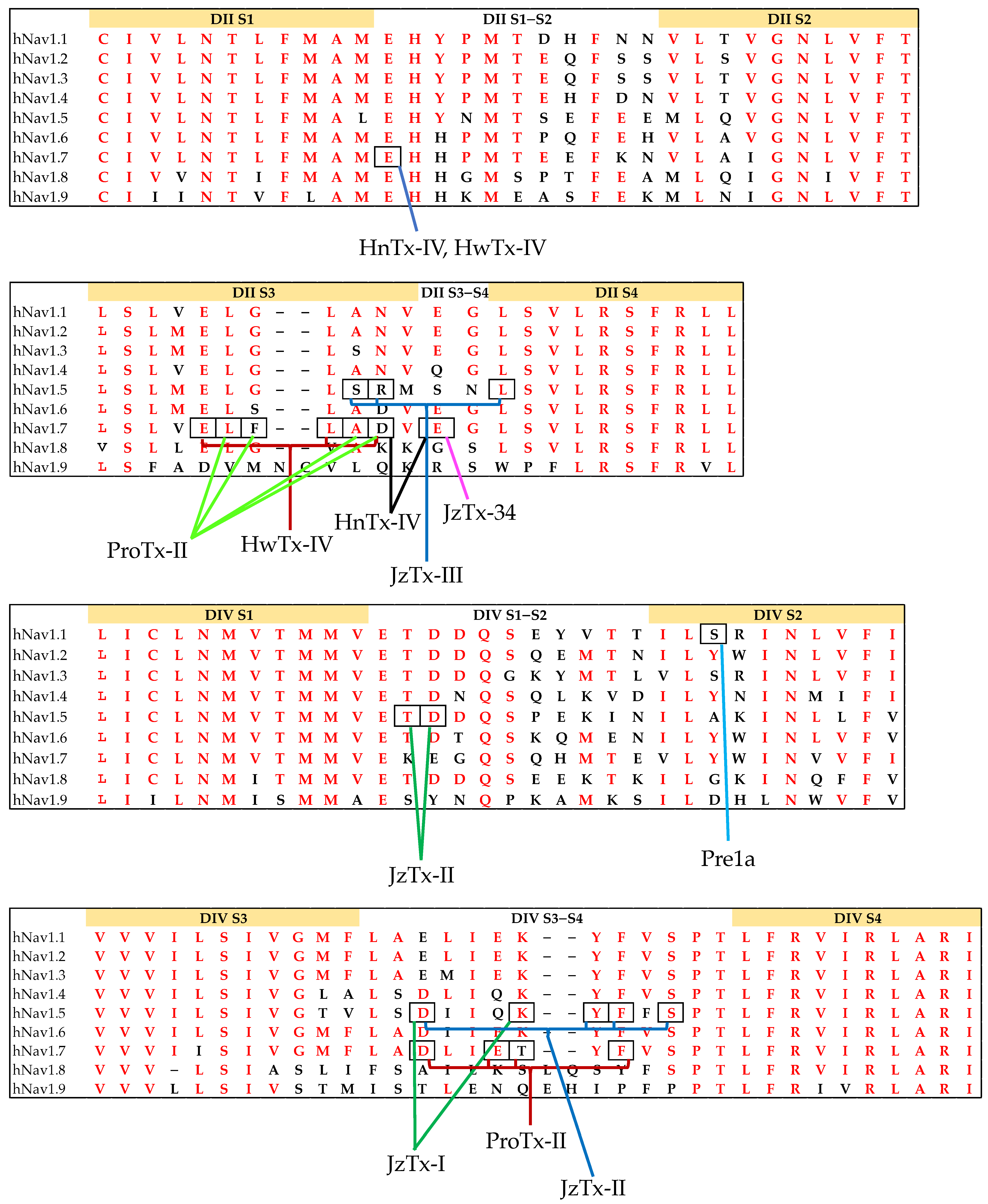
| Features | Examples |
|---|---|
| Hyperpolarizing shift in voltage-dependence of activation | PhTx-2 [89], VTX [94], Hv1 [95], Ar1 [96], Hv1b [97], PnTx2-6 [93], JzTx-II [98] |
| Hyperpolarizing shift in steady-state inactivation | VTX [94,99], PhTx-2 [89], Ar1 [89], Hv1b [97], PnTx2-6 [93] |
| No significant effect in voltage-dependence of steady-state inactivation | JzTx-I [100,101], JzTx-II [98] |
| Reduced peak inward current amplitude | VTX [94], Ar1 [96], Hv1b [97], PnTx2-6 [93] |
| No change in peak inward current amplitude | JzTx-I [100] |
| Increased peak inward current amplitude | Hm1a [20] |
| Increased recovery rate from inactivation | VTX [94], Ar1 [96], JzTx-I [102], JzTx-II [98] |
| Decreased recovery rate from inactivation | PnTx2-6 [93] |
| Features | Examples |
|---|---|
| Depolarizing shift in voltage-dependence of activation | ProTx-I [103], ProTx-II [77,103,104], JzTx-III [105,106], CcoTx-I [73], CcoTx-2 [73], CcoTx-3 [73], PaurTx-3 [73], JzTx-V [79,107], JzTx-IX [108], Hm-3 [109], Cd1a [110], Pre1a [111], Pn3a [61], Df1a [92], JxTx-XI [91], JzTx-35 [112] |
| No effect in voltage-dependence of activation | HwTx-IV [104,113,114,115], HnTx-III [116,117], JzTx-34 [118,119], Hm-1 [120], Hm-2 [120], Hd1a [121], GpTx-1 [122], Hl1a [123], PnTx1 [124], ProTx-III [80], Pre1a [111], JzTx-14 [125] |
| Hyperpolarizing shift in voltage-dependence of steady-state inactivation | HnTx-III [116], HnTx-IV [116], JzTx-V [79], Hm-1 [120], Hm-2 [120], JzTx-35 [112], PnTx4 (5-5) [126], Df1a [92], JzTx-34 [119] |
| Delay in channel inactivation | ProTx-II [104], JzTx-XI [91], Df1a [92], JzTx-14 [125] |
| Decreased channel recovery from inactivation | HnTx-III [116], JzTx-XI [91], Pn3a [61] |
| No effect in channel recovery from inactivation | HnTx-IV [116], JzTx-34 [118], HnTx-III [127], Hd1a [121] |
| Hyperpolarizing shift in voltage-dependence of activation | Df1a [92] |
| Depolarizing shift in voltage-dependence of steady-state inactivation | Df1a [92] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dongol, Y.; C. Cardoso, F.; Lewis, R.J. Spider Knottin Pharmacology at Voltage-Gated Sodium Channels and Their Potential to Modulate Pain Pathways. Toxins 2019, 11, 626. https://doi.org/10.3390/toxins11110626
Dongol Y, C. Cardoso F, Lewis RJ. Spider Knottin Pharmacology at Voltage-Gated Sodium Channels and Their Potential to Modulate Pain Pathways. Toxins. 2019; 11(11):626. https://doi.org/10.3390/toxins11110626
Chicago/Turabian StyleDongol, Yashad, Fernanda C. Cardoso, and Richard J. Lewis. 2019. "Spider Knottin Pharmacology at Voltage-Gated Sodium Channels and Their Potential to Modulate Pain Pathways" Toxins 11, no. 11: 626. https://doi.org/10.3390/toxins11110626
APA StyleDongol, Y., C. Cardoso, F., & Lewis, R. J. (2019). Spider Knottin Pharmacology at Voltage-Gated Sodium Channels and Their Potential to Modulate Pain Pathways. Toxins, 11(11), 626. https://doi.org/10.3390/toxins11110626






