Safety and Efficacy of PrabotulinumtoxinA (Nabota®) Injection for Cervical and Shoulder Girdle Myofascial Pain Syndrome: A Pilot Study
Abstract
:1. Introduction
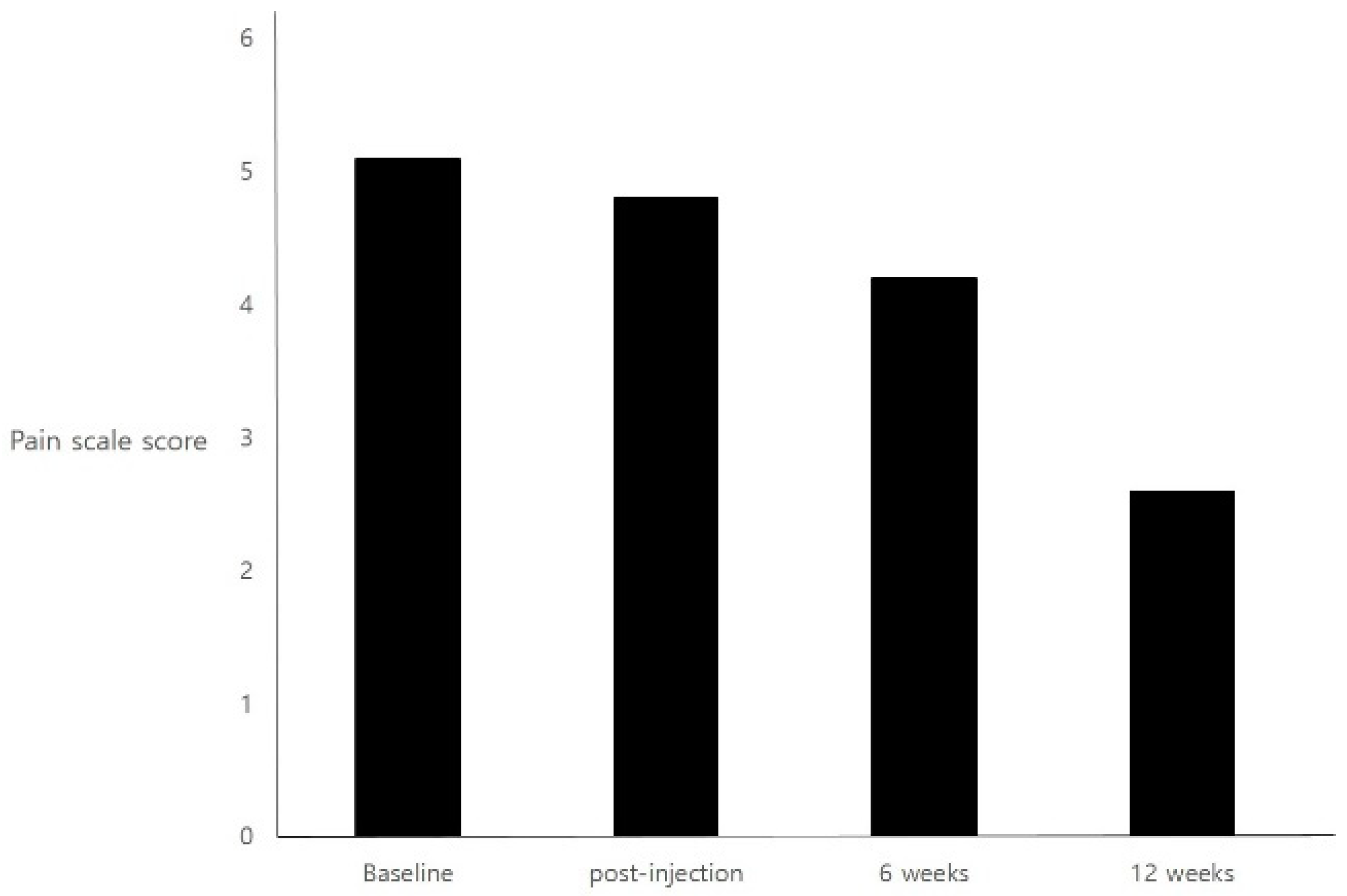
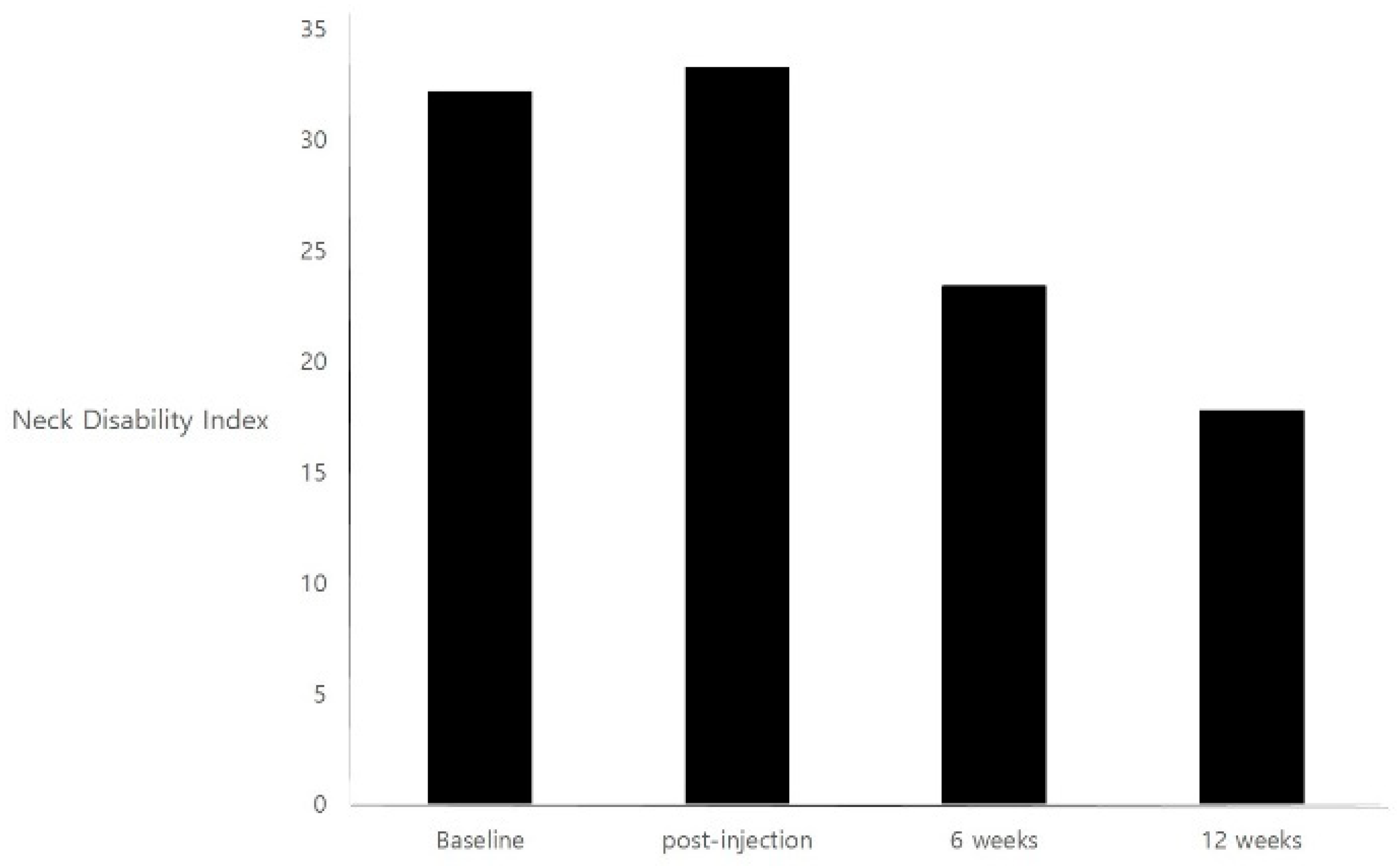
2. Results
3. Discussion
4. Study Limitations
5. Conclusions
6. Material and Methods
Subjects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borg-Stein, J.; Iaccarino, M.A. Myofascial pain syndrome treatments. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Gobel, H.; Heinze, A.; Reichel, G.; Hefter, H.; Benecke, R. Efficacy and safety of a single botulinum type A toxin complex treatment (Dysport) for the relief of upper back myofascial pain syndrome: Results from a randomized double-blind placebo-controlled multicentre study. Pain 2006, 125, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, F.M.; Bearn, L.; Rothrock, R.; King, L. Evidence against trigger point injection technique for the treatment of cervicothoracic myofascial pain with botulinum toxin type A. Anesthesiology 2005, 103, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Nicol, A.L.; Wu, I.I.; Ferrante, F.M. Botulinum toxin type a injections for cervical and shoulder girdle myofascial pain using an enriched protocol design. Anesth. Analg. 2014, 118, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Wang, D. An update on botulinum toxin a injections of trigger points for myofascial pain. Curr. Pain Headache Rep. 2014, 18, 386. [Google Scholar] [CrossRef] [PubMed]
- Won, C.H.; Kim, H.K.; Kim, B.J.; Kang, H.; Hong, J.P.; Lee, S.Y.; Kim, C.S. Comparative trial of a novel botulinum neurotoxin type A versus onabotulinumtoxinA in the treatment of glabellar lines: A multicenter, randomized, double-blind, active-controlled study. Int. J. Dermatol. 2015, 54, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.S.; Park, Y.G.; Paik, N.J.; Oh, B.M.; Chun, M.H.; Yang, H.E.; Kim, D.H.; Yi, Y.; Seo, H.G.; Kim, K.D.; et al. Efficacy and safety of NABOTA in post-stroke upper limb spasticity: A phase 3 multicenter, double-blinded, randomized controlled trial. J. Neurol. Sci. 2015, 357, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Khalifeh, M.; Mehta, K.; Varguise, N.; Suarez-Durall, P.; Enciso, R. Botulinum toxin type A for the treatment of head and neck chronic myofascial pain syndrome: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2016, 147, 959–973. [Google Scholar] [CrossRef] [PubMed]
- Graboski, C.L.; Gray, D.S.; Burnham, R.S. Botulinum toxin A versus bupivacaine trigger point injections for the treatment of myofascial pain syndrome: A randomised double blind crossover study. Pain 2005, 118, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Climent, J.M.; Kuan, T.S.; Fenollosa, P.; Martin-Del-Rosario, F. Botulinum toxin for the treatment of myofascial pain syndromes involving the neck and back: A review from a clinical perspective. Evid. Based Complement. Alternat. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.Y.; Fernandez-de-Las-Penas, C.; Yue, S.W. Myofascial trigger points: Spontaneous electrical activity and its consequences for pain induction and propagation. Chin. Med. 2011, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, H.J. Botulinum Toxin for the Treatment of Neuropathic Pain. Toxins 2017, 9, E260. [Google Scholar] [CrossRef] [PubMed]
| Patient | Age (years) | Gender | Height (cm) | Weight (kg) | Duration of Pain (Months) | Pain Rating Scale | |||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post-Injection | 6 Weeks | 12 Weeks | ||||||
| 1 | 26 | Female | 158 | 41 | 10 | 5 | 5 | 5 | 5 |
| 2 | 42 | Female | 165 | 63 | 10 | 5 | 4 | 3 | 1 |
| 3 | 33 | Female | 170 | 68 | 24 | 5 | 5 | 3 | 2 |
| 4 | 36 | Female | 161 | 56 | 60 | 5 | 5 | 5 | 2 |
| 5 | 35 | Female | 160 | 56 | 10 | 6 | 5 | 4 | 3 |
| 6 | 39 | Female | 167 | 61 | 24 | 5 | 5 | 5 | 3 |
| 7 | 58 | Female | 154 | 51 | 24 | 5 | 5 | 5 | 4 |
| 8 | 44 | Female | 159 | 58 | 30 | 5 | 8 | 2 | 1 |
| 9 | 36 | Female | 162 | 53 | 12 | 5 | 5 | 5 | 3 |
| 10 | 22 | Female | 163 | 63 | 12 | 5 | 5 | 5 | 2 |
| 11 | 23 | Female | 162 | 58 | 12 | 5 | 5 | 2 | 1 |
| 12 | 24 | Female | 160 | 57 | 15 | 5 | 3 | 5 | 4 |
| Variable | Value (Mean ± Standard Deviation) |
|---|---|
| Age | 34.8 ± 10.4 |
| Female Gender | 12 a |
| Height (cm) | 161.8 ± 4.2 |
| Weight (Kg) | 57.1 ± 6.9 |
| Duration of Pain (Months) | 20.3 ± 14.3 |
| Patient | Anterior Musculature (Units) | Posterior Musculature (Units) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Anterior Scalenes | Middle Scalenes | Pectoralis Major | Sternocleidomastoid | Levator Scapulae | Trapezius (Anterior Border) | Trapezius (Main Body) | Splenius Capitis | Semispinalis Capitis | |
| 1 | 6.25 | 6.25 | 6.25 | 25 | 125 | 6.25 | |||
| 2 | 6.25 | 6.25 | 6.25 | 25 | 25 | 6.25 | 6.25 | ||
| 3 | 6.25 | 6.25 | 25 | 25 | 6.25 | 6.25 | |||
| 4 | 6.25 | 6.25 | 12.5 | 25 | 25 | 6.25 | 6.25 | ||
| 5 | 6.25 | 6.25 | 6.25 | 6.25 | 25 | 25 | 6.25 | 6.25 | |
| 6 | 6.25 | 6.25 | 25 | 25 | 6.25 | 6.25 | |||
| 7 | 6.25 | 6.25 | 12.5 | 25 | 25 | 6.25 | 6.25 | ||
| 8 | 6.25 | 6.25 | 12.5 | 12.5 | 25 | 25 | 6.25 | 6.25 | |
| 9 | 12.5 | 25 | 50 | 6.25 | 6.25 | ||||
| 10 | 6.25 | 6.25 | 25 | 50 | 6.25 | 6.25 | |||
| 11 | 6.25 | 6.25 | 25 | 50 | 6.25 | 6.25 | |||
| 12 | 6.25 | 6.25 | 25 | 25 | 6.25 | 6.25 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-y.; Kim, J.M. Safety and Efficacy of PrabotulinumtoxinA (Nabota®) Injection for Cervical and Shoulder Girdle Myofascial Pain Syndrome: A Pilot Study. Toxins 2018, 10, 355. https://doi.org/10.3390/toxins10090355
Kim D-y, Kim JM. Safety and Efficacy of PrabotulinumtoxinA (Nabota®) Injection for Cervical and Shoulder Girdle Myofascial Pain Syndrome: A Pilot Study. Toxins. 2018; 10(9):355. https://doi.org/10.3390/toxins10090355
Chicago/Turabian StyleKim, Da-ye, and Jae Min Kim. 2018. "Safety and Efficacy of PrabotulinumtoxinA (Nabota®) Injection for Cervical and Shoulder Girdle Myofascial Pain Syndrome: A Pilot Study" Toxins 10, no. 9: 355. https://doi.org/10.3390/toxins10090355
APA StyleKim, D.-y., & Kim, J. M. (2018). Safety and Efficacy of PrabotulinumtoxinA (Nabota®) Injection for Cervical and Shoulder Girdle Myofascial Pain Syndrome: A Pilot Study. Toxins, 10(9), 355. https://doi.org/10.3390/toxins10090355





