Biological Stoichiometry Regulates Toxin Production in Microcystis aeruginosa (UTEX 2385)
Abstract
1. Introduction
2. Results
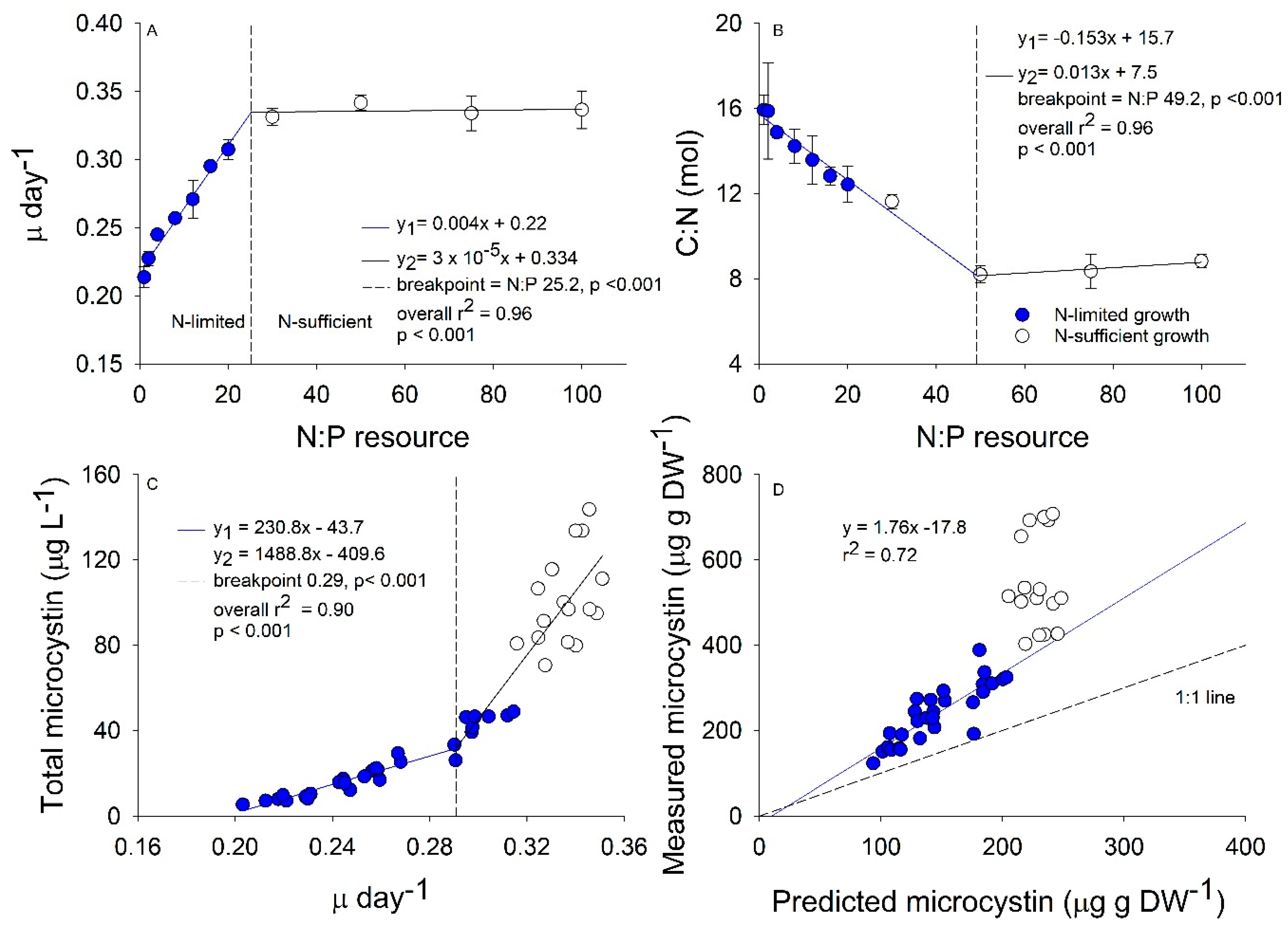
2.1. Growth and Stoichiometry Experiment
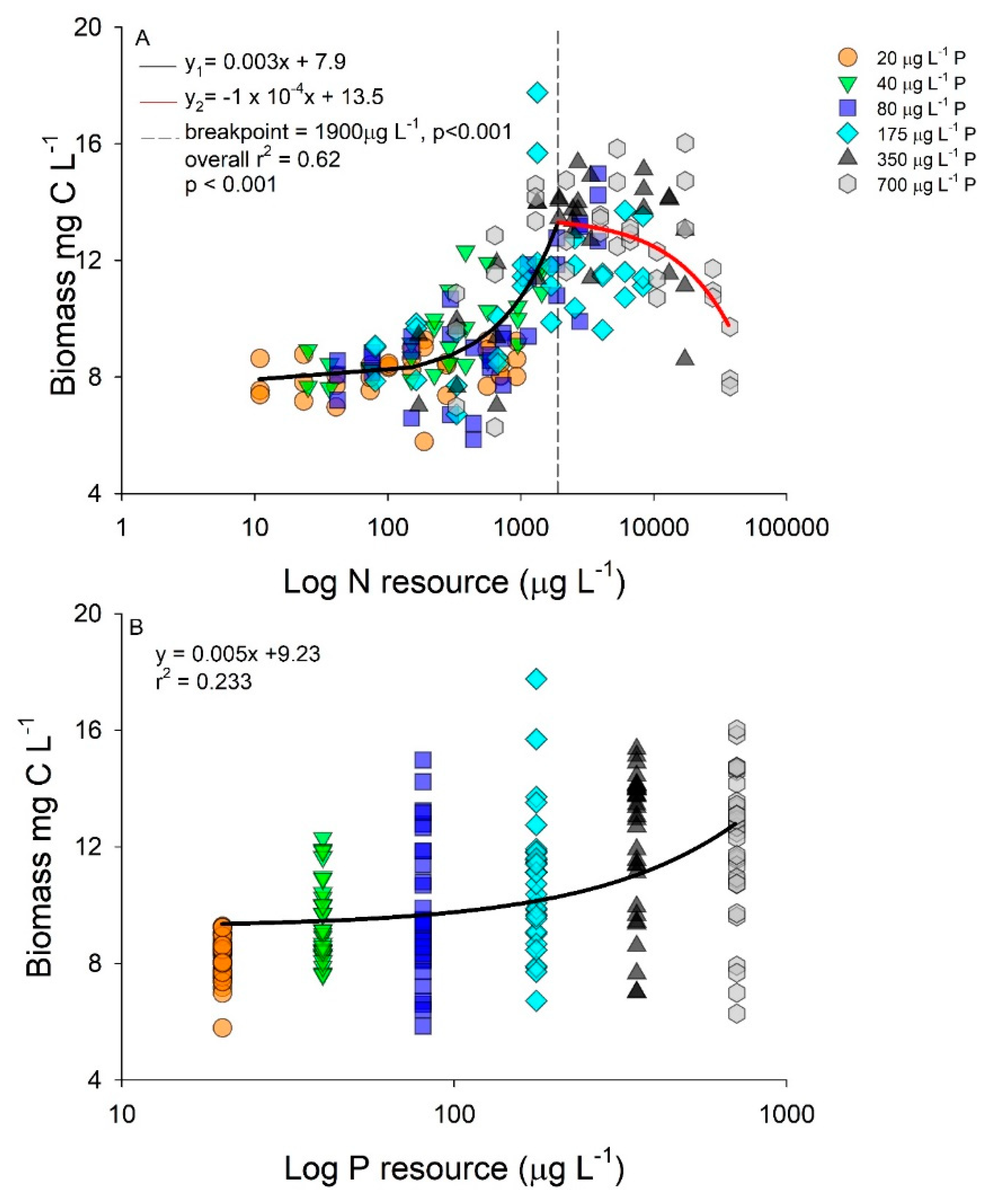
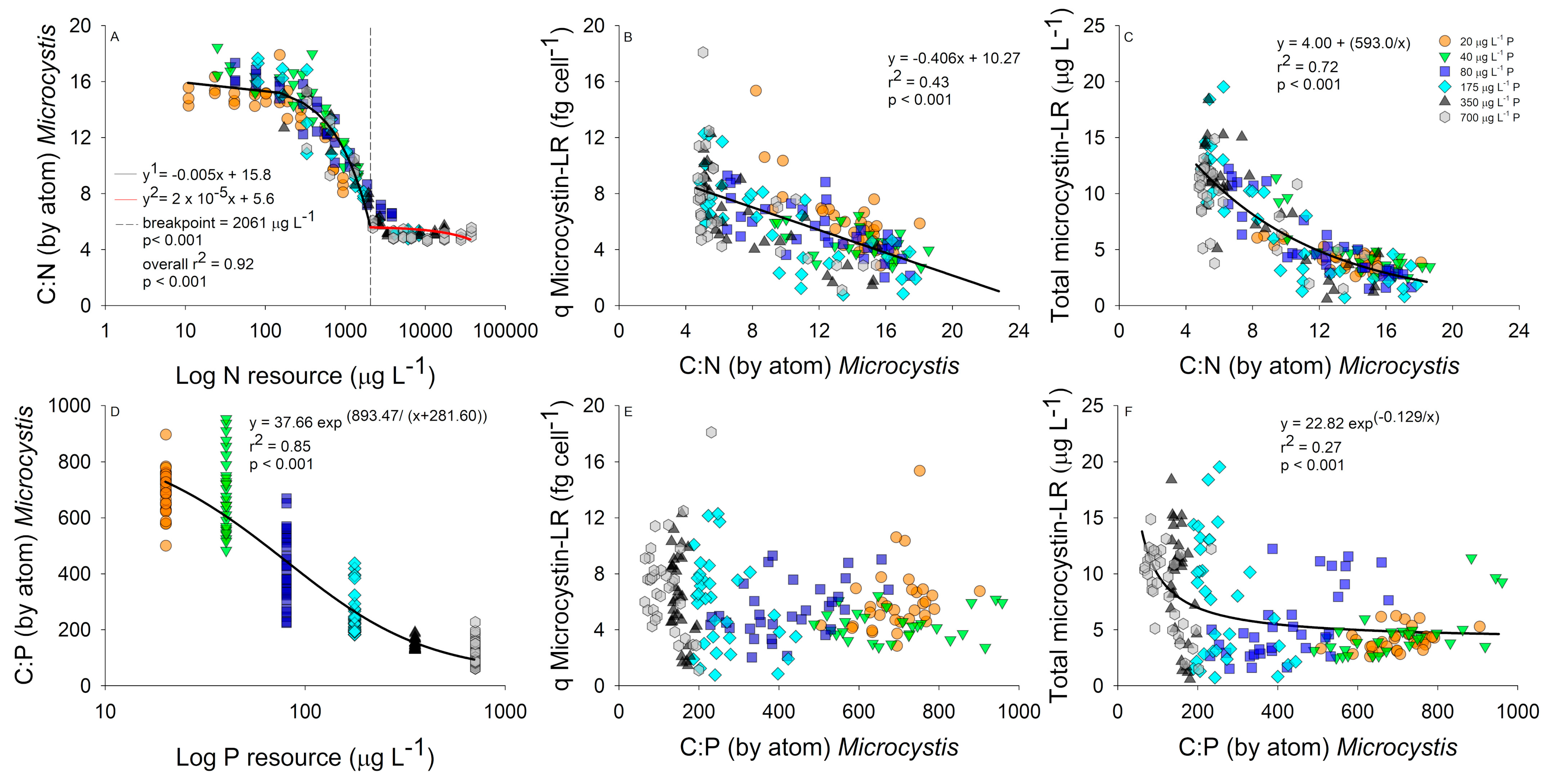
2.2. Interactive Effects of N and P Concentration on Stoichiometry and Toxin Cell Quota
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Growth and Stoichiometry Experiment
5.2. Interactive Effects of N and P Concentration on Stoichiometry and Toxin Cell Quota
5.3. Elemental Composition and Growth Rate
5.4. Cell Counts and Cyanotoxin Analysis
5.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.W.; Lazorchak, J.M.; Howard, M.D.A.; Johnson, M.V.V.; Morton, S.L.; Perkins, D.A.K.; Reavie, E.D.; Scott, G.I.; Smith, S.A.; Steevens, J.A. Are harmful algal blooms becoming the greatest inland water quality threat to public health and aquatic ecosystems? Environ. Toxicol. Chem. 2016, 35, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef]
- Deblois, C.P.; Juneau, P. Relationship between photosynthetic processes and microcystin in Microcystis aeruginosa grown under different photon irradiances. Harmful Algae 2010, 9, 18–24. [Google Scholar] [CrossRef]
- Paerl, H.W.; Scott, J.T.; McCarthy, M.J.; Newell, S.E.; Gardner, W.S.; Havens, K.E.; Hoffman, D.K.; Wilhelm, S.W.; Wurtsbaugh, W.A. It takes two to tango: When and where dual nutrient (N & P) reductions are needed to protect lakes and downstream ecosystems. Environ. Sci. Technol. 2016, 50, 10805–10813. [Google Scholar]
- Van de Waal, D.B.; Verspagen, J.M.H.; Lürling, M.; Van Donk, E.; Visser, P.M.; Huisman, J. The ecological stoichiometry of toxins produced by harmful cyanobacteria: An experimental test of the carbon-nutrient balance hypothesis. Ecol. Lett. 2009, 12, 1326–1335. [Google Scholar] [CrossRef]
- Dolman, A.M.; Rücker, J.; Pick, F.R.; Fastner, J.; Rohrlack, T.; Mischke, U.; Wiedner, C. Cyanobacteria and cyanotoxins: The influence of nitrogen versus phosphorus. PLoS ONE 2012, 7, e38757. [Google Scholar] [CrossRef]
- Yuan, L.L.; Pollard, A.I.; Pather, S.; Oliver, J.L.; D’Anglada, L. Managing microcystin: Identifying national-scale thresholds for total nitrogen and chlorophyll a. Freshw. Biol. 2014, 59, 1970–1981. [Google Scholar] [CrossRef]
- Harris, T.D.; Smith, V.H.; Graham, J.L.; Van de Waal, D.B.; Tedesco, L.P.; Clercin, N. Combined effects of nitrogen to phosphorus and nitrate to ammonia ratios on cyanobacterial metabolite concentrations in eutrophic Midwestern USA reservoirs. Inland Waters 2016, 6, 199–210. [Google Scholar] [CrossRef]
- Scott, J.T.; McCarthy, M.J.; Otten, T.G.; Steffen, M.M.; Baker, B.C.; Grantz, E.M.; Wilhelm, S.W.; Paerl, H.W. Comment: An alternative interpretation of the relationship between TN:TP and microcystins in Canadian lakes. Can. J. Fish. Aquat. Sci. 2013, 70, 1265–1268. [Google Scholar] [CrossRef]
- Orr, P.T.; Jones, G.J. Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnol. Oceanogr. 1998, 43, 1604–1614. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002; ISBN 0691074917. [Google Scholar]
- Tillett, D.; Neilan, B.A.; Dittmann, E.; Börner, T.; Erhard, M.; Von Döhren, H. Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC7806: An integrated peptide-polyketide synthetase system. Chem. Biol. 2000, 7, 753–764. [Google Scholar] [CrossRef]
- Van de Waal, D.B.; Smith, V.H.; Declerck, S.A.J.; Stam, E.C.M.; Elser, J.J. Stoichiometric regulation of phytoplankton toxins. Ecol. Lett. 2014, 17, 736–742. [Google Scholar] [CrossRef]
- Long, B.M.; Jones, G.J.; Orr, P.T. Cellular microcystin content in N-limited Microcystis aeruginosa can be predicted from growth rate. Appl. Environ. Microbiol. 2001, 67, 278–283. [Google Scholar] [CrossRef]
- Glibert, P.M. Eutrophication, harmful algae and biodiversity—Challenging paradigms in a world of complex nutrient changes. Mar. Pollut. Bull. 2017, 124, 591–606. [Google Scholar] [CrossRef]
- Hamilton, J.G.; Zangerl, A.R.; DeLucia, E.H.; Berenbaum, M.R. The carbon-nutrient balance hypothesis: Its rise and fall. Ecol. Lett. 2001, 4, 86–95. [Google Scholar] [CrossRef]
- Harke, M.J.; Gobler, C.J. Daily transcriptome changes reveal the role of nitrogen in controlling microcystin synthesis and nutrient transport in the toxic cyanobacterium, Microcystis aeruginosa. BMC Genom. 2015, 16, 1–18. [Google Scholar] [CrossRef]
- Kardinaal, W.E.A.; Tonk, L.; Janse, I.; Hol, S.; Slot, P.; Huisman, J.; Visser, P.M. Competition for light between toxic and nontoxic strains of the harmful cyanobacterium Microcystis. Appl. Environ. Microbiol. 2007, 73, 2939–2946. [Google Scholar] [CrossRef]
- Vézie, C.; Rapala, J.; Vaitomaa, J.; Seitsonen, J.; Sivonen, K. Effect of nitrogen and phosphorus on growth of toxic and nontoxic Microcystis strains and on intracellular microcystin concentrations. Microb. Ecol. 2002, 43, 443–454. [Google Scholar] [CrossRef]
- Briand, E.; Bormans, M.; Quiblier, C.; Salençon, M.J.; Humbert, J.F. Evidence of the cost of the production of microcystins by Microcystis aeruginosa under differing light and nitrate environmental conditions. PLoS ONE 2012, 7, e29981. [Google Scholar] [CrossRef]
- Zilliges, Y.; Kehr, J.C.; Meissner, S.; Ishida, K.; Mikkat, S.; Hagemann, M.; Kaplan, A.; Börner, T.; Dittmann, E. The cyanobacterial hepatotoxin microcystin binds to proteins and increases the fitness of Microcystis under oxidative stress conditions. PLoS ONE 2011, 6, e17615. [Google Scholar] [CrossRef] [PubMed]
- Gobler, C.J.; Harke, M.J.; Burkholder, J.M.; Davis, T.W.; Stow, C.A.; Johengen, T.; Van de Waal, D.B. The dual role of nitrogen supply in controlling the growth and toxicity of cyanobacterial blooms. Harmful Algae 2016, 54, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Muro-Pastor, M.I.; Reyes, J.C.; Florencio, F.J. Cyanobacteria perceive nitrogen status by sensing intracellular 2-Oxoglutarate levels. J. Biol. Chem. 2001, 276, 38320–38328. [Google Scholar] [PubMed]
- Tandeau De Marsac, N.; Lee, H.M.; Hisbergues, M.; Castets, A.M.; Bédu, S. Control of nitrogen and carbon metabolism in cyanobacteria. J. Appl. Phycol. 2001, 13, 287–292. [Google Scholar] [CrossRef]
- Huergo, L.F.; Dixon, R. The emergence of 2-Oxoglutarate as a master regulator metabolite. Microbiol. Mol. Biol. Rev. 2015, 79, 419–435. [Google Scholar] [CrossRef]
- Krasikov, V.; Aguirre von Wobeser, E.; Dekker, H.L.; Huisman, J.; Matthijs, H.C.P. Time-series resolution of gradual nitrogen starvation and its impact on photosynthesis in the cyanobacterium Synechocystis PCC 6803. Physiol. Plant. 2012, 145, 426–439. [Google Scholar] [CrossRef]
- Hagemann, M.; Lockau, W.; Forchhammer, K.; Ziegler, K.; Maheswaran, M. PII-regulated arginine synthesis controls accumulation of cyanophycin in Synechocystis sp. strain PCC 6803. J. Bacteriol. 2006, 188, 2730–2734. [Google Scholar]
- Lee, S.; Jang, M.-H.; Kim, H.-S.; Yoon, B.-D.; Oh, H.-M. Variation of microcystin content of Microcystis aeruginosa relative to medium N:P ratio and growth stage. J. Appl. Microbiol. 2000, 89, 323–329. [Google Scholar] [CrossRef]
- Saxton, M.A.; Arnold, R.J.; Bourbonniere, R.A.; McKay, R.M.L.; Wilhelm, S.W. Plasticity of total and intracellular phosphorus quotas in Microcystis aeruginosa cultures and Lake Erie algal assemblages. Front. Microbiol. 2012, 3, 1–9. [Google Scholar] [CrossRef]
- Redfield, A.C. The biological control of chemical factors in the environment. Am. Sci. 1958, 46, 205–221. [Google Scholar]
- Guildford, S.J.; Hecky, R.E. Total nitrogen, total phosphorus, and nutrient limitation in lakes and oceans: Is there a common relationship? Limnol. Oceanogr. 2000, 45, 1213–1223. [Google Scholar] [CrossRef]
- Orihel, D.M.; Bird, D.F.; Brylinsky, M.; Chen, H.; Donald, D.B.; Huang, D.Y.; Giani, A.; Kinniburgh, D.; Kling, H.; Kotak, B.G.; et al. High microcystin concentrations occur only at low nitrogen-to-phosphorus ratios in nutrient-rich Canadian lakes. Can. J. Fish. Aquat. Sci. 2012, 69, 1457–1462. [Google Scholar] [CrossRef]
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. Wiley Interdiscip. Rev. Water 2019, 6, e1373. [Google Scholar] [CrossRef]
- Scott, J.T.; McCarthy, M.J.; Paerl, H.W. Nitrogen transformations differentially affect nutrient-limited primary production in lakes of varying trophic state. Limnol. Oceanogr. Lett. 2019, 4, 96–104. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 1992. [Google Scholar]
- Haddad, S.P.; Bobbitt, J.M.; Taylor, R.B.; Lovin, L.M.; Conkle, J.L.; Chambliss, C.K.; Brooks, B.W. Determination of microcystins, nodularin, anatoxin-a, cylindrospermopsin, and saxitoxin in water and fish tissue using isotope dilution liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2019, 1599, 66–74. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Falster, D.; Warton, D.; Wright, I. Standardised Major Axis Tests and Routines, Ver 2.0. 2006. [Google Scholar]
- Warton, D.; Wright, I.; Falster, D.; Westoby, M. Bivariate line-fitting methods for allometry. Biol. Rev. 2006, 81, 259–291. [Google Scholar] [CrossRef]
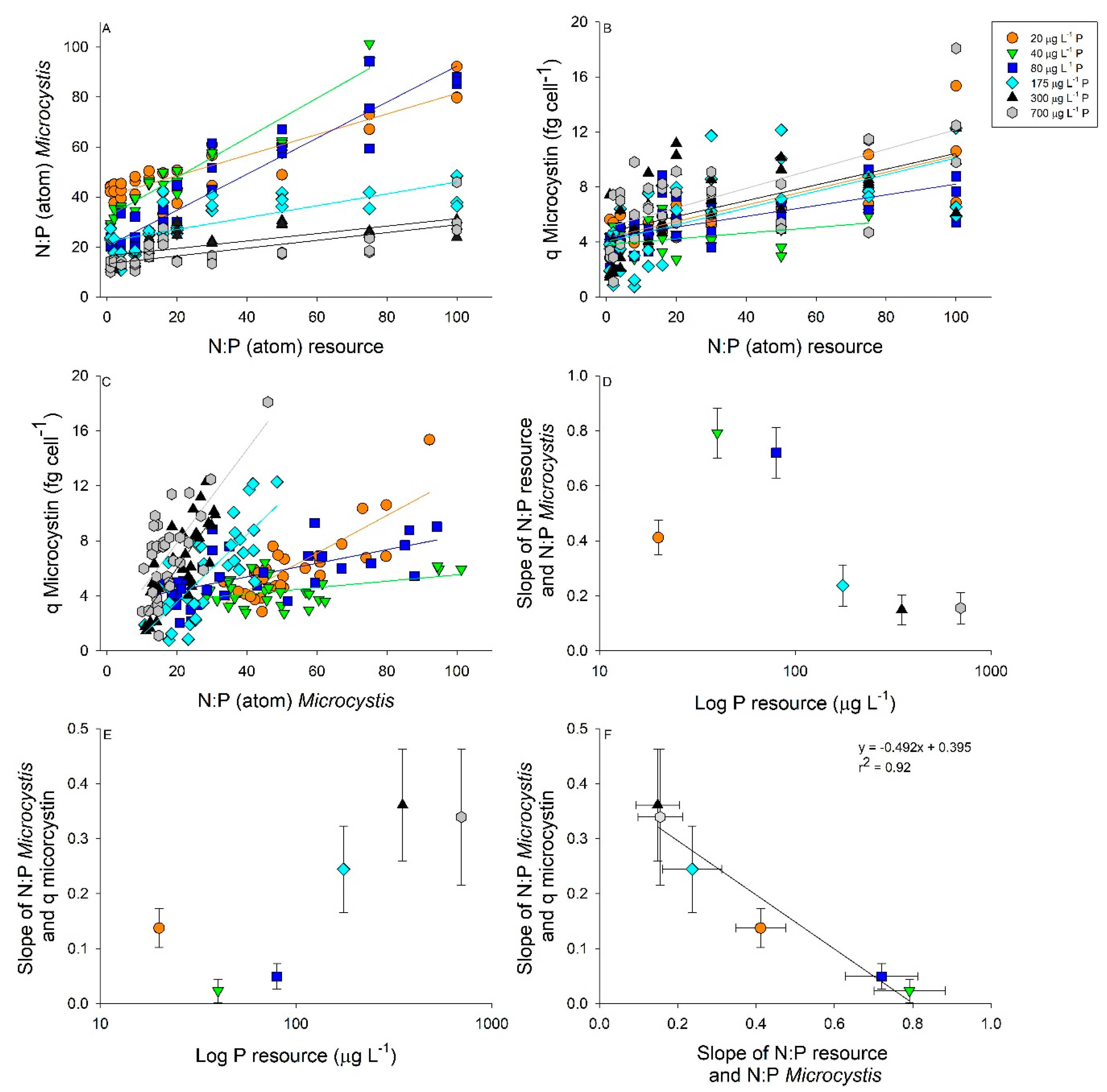
| Regressions for Resource and Microcystis N:P as Shown in Figure 4A | ||||
| P µg L−1 | Equation | r2 | p-Value | Slope Comparisons |
| 20 | y = 0.412x + 40.27 | 0.84 | <0.0001 | a |
| 40 | y = 0.792x + 31.99 | 0.92 | <0.0001 | b |
| 80 | y = 0.721x + 20.29 | 0.89 | <0.0001 | b |
| 175 | y = 0.237x + 22.26 | 0.56 | <0.0001 | c |
| 350 | y = 0.149x + 16.44 | 0.49 | <0.0001 | c |
| 700 | y = 0.156x + 13.31 | 0.48 | <0.0001 | c |
| Regressions for Resource N:P and Microcystin Cell Quota as Shown in Figure 4B | ||||
| P µg L−1 | Equation | r2 | p-Value | Slope Comparisons |
| 20 | y = 0.060x + 4.29 | 0.62 | <0.0001 | a |
| 40 | y = 0.021x + 3.81 | 0.14 | 0.0232 | b |
| 80 | y = 0.039x + 4.30 | 0.39 | <0.0001 | ab |
| 175 | y = 0.061x + 4.03 | 0.33 | 0.0003 | a |
| 350 | y = 0.057 + 4.70 | 0.35 | 0.0002 | a |
| 700 | y = 0.071 + 5.06 | 0.44 | <0.0001 | a |
| Regressions for Microcystis N:P and Microcystin Cell Quota as Shown in Figure 4C | ||||
| P µg L−1 | Equation | r2 | p-Value | Slope Comparisons |
| 20 | y = 0.137x − 1.15 | 0.66 | <0.0001 | a |
| 40 | y = 0.023x + 3.20 | 0.13 | 0.0322 | b |
| 80 | y = 0.049x + 3.40 | 0.36 | <0.0001 | b |
| 175 | y = 0.244x − 1.33 | 0.55 | <0.0001 | c |
| 350 | y = 0.361x − 1.20 | 0.62 | <0.0001 | c |
| 700 | y = 0.339x + 1.07 | 0.49 | <0.0001 | c |
| Factor | Df | F-Value | p-Value | ||||||||
| N:P resource | 10 | 16.83 | <0.0001 | ||||||||
| P-level | 5 | 6.44 | <0.0001 | ||||||||
| N:P × P-level | 49 | 1.51 | 0.035 | ||||||||
| Residuals | 128 | ||||||||||
| Tukey Post Hoc by P Resource | |||||||||||
| 1 | 2 | 4 | 8 | 12 | 16 | 20 | 30 | 50 | 75 | 100 | |
| 20 | a | a | a | ab | a | a | a | ab | ab | a | a |
| 40 | a | a | a | ab | a | a | a | a | a | a | |
| 80 | a | a | a | ab | a | a | a | ab | ab | a | a |
| 175 | a | a | a | a | a | a | ab | b | b | a | a |
| 350 | a | a | a | ab | a | a | b | ab | b | a | a |
| 700 | a | a | a | b | a | a | ab | b | ab | a | a |
| Tukey Post Hoc by N:P Resource within P Resource Level | |||||||||||
| 20 | 40 | 80 | 175 | 350 | 700 | ||||||
| 1 | a | a | a | ac | a | a | |||||
| 2 | a | a | a | abc | a | a | |||||
| 4 | ab | a | ab | abc | a | ab | |||||
| 8 | a | a | ab | ab | ab | ab | |||||
| 12 | ab | a | ab | abc | ab | ab | |||||
| 16 | ab | a | ab | abc | ab | ab | |||||
| 20 | ab | a | ab | abc | b | ab | |||||
| 30 | ab | a | ab | abc | ab | ab | |||||
| 50 | ab | a | ab | bc | ab | ab | |||||
| 75 | b | a | b | abc | ab | b | |||||
| 100 | b | b | abc | b | b | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, N.D.; Osburn, F.S.; Wang, J.; Taylor, R.B.; Boedecker, A.R.; Chambliss, C.K.; Brooks, B.W.; Scott, J.T. Biological Stoichiometry Regulates Toxin Production in Microcystis aeruginosa (UTEX 2385). Toxins 2019, 11, 601. https://doi.org/10.3390/toxins11100601
Wagner ND, Osburn FS, Wang J, Taylor RB, Boedecker AR, Chambliss CK, Brooks BW, Scott JT. Biological Stoichiometry Regulates Toxin Production in Microcystis aeruginosa (UTEX 2385). Toxins. 2019; 11(10):601. https://doi.org/10.3390/toxins11100601
Chicago/Turabian StyleWagner, Nicole D., Felicia S. Osburn, Jingyu Wang, Raegyn B. Taylor, Ashlynn R. Boedecker, C. Kevin Chambliss, Bryan W. Brooks, and J. Thad Scott. 2019. "Biological Stoichiometry Regulates Toxin Production in Microcystis aeruginosa (UTEX 2385)" Toxins 11, no. 10: 601. https://doi.org/10.3390/toxins11100601
APA StyleWagner, N. D., Osburn, F. S., Wang, J., Taylor, R. B., Boedecker, A. R., Chambliss, C. K., Brooks, B. W., & Scott, J. T. (2019). Biological Stoichiometry Regulates Toxin Production in Microcystis aeruginosa (UTEX 2385). Toxins, 11(10), 601. https://doi.org/10.3390/toxins11100601





