Advanced Proteomics as a Powerful Tool for Studying Toxins of Human Bacterial Pathogens
Abstract
1. Introduction
2. Bacterial Exotoxins, the Key Arsenal of Pathogens
2.1. Enterotoxins Modulating Signal Transduction Pathways
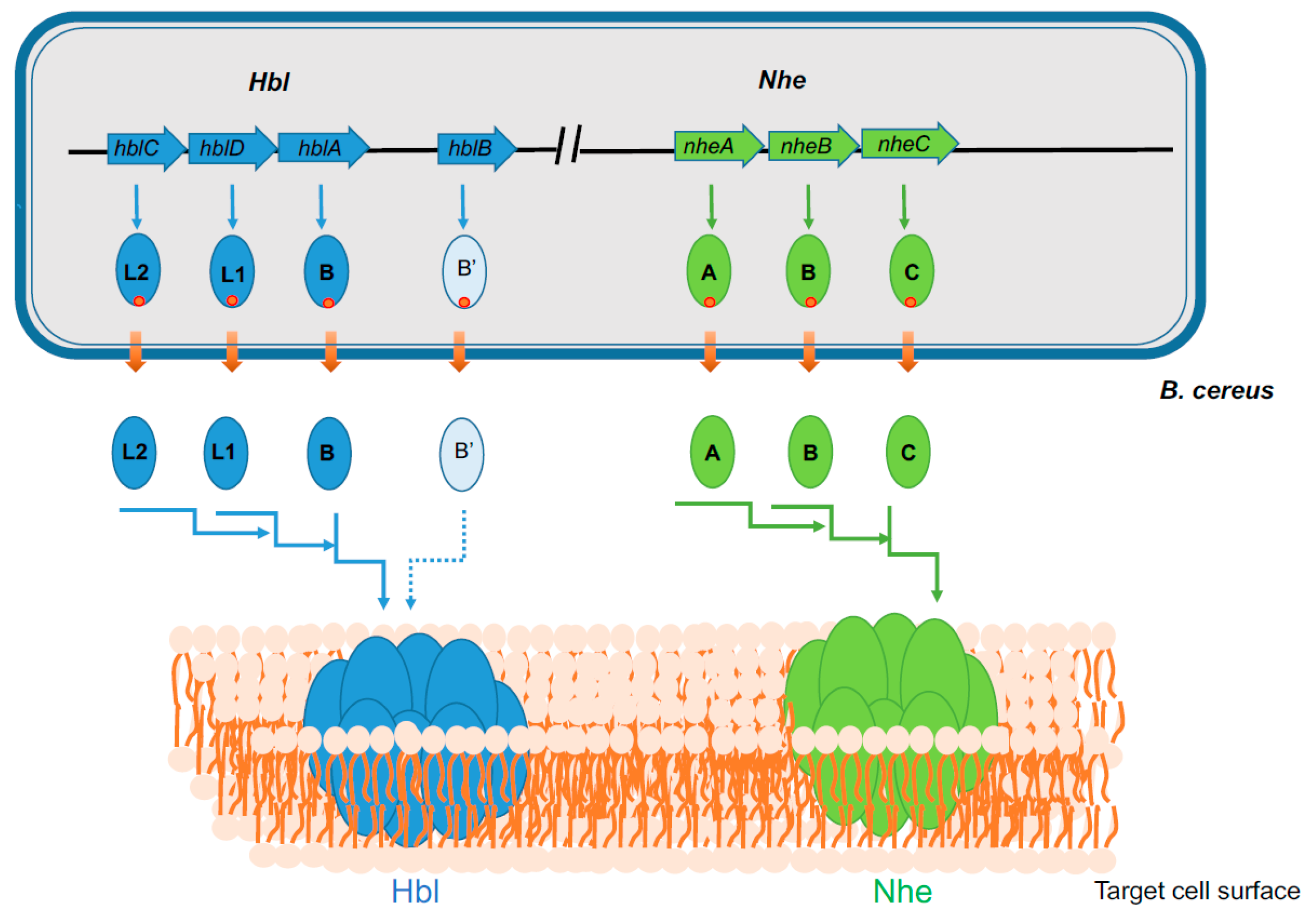
2.2. Membrane Damaging Toxins
2.3. AB Toxins
2.4. Toxins Injected via Secretion Systems
3. Next-Generation Proteomics, a Powerful Toolbox to Study Bacterial Toxins
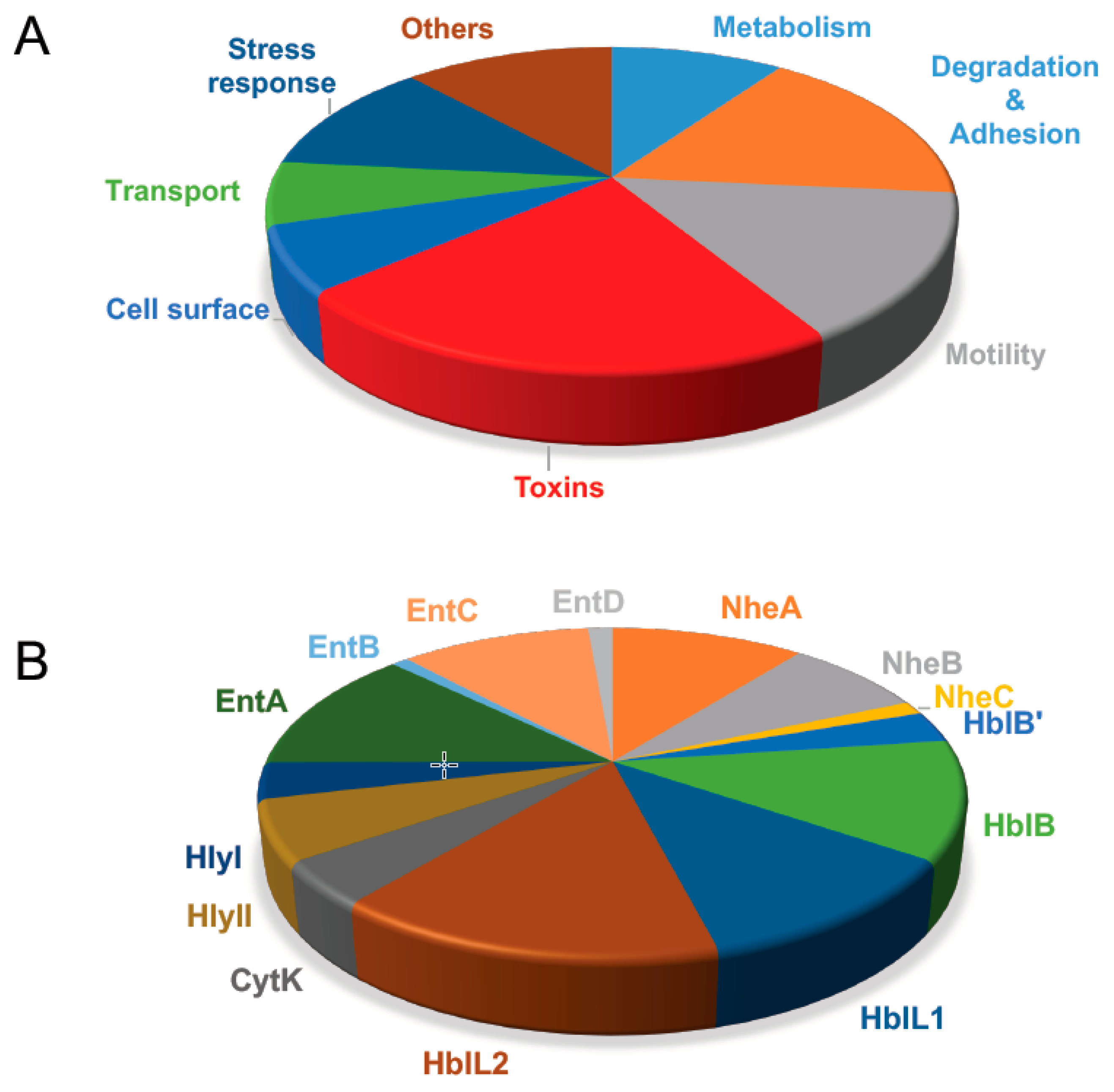
3.1. Proteomics Potential for Toxin Detection
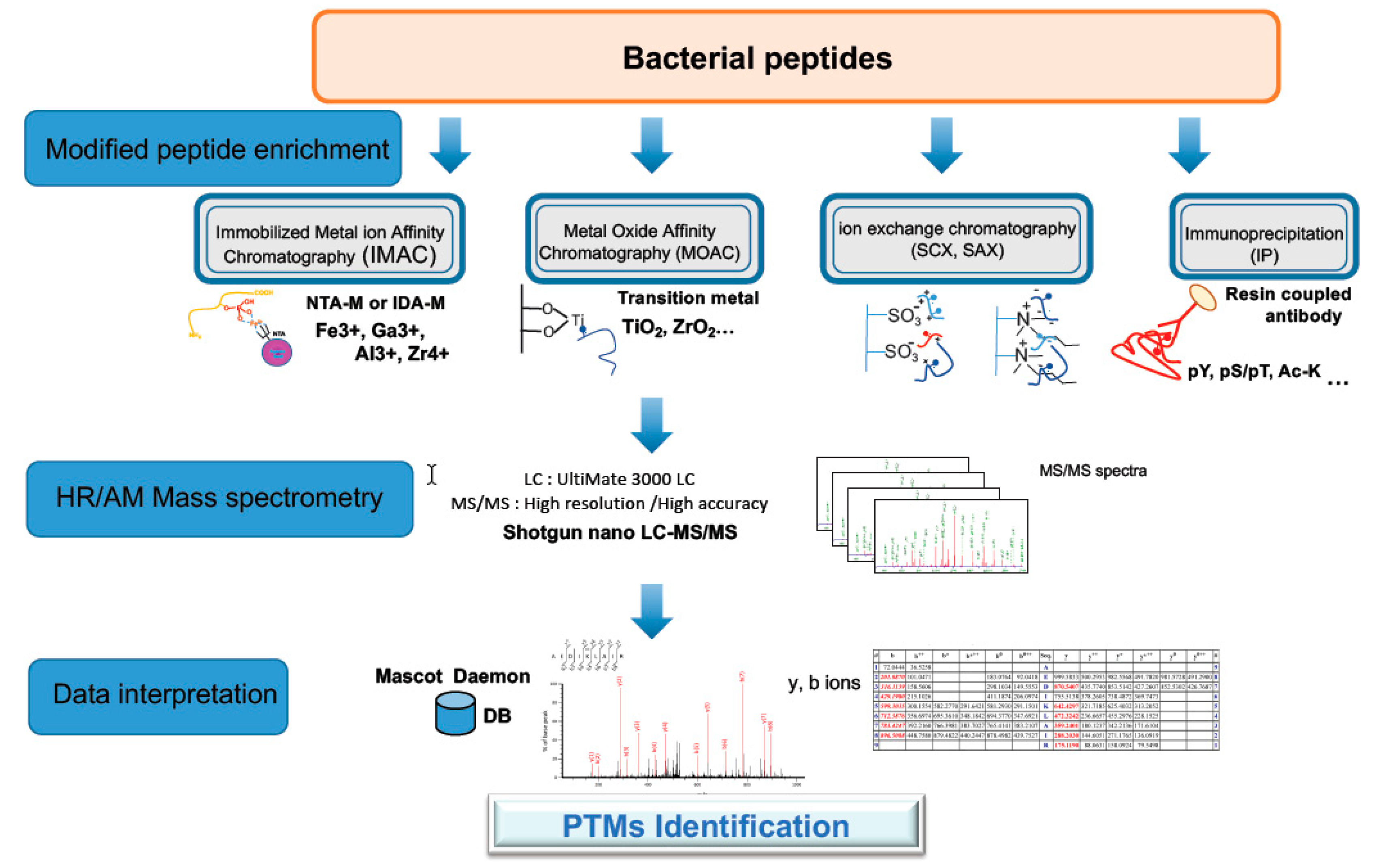
3.2. Proteomics Analyses for Detection of Post-Translational Modifications (PTMs)
3.3. Protein Oxidation
3.4. Protein S-Thiolation
3.5. Protein Phosphorylation
3.6. Protein Acetylation
3.7. Emerging Topics
4. Proteogenomics, Metaproteomics, and New Tools for a More Integrated Vision of Bacterial Toxins
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Cavaillon, J.M. Exotoxins and endotoxins: Inducers of inflammatory cytokines. Toxicon 2018, 149, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Yang, Y.; Zhang, J.R. Molecular basis of host specificity in human pathogenic bacteria. Emerg. Microbes Infect. 2014, 3, e23. [Google Scholar] [CrossRef] [PubMed]
- Tauxe, R.V. Emerging foodborne pathogens. Int. J. Food Microbiol. 2002, 78, 31–41. [Google Scholar] [CrossRef]
- Rudkin, J.K.; McLoughlin, R.M.; Preston, A.; Massey, R.C. Bacterial toxins: Offensive, defensive, or something else altogether? PLoS Pathog. 2017, 13, e1006452. [Google Scholar] [CrossRef] [PubMed]
- Jackie, J.; Lau, W.K.; Feng, H.T.; Li, S.F.Y. Detection of Endotoxins: From Inferring the Responses of Biological Hosts to the Direct Chemical Analysis of Lipopolysaccharides. Crit. Rev. Anal. Chem. 2019, 49, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Okano, S.; Kasai, N. Inactivation of Escherichia coli endotoxin by soft hydrothermal processing. Appl. Environ. Microbiol. 2009, 75, 5058–5063. [Google Scholar] [CrossRef] [PubMed]
- Popoff, M.R. Bacterial exotoxins. Contrib. Microbiol. 2005, 12, 28–54. [Google Scholar] [CrossRef]
- Martinovic, T.; Andjelkovic, U.; Gajdosik, M.S.; Resetar, D.; Josic, D. Foodborne pathogens and their toxins. J. Proteom. 2016, 147, 226–235. [Google Scholar] [CrossRef]
- Chakraborty, A.; Ghosh, S.; Chowdhary, G.; Maulik, U.; Chakrabarti, S. DBETH: A Database of Bacterial Exotoxins for Human. Nucleic Acids Res. 2012, 40, D615–D620. [Google Scholar] [CrossRef]
- Luna, V.A.; King, D.S.; Peak, K.K.; Reeves, F.; Heberlein-Larson, L.; Veguilla, W.; Heller, L.; Duncan, K.E.; Cannons, A.C.; Amuso, P.; et al. Bacillus anthracis virulent plasmid pX02 genes found in large plasmids of two other Bacillus species. J. Clin. Microbiol. 2006, 44, 2367–2377. [Google Scholar] [CrossRef]
- Sengupta, M.; Austin, S. Prevalence and significance of plasmid maintenance functions in the virulence plasmids of pathogenic bacteria. Infect. Immun. 2011, 79, 2502–2509. [Google Scholar] [CrossRef] [PubMed]
- Armengaud, J.; Christie-Oleza, J.A.; Clair, G.; Malard, V.; Duport, C. Exoproteomics: Exploring the world around biological systems. Expert Rev. Proteom. 2012, 9, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Lemichez, E.; Barbieri, J.T. General aspects and recent advances on bacterial protein toxins. Cold Spring Harb. Perspect. Med. 2013, 3, a013573. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.J. Enterotoxins. In Veterinary Toxicology Basic and Clinical Principles, 3rd ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 759–761. [Google Scholar] [CrossRef]
- Pettersen, V.K.; Steinsland, H.; Wiker, H.G. Comparative Proteomics of Enterotoxigenic Escherichia coli Reveals Differences in Surface Protein Production and Similarities in Metabolism. J. Proteome Res. 2018, 17, 325–336. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, Z.; Luo, Y.; Cox, E.; Devriendt, B. Heat-Stable Enterotoxins of Enterotoxigenic Escherichia coli and Their Impact on Host Immunity. Toxins 2019, 11, 24. [Google Scholar] [CrossRef]
- Batisson, I.; Der Vartanian, M.; Gaillard-Martinie, B.; Contrepois, M. Full capacity of recombinant Escherichia coli heat-stable enterotoxin fusion proteins for extracellular secretion, antigenicity, disulfide bond formation, and activity. Infect. Immun. 2000, 68, 4064–4074. [Google Scholar] [CrossRef]
- Dreyfus, L.A.; Harville, B.; Howard, D.E.; Shaban, R.; Beatty, D.M.; Morris, S.J. Calcium influx mediated by the Escherichia coli heat-stable enterotoxin B (STB). Proc. Natl. Acad. Sci. USA 1993, 90, 3202–3206. [Google Scholar] [CrossRef]
- Oda, M.; Terao, Y.; Sakurai, J.; Nagahama, M. Membrane-Binding Mechanism of Clostridium perfringens Alpha-Toxin. Toxins 2015, 7, 5268–5275. [Google Scholar] [CrossRef]
- Agarwal, S.; Kim, H.; Chan, R.B.; Agarwal, S.; Williamson, R.; Cho, W.; Paolo, G.D.; Satchell, K.J. Autophagy and endosomal trafficking inhibition by Vibrio cholerae MARTX toxin phosphatidylinositol-3-phosphate-specific phospholipase A1 activity. Nat. Commun. 2015, 6, 8745. [Google Scholar] [CrossRef]
- Gonzalez-Bullon, D.; Uribe, K.B.; Martin, C.; Ostolaza, H. Phospholipase A activity of adenylate cyclase toxin mediates translocation of its adenylate cyclase domain. Proc. Natl. Acad. Sci. USA 2017, 114, E6784–E6793. [Google Scholar] [CrossRef]
- Escajadillo, T.; Nizet, V. Pharmacological Targeting of Pore-Forming Toxins as Adjunctive Therapy for Invasive Bacterial Infection. Toxins 2018, 10, 542. [Google Scholar] [CrossRef] [PubMed]
- Dal Peraro, M.; Van Der Goot, F.G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. Microbiol. 2016, 14, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, S.; Pulagam, L.P.; Steinhoff, H.J.; Klare, J.P. In Vivo EPR on spin labeled colicin A reveals an oligomeric assembly of the pore-forming domain in E. coli membranes. Phys. Chem. Chem. Phys. PCCP 2015, 17, 4875–4878. [Google Scholar] [CrossRef] [PubMed]
- Ridley, H.; Johnson, C.L.; Lakey, J.H. Interfacial interactions of pore-forming colicins. Adv. Exp. Med. Biol. 2010, 677, 81–90. [Google Scholar] [CrossRef]
- Brauning, B.; Groll, M. Structural and Mechanistic Features of ClyA-Like alpha-Pore-Forming Toxins. Toxins 2018, 10, 343. [Google Scholar] [CrossRef]
- Hunt, S.; Green, J.; Artymiuk, P.J. Hemolysin E (HlyE, ClyA, SheA) and related toxins. Adv. Exp. Med. Biol. 2010, 677, 116–126. [Google Scholar] [CrossRef]
- Roderer, D.; Glockshuber, R. Assembly mechanism of the alpha-pore-forming toxin cytolysin A from Escherichia coli. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2017, 372, 20160211. [Google Scholar] [CrossRef]
- Wagner, N.J.; Lin, C.P.; Borst, L.B.; Miller, V.L. YaxAB, a Yersinia enterocolitica pore-forming toxin regulated by RovA. Infect. Immun. 2013, 81, 4208–4219. [Google Scholar] [CrossRef]
- Stenfors Arnesen, L.P.; Fagerlund, A.; Granum, P.E. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 2008, 32, 579–606. [Google Scholar] [CrossRef]
- Jessberger, N.; Dietrich, R.; Schwemmer, S.; Tausch, F.; Schwenk, V.; Didier, A.; Martlbauer, E. Binding to The Target Cell Surface Is The Crucial Step in Pore Formation of Hemolysin BL from Bacillus cereus. Toxins 2019, 11, 281. [Google Scholar] [CrossRef]
- Sastalla, I.; Fattah, R.; Coppage, N.; Nandy, P.; Crown, D.; Pomerantsev, A.P.; Leppla, S.H. The Bacillus cereus Hbl and Nhe tripartite enterotoxin components assemble sequentially on the surface of target cells and are not interchangeable. PLoS ONE 2013, 8, e76955. [Google Scholar] [CrossRef]
- Granum, P.E.; O’Sullivan, K.; Lund, T. The sequence of the non-haemolytic enterotoxin operon from Bacillus cereus. FEMS Microbiol. Lett. 1999, 177, 225–229. [Google Scholar] [CrossRef]
- Ryan, P.A.; Macmillan, J.D.; Zilinskas, B.A. Molecular cloning and characterization of the genes encoding the L1 and L2 components of hemolysin BL from Bacillus cereus. J. Bacteriol. 1997, 179, 2551–2556. [Google Scholar] [CrossRef]
- Lindback, T.; Hardy, S.P.; Dietrich, R.; Sodring, M.; Didier, A.; Moravek, M.; Fagerlund, A.; Bock, S.; Nielsen, C.; Casteel, M.; et al. Cytotoxicity of the Bacillus cereus Nhe enterotoxin requires specific binding order of its three exoprotein components. Infect. Immun. 2010, 78, 3813–3821. [Google Scholar] [CrossRef]
- Zhu, K.; Didier, A.; Dietrich, R.; Heilkenbrinker, U.; Waltenberger, E.; Jessberger, N.; Martlbauer, E.; Benz, R. Formation of small transmembrane pores: An intermediate stage on the way to Bacillus cereus non-hemolytic enterotoxin (Nhe) full pores in the absence of NheA. Biochem. Biophys. Res. Commun. 2016, 469, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Ganash, M.; Phung, D.; Sedelnikova, S.E.; Lindback, T.; Granum, P.E.; Artymiuk, P.J. Structure of the NheA component of the Nhe toxin from Bacillus cereus: Implications for function. PLoS ONE 2013, 8, e74748. [Google Scholar] [CrossRef] [PubMed]
- Madegowda, M.; Eswaramoorthy, S.; Burley, S.K.; Swaminathan, S. X-ray crystal structure of the B component of Hemolysin BL from Bacillus cereus. Proteins 2008, 71, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Aaij, R.; Adeva, B.; Adinolfi, M.; Affolder, A.; Ajaltouni, Z.; Akar, S.; Albrecht, J.; Alessio, F.; Alexander, M.; Ali, S.; et al. Precision measurement of the mass and lifetime of the Xi(b)(0) baryon. Phys. Rev. Lett. 2014, 113, 032001. [Google Scholar] [CrossRef]
- Sugawara, T.; Yamashita, D.; Kato, K.; Peng, Z.; Ueda, J.; Kaneko, J.; Kamio, Y.; Tanaka, Y.; Yao, M. Structural basis for pore-forming mechanism of staphylococcal alpha-hemolysin. Toxicon 2015, 108, 226–231. [Google Scholar] [CrossRef]
- Yamashita, D.; Sugawara, T.; Takeshita, M.; Kaneko, J.; Kamio, Y.; Tanaka, I.; Tanaka, Y.; Yao, M. Molecular basis of transmembrane beta-barrel formation of staphylococcal pore-forming toxins. Nat. Commun. 2014, 5, 4897. [Google Scholar] [CrossRef]
- Lund, T.; De Buyser, M.L.; Granum, P.E. A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol. Microbiol. 2000, 38, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Baida, G.; Budarina, Z.I.; Kuzmin, N.P.; Solonin, A.S. Complete nucleotide sequence and molecular characterization of hemolysin II gene from Bacillus cereus. FEMS Microbiol. Lett. 1999, 180, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.R.; Kaus, K.; De, S.; Olson, R.; Alexandrescu, A.T. NMR structure of the Bacillus cereus hemolysin II C-terminal domain reveals a novel fold. Sci. Rep. 2017, 7, 3277. [Google Scholar] [CrossRef] [PubMed]
- Cirauqui, N.; Abriata, L.A.; van der Goot, F.G.; Dal Peraro, M. Structural, physicochemical and dynamic features conserved within the aerolysin pore-forming toxin family. Sci. Rep. 2017, 7, 13932. [Google Scholar] [CrossRef]
- Heuck, A.P.; Moe, P.C.; Johnson, B.B. The cholesterol-dependent cytolysin family of gram-positive bacterial toxins. Sub-Cell. Biochem. 2010, 51, 551–577. [Google Scholar] [CrossRef]
- Hotze, E.M.; Tweten, R.K. Membrane assembly of the cholesterol-dependent cytolysin pore complex. Biochim. Biophys. Acta 2012, 1818, 1028–1038. [Google Scholar] [CrossRef]
- Maurer, J.; Hupp, S.; Bischoff, C.; Foertsch, C.; Mitchell, T.J.; Chakraborty, T.; Iliev, A.I. Distinct Neurotoxicity Profile of Listeriolysin O from Listeria monocytogenes. Toxins 2017, 9, 34. [Google Scholar] [CrossRef]
- Dang, T.X.; Milligan, R.A.; Tweten, R.K.; Wilson-Kubalek, E.M. Helical crystallization on nickel-lipid nanotubes: Perfringolysin O as a model protein. J. Struct. Biol. 2005, 152, 129–139. [Google Scholar] [CrossRef]
- Nakouzi, A.; Rivera, J.; Rest, R.F.; Casadevall, A. Passive administration of monoclonal antibodies to anthrolysin O prolong survival in mice lethally infected with Bacillus anthracis. BMC Microbiol. 2008, 8, 159. [Google Scholar] [CrossRef]
- Kilcullen, K.; Teunis, A.; Popova, T.G.; Popov, S.G. Cytotoxic Potential of Bacillus cereus Strains ATCC 11778 and 14579 Against Human Lung Epithelial Cells Under Microaerobic Growth Conditions. Front. Microbiol. 2016, 7, 69. [Google Scholar] [CrossRef]
- Ramarao, N.; Sanchis, V. The pore-forming haemolysins of Bacillus cereus: A review. Toxins 2013, 5, 1119–1139. [Google Scholar] [CrossRef] [PubMed]
- Ostolaza, H.; Gonzalez-Bullon, D.; Uribe, K.B.; Martin, C.; Amuategi, J.; Fernandez-Martinez, X. Membrane Permeabilization by Pore-Forming RTX Toxins: What Kind of Lesions Do These Toxins Form? Toxins 2019, 11, 354. [Google Scholar] [CrossRef]
- Wiles, T.J.; Mulvey, M.A. The RTX pore-forming toxin alpha-hemolysin of uropathogenic Escherichia coli: Progress and perspectives. Future Microbiol. 2013, 8, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Odumosu, O.; Nicholas, D.; Yano, H.; Langridge, W. AB toxins: A paradigm switch from deadly to desirable. Toxins 2010, 2, 1612–1645. [Google Scholar] [CrossRef]
- Simon, N.C.; Aktories, K.; Barbieri, J.T. Novel bacterial ADP-ribosylating toxins: Structure and function. Nat. Rev. Microbiol. 2014, 12, 599–611. [Google Scholar] [CrossRef]
- Friebe, S.; van der Goot, F.G.; Burgi, J. The Ins and Outs of Anthrax Toxin. Toxins 2016, 8, 69. [Google Scholar] [CrossRef]
- Gaytan, M.O.; Martinez-Santos, V.I.; Soto, E.; Gonzalez-Pedrajo, B. Type Three Secretion System in Attaching and Effacing Pathogens. Front. Cell. Infect. Microbiol. 2016, 6, 129. [Google Scholar] [CrossRef]
- Abby, S.S.; Rocha, E.P. The non-flagellar type III secretion system evolved from the bacterial flagellum and diversified into host-cell adapted systems. PLoS Genet. 2012, 8, e1002983. [Google Scholar] [CrossRef]
- Bayer-Santos, E.; Cenens, W.; Matsuyama, B.Y.; Oka, G.U.; Di Sessa, G.; Mininel, I.D.V.; Alves, T.L.; Farah, C.S. The opportunistic pathogen Stenotrophomonas maltophilia utilizes a type IV secretion system for interbacterial killing. PLoS Pathog. 2019, 15, e1007651. [Google Scholar] [CrossRef]
- Davidson, A.R.; Maxwell, K.L. Type VI secretion system baseplate. Nat. Microbiol. 2018, 3, 1330–1331. [Google Scholar] [CrossRef]
- Hachani, A.; Wood, T.E.; Filloux, A. Type VI secretion and anti-host effectors. Curr. Opin. Microbiol. 2016, 29, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Garcia, F.; Ruiz-Perez, F.; Cataldi, A.; Larzabal, M. Type VI Secretion System in Pathogenic Escherichia coli: Structure, Role in Virulence, and Acquisition. Front. Microbiol. 2019, 10, 1965. [Google Scholar] [CrossRef] [PubMed]
- Gohar, M.; Okstad, O.A.; Gilois, N.; Sanchis, V.; Kolsto, A.B.; Lereclus, D. Two-dimensional electrophoresis analysis of the extracellular proteome of Bacillus cereus reveals the importance of the PlcR regulon. Proteomics 2002, 2, 784–791. [Google Scholar] [CrossRef]
- Gohar, M.; Gilois, N.; Graveline, R.; Garreau, C.; Sanchis, V.; Lereclus, D. A comparative study of Bacillus cereus, Bacillus thuringiensis and Bacillus anthracis extracellular proteomes. Proteomics. 2005, 5, 3696–3711. [Google Scholar] [CrossRef] [PubMed]
- Gilois, N.; Ramarao, N.; Bouillaut, L.; Perchat, S.; Aymerich, S.; Nielsen-Leroux, C.; Lereclus, D.; Gohar, M. Growth-related variations in the Bacillus cereus secretome. Proteomics 2007, 7, 1719–1728. [Google Scholar] [CrossRef]
- Clair, G.; Roussi, S.; Armengaud, J.; Duport, C. Expanding the known repertoire of virulence factors produced by Bacillus cereus through early secretome profiling in three redox conditions. Mol. Cell. Proteom. 2010, 9, 1486–1498. [Google Scholar] [CrossRef]
- Madeira, J.P.; Alpha-Bazin, B.; Armengaud, J.; Duport, C. Time dynamics of the Bacillus cereus exoproteome are shaped by cellular oxidation. Front. Microbiol. 2015, 6, 342. [Google Scholar] [CrossRef]
- Madeira, J.P.; Omer, H.; Alpha-Bazin, B.; Armengaud, J.; Duport, C. Cellular and exoproteome dynamics of Bacillus cereus ATCC 14579 with and without pBClin15. Data Brief 2016. submitted. [Google Scholar]
- Didier, A.; Dietrich, R.; Martlbauer, E. Antibody Binding Studies Reveal Conformational Flexibility of the Bacillus cereus Non-Hemolytic Enterotoxin (Nhe) A-Component. PLoS ONE 2016, 11, e0165135. [Google Scholar] [CrossRef]
- Lindback, T.; Fagerlund, A.; Rodland, M.S.; Granum, P.E. Characterization of the Bacillus cereus Nhe enterotoxin. Microbiology 2004, 150, 3959–3967. [Google Scholar] [CrossRef]
- Omer, H.; Alpha-Bazin, B.; Brunet, J.L.; Armengaud, J.; Duport, C. Proteomics identifies Bacillus cereus EntD as a pivotal protein for the production of numerous virulence factors. Front. Microbiol. 2015, 6, 1004. [Google Scholar] [CrossRef] [PubMed]
- Ebner, P.; Rinker, J.; Gotz, F. Excretion of cytoplasmic proteins in Staphylococcus is most likely not due to cell lysis. Curr. Genet. 2016, 62, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Gotz, F.; Yu, W.; Dube, L.; Prax, M.; Ebner, P. Excretion of cytosolic proteins (ECP) in bacteria. Int. J. Med Microbiol. 2015, 305, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Clair, G.; Armengaud, J.; Duport, C. Restricting Fermentative Potential by Proteome Remodeling: An adaptative strategy evidenced in Bacillus cereus. Mol. Cell. Proteom. 2012, 11, M111-013102. [Google Scholar] [CrossRef] [PubMed]
- Clair, G.; Lorphelin, A.; Armengaud, J.; Duport, C. OhrRA functions as a redox-responsive system controlling toxinogenesis in Bacillus cereus. J. Proteom. 2013, 94, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Laouami, S.; Clair, G.; Armengaud, J.; Duport, C. Proteomic evidences for rex regulation of metabolism in toxin-producing Bacillus cereus ATCC 14579. PLoS ONE 2014, 9, e107354. [Google Scholar] [CrossRef][Green Version]
- Madeira, J.P.; Omer, H.; Alpha-Bazin, B.; Armengaud, J.; Duport, C. Deciphering the interactions between the Bacillus cereus linear plasmid, pBClin15, and its host by high-throughput comparative proteomics. J. Proteom. 2016, in press. [Google Scholar] [CrossRef]
- Ribet, D.; Cossart, P. Pathogen-mediated posttranslational modifications: A re-emerging field. Cell 2010, 143, 694–702. [Google Scholar] [CrossRef]
- Ribet, D.; Cossart, P. Post-translational modifications in host cells during bacterial infection. FEBS Lett. 2010, 584, 2748–2758. [Google Scholar] [CrossRef]
- Soares, N.C.; Blackburn, J.M. Mass Spectrometry Targeted Assays as a Tool to Improve Our Understanding of Post-translational Modifications in Pathogenic Bacteria. Front. Microbiol. 2016, 7, 1216. [Google Scholar] [CrossRef]
- Cain, J.A.; Solis, N.; Cordwell, S.J. Beyond gene expression: The impact of protein post-translational modifications in bacteria. J. Proteom. 2014, 97, 265–286. [Google Scholar] [CrossRef] [PubMed]
- Mijakovic, I.; Grangeasse, C.; Turgay, K. Exploring the diversity of protein modifications: Special bacterial phosphorylation systems. FEMS Microbiol. Rev. 2016, 40, 398–417. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Van Remmen, H.; Richardson, A.; Wehr, N.B.; Levine, R.L. Methionine oxidation and aging. Biochim. Biophys. Acta 2005, 1703, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Vogt, W. Oxidation of methionyl residues in proteins: Tools, targets, and reversal. Free Radic. Biol. Med. 1995, 18, 93–105. [Google Scholar] [CrossRef]
- Leonard, S.E.; Carroll, K.S. Chemical ‘omics’ approaches for understanding protein cysteine oxidation in biology. Curr. Opin. Chem. Biol. 2011, 15, 88–102. [Google Scholar] [CrossRef]
- Reardon-Robinson, M.E.; Ton-That, H. Disulfide-Bond-Forming Pathways in Gram-Positive Bacteria. J. Bacteriol. 2015, 198, 746–754. [Google Scholar] [CrossRef]
- Cowell, J.L.; Grushoff-Kosyk, P.S.; Bernheimer, A.W. Purification of cereolysin and the electrophoretic separation of the active (reduced) and inactive (oxidized) forms of the purified toxin. Infect. Immun. 1976, 14, 144–154. [Google Scholar]
- Kim, G.; Weiss, S.J.; Levine, R.L. Methionine oxidation and reduction in proteins. Biochim. Biophys. Acta 2014, 1840, 901–905. [Google Scholar] [CrossRef]
- Imber, M.; Pietrzyk-Brzezinska, A.J.; Antelmann, H. Redox regulation by reversible protein S-thiolation in Gram-positive bacteria. Redox Biol. 2019, 20, 130–145. [Google Scholar] [CrossRef]
- Chandrangsu, P.; Loi, V.V.; Antelmann, H.; Helmann, J.D. The Role of Bacillithiol in Gram-Positive Firmicutes. Antioxid. Redox Signal. 2018, 28, 445–462. [Google Scholar] [CrossRef]
- Xiong, Y.; Uys, J.D.; Tew, K.D.; Townsend, D.M. S-glutathionylation: From molecular mechanisms to health outcomes. Antioxid. Redox Signal. 2011, 15, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Portman, J.L.; Huang, Q.; Reniere, M.L.; Iavarone, A.T.; Portnoy, D.A. Activity of the Pore-Forming Virulence Factor Listeriolysin O Is Reversibly Inhibited by Naturally Occurring S-Glutathionylation. Infect. Immun. 2017, 85, e00959-16. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Sun, X.; Xiao, C.; Yin, X.; Shan, W.; Chen, Z.; He, Q.Y. Phosphoproteome analysis of the pathogenic bacterium Helicobacter pylori reveals over-representation of tyrosine phosphorylation and multiply phosphorylated proteins. Proteomics 2011, 11, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Basell, K.; Otto, A.; Junker, S.; Zuhlke, D.; Rappen, G.M.; Schmidt, S.; Hentschker, C.; Macek, B.; Ohlsen, K.; Hecker, M.; et al. The phosphoproteome and its physiological dynamics in Staphylococcus aureus. Int. J. Med. Microbiol. 2014, 304, 121–132. [Google Scholar] [CrossRef]
- Misra, S.K.; Moussan Desiree Ake, F.; Wu, Z.; Milohanic, E.; Cao, T.N.; Cossart, P.; Deutscher, J.; Monnet, V.; Archambaud, C.; Henry, C. Quantitative proteome analyses identify PrfA-responsive proteins and phosphoproteins in Listeria monocytogenes. J. Proteome Res. 2014, 13, 6046–6057. [Google Scholar] [CrossRef]
- Scholz, R.; Imami, K.; Scott, N.E.; Trimble, W.S.; Foster, L.J.; Finlay, B.B. Novel Host Proteins and Signaling Pathways in Enteropathogenic E. coli Pathogenesis Identified by Global Phosphoproteome Analysis. Mol. Cell. Proteom. 2015, 14, 1927–1945. [Google Scholar] [CrossRef]
- Ouidir, T.; Jarnier, F.; Cosette, P.; Jouenne, T.; Hardouin, J. Extracellular Ser/Thr/Tyr phosphorylated proteins of Pseudomonas aeruginosa PA14 strain. Proteomics 2014, 14, 2017–2030. [Google Scholar] [CrossRef]
- Gaviard, C.; Jouenne, T.; Hardouin, J. Proteomics of Pseudomonas aeruginosa: The increasing role of post-translational modifications. Expert Rev. Proteom. 2018, 15, 757–772. [Google Scholar] [CrossRef]
- Gaviard, C.; Broutin, I.; Cosette, P.; De, E.; Jouenne, T.; Hardouin, J. Lysine Succinylation and Acetylation in Pseudomonas aeruginosa. J. Proteome Res. 2018, 17, 2449–2459. [Google Scholar] [CrossRef]
- Christie-Oleza, J.A.; Pina-Villalonga, J.M.; Guerin, P.; Miotello, G.; Bosch, R.; Nogales, B.; Armengaud, J. Shotgun nanoLC-MS/MS proteogenomics to document MALDI-TOF biomarkers for screening new members of the Ruegeria genus. Environ. Microbiol. 2013, 15, 133–147. [Google Scholar] [CrossRef]
- Kentache, T.; Jouenne, T.; De, E.; Hardouin, J. Proteomic characterization of Nalpha- and Nepsilon-acetylation in Acinetobacter baumannii. J. Proteom. 2016, 144, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Carabetta, V.J.; Cristea, I.M. Regulation, Function, and Detection of Protein Acetylation in Bacteria. J. Bacteriol. 2017, 199, e00107-17. [Google Scholar] [CrossRef]
- Christensen, D.G.; Baumgartner, J.T.; Xie, X.; Jew, K.M.; Basisty, N.; Schilling, B.; Kuhn, M.L.; Wolfe, A.J. Mechanisms, Detection, and Relevance of Protein Acetylation in Prokaryotes. mBio 2019, 10, e02708-18. [Google Scholar] [CrossRef] [PubMed]
- Ouidir, T.; Kentache, T.; Hardouin, J. Protein lysine acetylation in bacteria: Current state of the art. Proteomics 2016, 16, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Sang, Y.; Lu, J.; Yao, Y.F. Protein Acetylation and Its Role in Bacterial Virulence. Trends Microbiol. 2017, 25, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Ouidir, T.; Cosette, P.; Jouenne, T.; Hardouin, J. Proteomic profiling of lysine acetylation in Pseudomonas aeruginosa reveals the diversity of acetylated proteins. Proteomics 2015, 15, 2152–2157. [Google Scholar] [CrossRef] [PubMed]
- Gaviard, C.; Cosette, P.; Jouenne, T.; Hardouin, J. LasB and CbpD Virulence Factors of Pseudomonas aeruginosa Carry Multiple Post-Translational Modifications on Their Lysine Residues. J. Proteome Res. 2019, 18, 923–933. [Google Scholar] [CrossRef]
- Soufi, B.; Soares, N.C.; Ravikumar, V.; Macek, B. Proteomics reveals evidence of cross-talk between protein modifications in bacteria: Focus on acetylation and phosphorylation. Curr. Opin. Microbiol. 2012, 15, 357–363. [Google Scholar] [CrossRef]
- Armengaud, J. A perfect genome annotation is within reach with the proteomics and genomics alliance. Curr. Opin. Microbiol. 2009, 12, 292–300. [Google Scholar] [CrossRef]
- Hartmann, E.M.; Armengaud, J. N-terminomics and proteogenomics, getting off to a good start. Proteomics 2014, 14, 2637–2646. [Google Scholar] [CrossRef]
- Nesvizhskii, A.I. Proteogenomics: Concepts, applications and computational strategies. Nat. Methods 2014, 11, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Ruggles, K.V.; Krug, K.; Wang, X.; Clauser, K.R.; Wang, J.; Payne, S.H.; Fenyo, D.; Zhang, B.; Mani, D.R. Methods, Tools and Current Perspectives in Proteogenomics. Mol. Cell. Proteom. 2017, 16, 959–981. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Mondal, A.K.; Kutum, R.; Dash, D. Proteogenomics of rare taxonomic phyla: A prospective treasure trove of protein coding genes. Proteomics 2016, 16, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, M. Metaproteomics: Much More than Measuring Gene Expression in Microbial Communities. mSystems 2019, 4, e00115-19. [Google Scholar] [CrossRef]
- Wilmes, P.; Heintz-Buschart, A.; Bond, P.L. A decade of metaproteomics: Where we stand and what the future holds. Proteomics 2015, 15, 3409–3417. [Google Scholar] [CrossRef]
- Armengaud, J. Next-generation proteomics faces new challenges in environmental biotechnology. Curr. Opin. Biotechnol. 2016, 38, 174–182. [Google Scholar] [CrossRef]
- Chapman, J.D.; Goodlett, D.R.; Masselon, C.D. Multiplexed and data-independent tandem mass spectrometry for global proteome profiling. Mass Spectrom. Rev. 2014, 33, 452–470. [Google Scholar] [CrossRef]
- Semanjski, M.; Macek, B. Shotgun proteomics of bacterial pathogens: Advances, challenges and clinical implications. Expert Rev. Proteom. 2016, 13, 139–156. [Google Scholar] [CrossRef]
- Gallois, N.; Alpha-Bazin, B.; Ortet, P.; Barakat, M.; Piette, L.; Long, J.; Berthomieu, C.; Armengaud, J.; Chapon, V. Proteogenomic insights into uranium tolerance of a Chernobyl’s Microbacterium bacterial isolate. J. Proteom. 2018, 177, 148–157. [Google Scholar] [CrossRef]
- Pujic, P.; Alloisio, N.; Fournier, P.; Roche, D.; Sghaier, H.; Miotello, G.; Armengaud, J.; Berry, A.M.; Normand, P. Omics of the early molecular dialogue between Frankia alni and Alnus glutinosa and the cellulase synton. Environ. Microbiol. 2019, 21, 3328–3345. [Google Scholar] [CrossRef]
- Richards, A.L.; Hebert, A.S.; Ulbrich, A.; Bailey, D.J.; Coughlin, E.E.; Westphall, M.S.; Coon, J.J. One-hour proteome analysis in yeast. Nat. Protoc. 2015, 10, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Taumer, C.; Griesbaum, L.; Kovacevic, A.; Soufi, B.; Nalpas, N.C.; Macek, B. Parallel reaction monitoring on a Q Exactive mass spectrometer increases reproducibility of phosphopeptide detection in bacterial phosphoproteomics measurements. J. Proteom. 2018, 189, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, C.; Gillet, L.; Rosenberger, G.; Amon, S.; Collins, B.C.; Aebersold, R. Data-independent acquisition-based SWATH-MS for quantitative proteomics: A tutorial. Mol. Syst. Biol. 2018, 14, e8126. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duport, C.; Alpha-Bazin, B.; Armengaud, J. Advanced Proteomics as a Powerful Tool for Studying Toxins of Human Bacterial Pathogens. Toxins 2019, 11, 576. https://doi.org/10.3390/toxins11100576
Duport C, Alpha-Bazin B, Armengaud J. Advanced Proteomics as a Powerful Tool for Studying Toxins of Human Bacterial Pathogens. Toxins. 2019; 11(10):576. https://doi.org/10.3390/toxins11100576
Chicago/Turabian StyleDuport, Catherine, Béatrice Alpha-Bazin, and Jean Armengaud. 2019. "Advanced Proteomics as a Powerful Tool for Studying Toxins of Human Bacterial Pathogens" Toxins 11, no. 10: 576. https://doi.org/10.3390/toxins11100576
APA StyleDuport, C., Alpha-Bazin, B., & Armengaud, J. (2019). Advanced Proteomics as a Powerful Tool for Studying Toxins of Human Bacterial Pathogens. Toxins, 11(10), 576. https://doi.org/10.3390/toxins11100576





