Histopathological Evaluation of the Exposure by Cyanobacteria Cultive Containing [d-Leu1]Microcystin-LR on Lithobates catesbeianus Tadpoles
Abstract
1. Introduction
2. Results
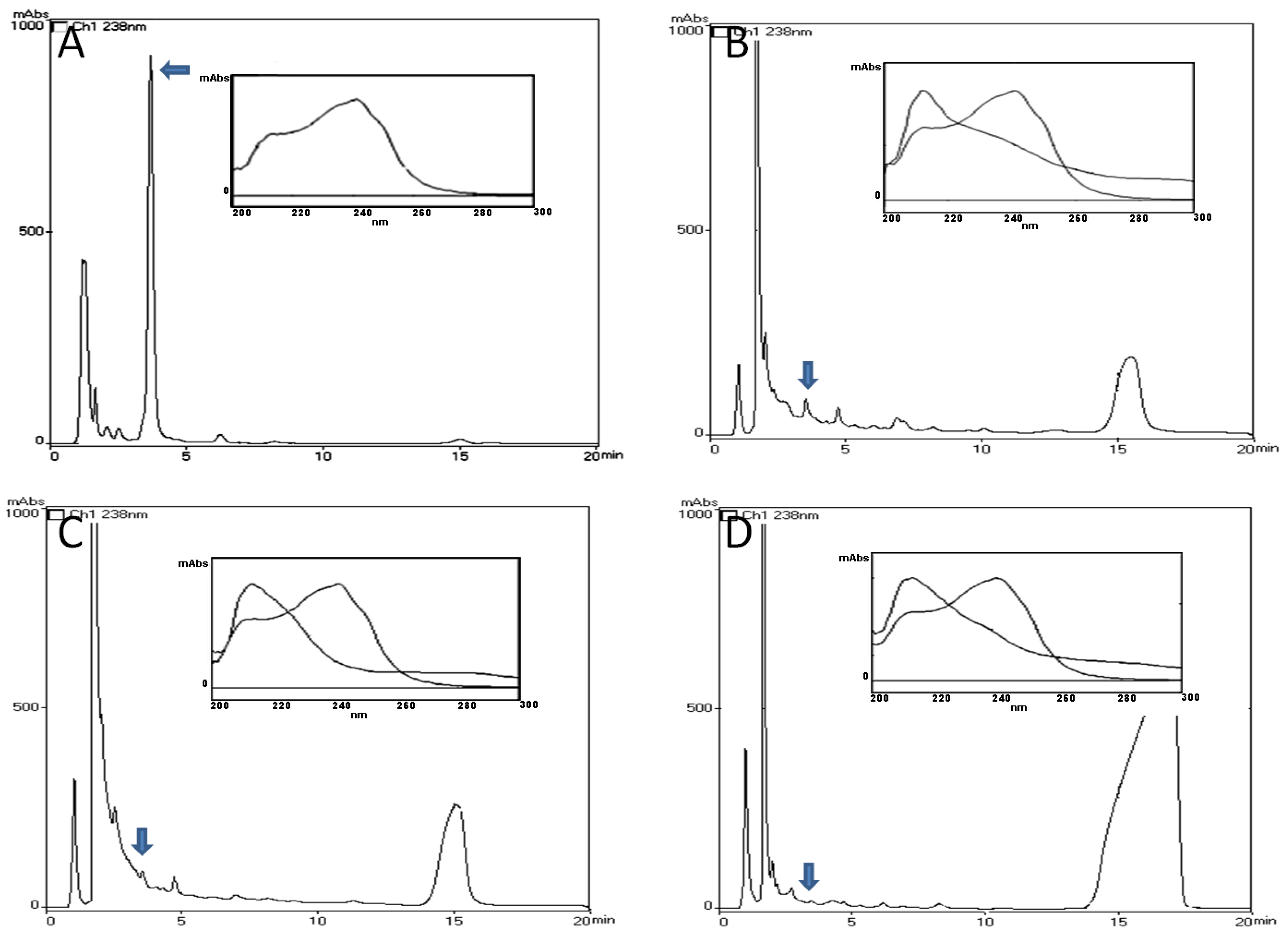
2.1. HPLC-PDA and MALDI-TOF Analysis
2.2. Histology Analysis
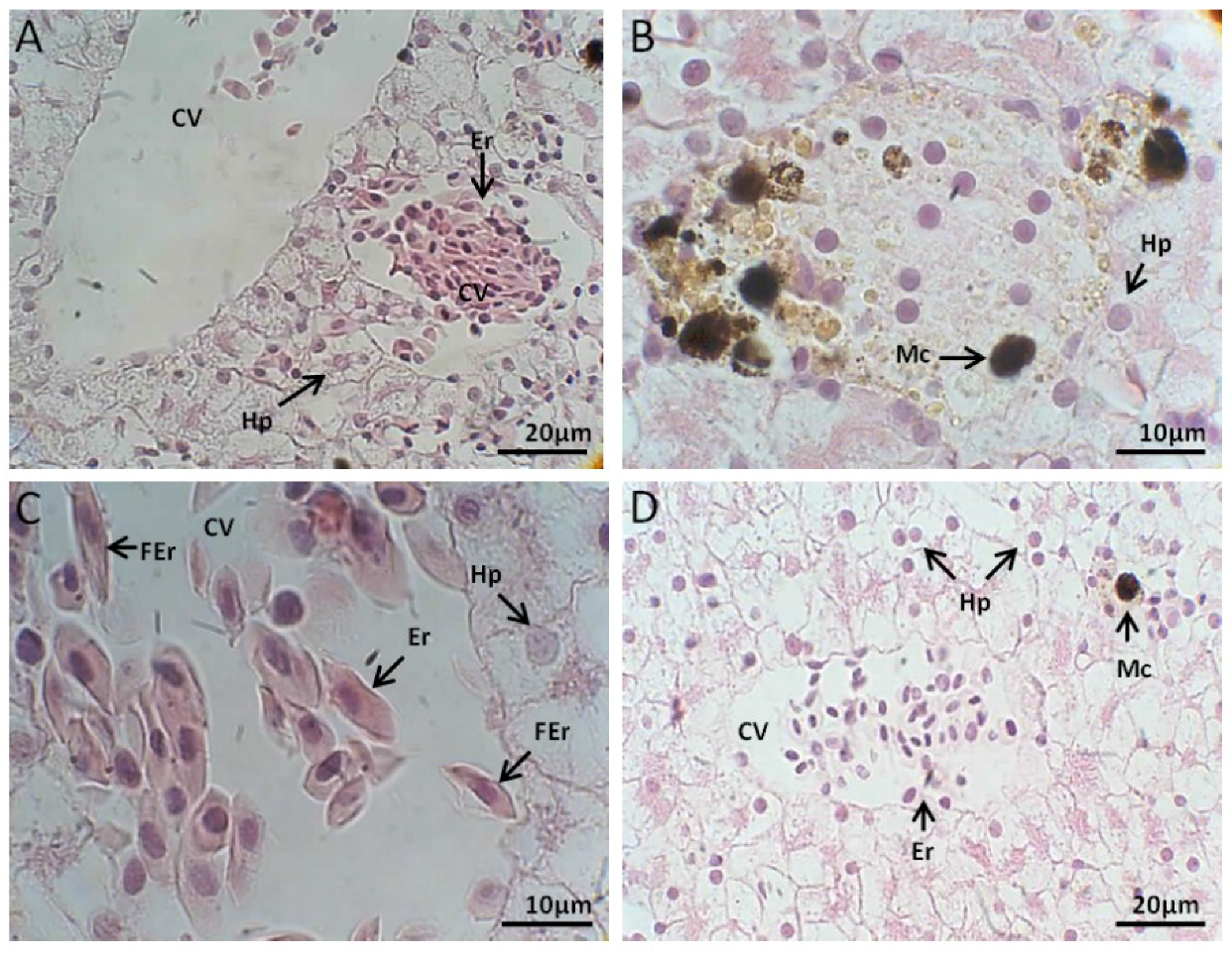
2.2.1. Liver Histology
Control Group
Exposed to M. aeruginosa Cells
Exposed to M. aeruginosa Disrupted Cells
Recovery
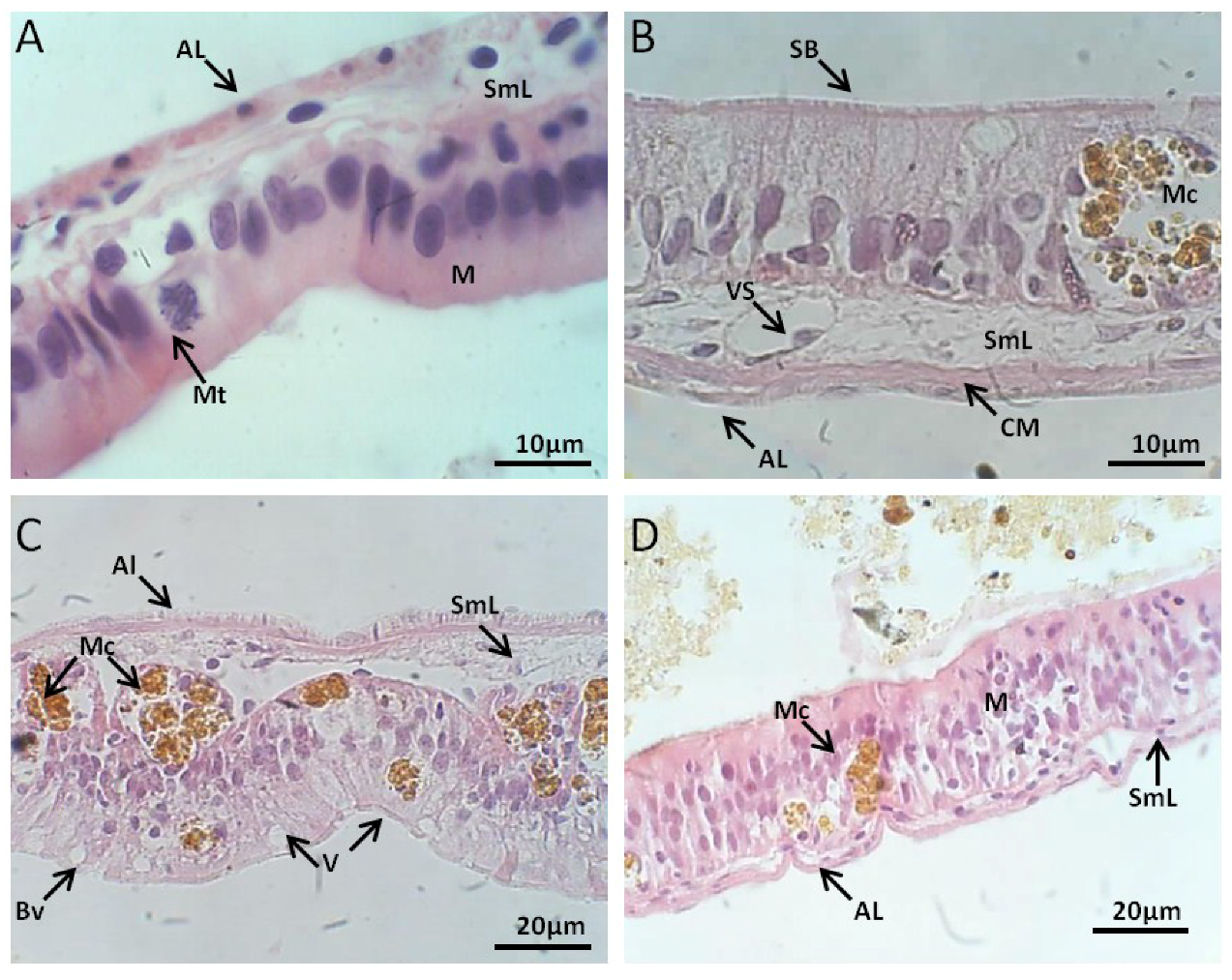
2.2.2. Intestinal Tract Histology
Control Group
Exposed to M. aeruginosa Cells
Exposed to M. aeruginosa Disrupted Cells
Recovery
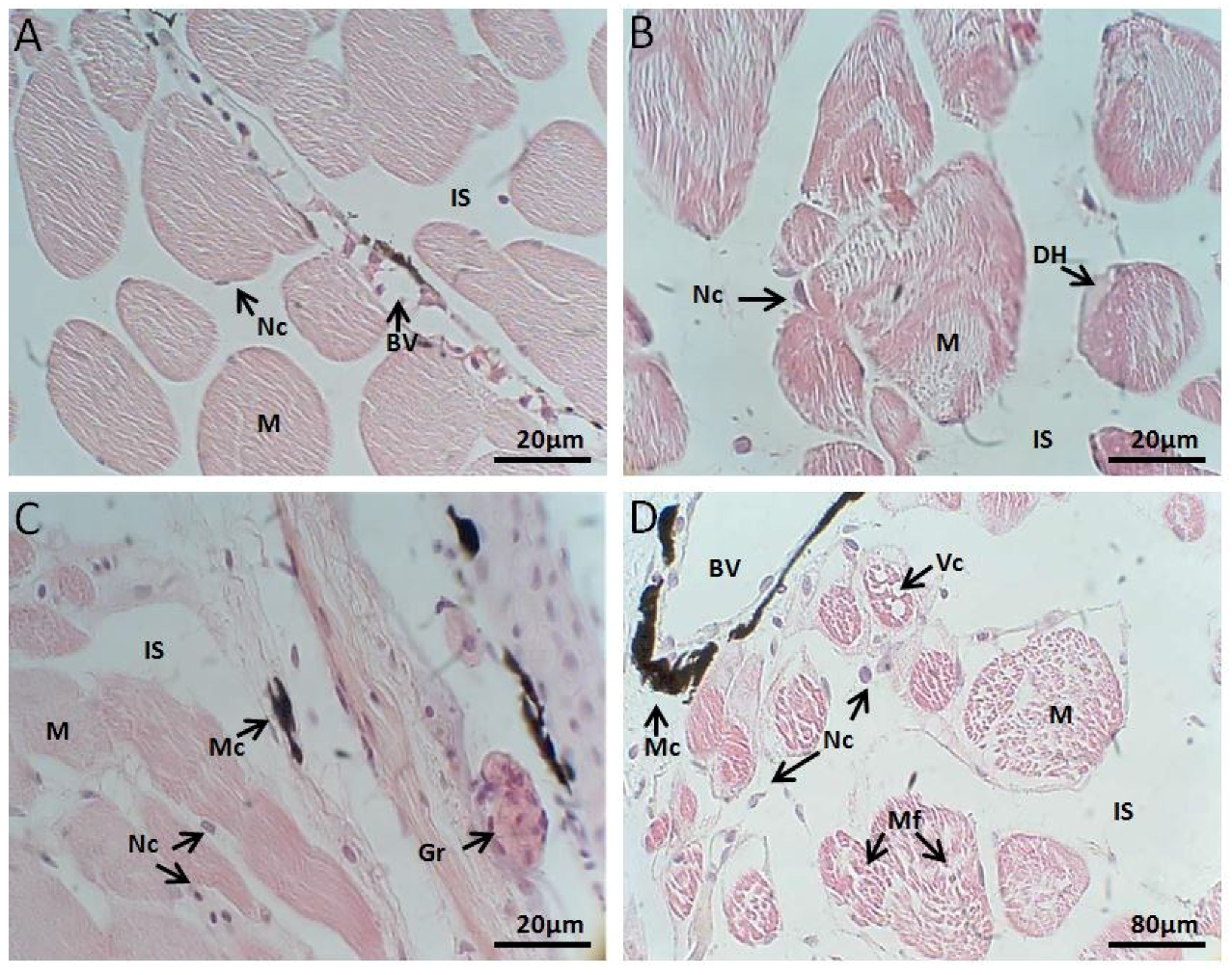
2.2.3. Muscle Histology
Control Group
Exposed to M. aeruginosa Cells
Exposed to M. aeruginosa Disrupted Cells
Recovery
3. Discussion
4. Materials and Methods
4.1. Cyanobacteria Culture and the Experimental Animals
4.2. M. aeruginosa NPLJ4 Toxins Characterization
4.3. Exposure of Tadpole to M. aeruginosa Cells or Cells Extract
4.4. HPLC-PDA Analysis
4.5. Determination of Unbound MCs Concentration in Tissues—MALDITOF Analysis
4.6. Histology Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Botes, D.P.; Tuinman, A.A.; Wessels, P.L.; Viljoen, C.C.; Kruger, H. The structure of cyanoginosin-LA, a cyclic heptapeptide toxin from the Microcystis aeruginosa. J. Chem. Soc. Perkin Trans. 1984, 2311–2318. [Google Scholar] [CrossRef]
- Runnegar, M.T.C.; Falconer, I.R. The in vivo and in vitro biological effects of the peptide hepatotoxin from the bluegreen alga Microcystis aeruginosa. S. Afr. J. Sci. 1982, 78, 363–366. [Google Scholar]
- Van Halderen, A.; Harding, W.R.; Wessels, J.C.; Schneider, D.J.; Heine, E.W.R.; Van der Merwe, J.; Fourie, J.M. Cyanobacterial (blue-green algae) poisoning of livestock in the Western’ Cape Province of South Africa. J. S. Afr. Vet. Assoc. 1995, 66, 260–264. [Google Scholar] [PubMed]
- White, S.H.; Duivenvoorden, L.J.; Fabbro, L.D.; Eaglesham, G.K. Mortality and toxin bioaccumulation in Bufo marinus following exposure to Cylindrospermopsis raciborskii cell extracts and live cultures. Environ. Pollut. 2007, 147, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Oberemm, A.; Becker, J.; Codd, G.A.; Steinberg, C. Effects of cyanobacterial toxins and aqueous crude extracts of cyanobacteria on the development of fish and amphibians. Environ. Toxicol. 1999, 14, 77–88. [Google Scholar] [CrossRef]
- Fisher, W.J.; Dietrich, D.R. Toxicity of the cyanobacterial cyclic heptapepitide toxins microcystin-LR and -RR in early stages of the African clawed frog (Xenopus laevis). Aquat. Toxicol. 2000, 49, 189–198. [Google Scholar] [CrossRef]
- Dvořáková, D.; Dvořáková, K.; Bláha, L.; Maršálek, B.; Knotková, Z. Effects of Cyanobacterial biomass and purified microcystins on malformations in Xenopus laevis: Teratogenesis assay (FETAX). Environ. Toxicol. 2002, 17, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.F.N.; Oliveira, V.M.; Oliveira, R.; Cunha, P.V.; Grisolia, C.K.; Pires, O.R., Jr. Histopathological Effects of [d-Leu1] Microcystin-LR variants on liver, skeletal muscle and intestinal tract of Hypophthalmichthys molitrix (Valenciennes, 1844). Toxicon 2010, 55, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Neffling, M.-R.; Lance, E.; Meriluoto, J. Detection of free and covalently bound microcystins in animal tissues by liquid chromatography–tandem mass spectrometry. Environ. Pollut. 2010, 158, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Kotak, B.G.; Zurawell, R.W.; Prepas, E.E.; Holmes, C.F.B. Microcystin-LR concentrations in aquatic food web compartments from lakes of varying trophic status. Can. J. Fish. Aquat. Sci. 1996, 53, 1974–1985. [Google Scholar] [CrossRef]
- Fischer, W.J.; Dietrich, D.R. Pathological and biochemical characterization of microcystin-induced hepatopancreas and kidney damage in carp (Cyprinus carpio). Toxicol. Appl. Pharmacol. 2000, 164, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, C.; Thermes, V.; De Luze, A.; Puiseux-Dao, S.; Bernard, C.; Joly, J.S.; Bourrat, F.; Edery, M. Effects of microcystin-LR on development of medaka fish embryos (Oryzias latipes). Toxicon 2004, 43, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Runnegar, M.; Berndt, N.; Kong, S.M.; Lee, E.Y.; Zhang, L.F. In vivo and in vitro binding of microcystin to protein phosphatase 1 and 2A. Biochem. Biophys. Res. Commun. 1995, 216, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Bagu, J.R.; Sykes, B.D.; Craig, M.M.; Holmes, C.F. A molecular basis for different interactions of marine toxins with protein phosphatase-1 Molecular models for bound motuporin, microcystins, okadaic acid, and calyculin A. J. Biol. Chem. 1997, 272, 5087–5097. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.; Huang, H.B.; Kwon, Y.G.; Greengard, P.; Nairn, A.C.; Kuriyan, J. Three-dimensional structure of the catalytic subunit of protein serine/threonine phosphatase-1. Nature 1995, 376, 745. [Google Scholar] [CrossRef] [PubMed]
- MacKintosh, R.W.; Dalby, K.N.; Campbell, D.G.; Cohen, P.T.; Cohen, P.; MacKintosh, C. The cyanobacterial toxin microcystin binds covalently to cysteine-273 on protein phosphatase 1. FEBS Lett. 1995, 371, 236–240. [Google Scholar] [PubMed]
- Campos, M.; Fadden, P.; Alms, G.; Qian, Z.; Haystead, T.A. Identification of protein phosphatase-1-binding proteins by microcystin-biotin affinity chromatography. J. Biol. Chem. 1996, 271, 28478–28484. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.E.; Grönberg, L.; Nygård, S.; Slotte, J.P.; Meriluoto, J.A. Hepatocellular uptake of 3H-dihydromicrocystin-LR, a cyclic peptide toxin. Biochim. Biophys. Acta (BBA) Biomembr. 1990, 1025, 60–66. [Google Scholar] [CrossRef]
- Rudolph-Böhner, S.; Mierke, D.F.; Moroder, L. Molecular structure of the cyanobacterial tumor-promoting microcystins. FEBS Lett. 1994, 349, 319–323. [Google Scholar] [CrossRef]
- Batista, T.; de Sousa, G.; Suput, J.S.; Rahmani, R.; Suput, D. Microcystin-LR causes the collapse of actin filaments in primary human hepatocytes. Aquat. Toxicol. 2003, 65, 85–91. [Google Scholar] [CrossRef]
- Blankson, H.; Grotterod, E.M.; Seglen, P.O. Prevention of toxin-induced cytoskeletal disruption and apoptotic liver cell death by the grapefruit flavonoid, naringin. Cell. Death Differ. 2000, 7, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.N.; Zeng, J.; Wang, F.; Zheng, W.; Tu, W.W.; Zhao, J.S.; Xu, J. Hyperphosphorylation of intermediate filament proteins is involved in microcystinLR-induced toxicity in HL7702 cells. Toxicol. Lett. 2012, 214, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.X.; Shen, H.M.; Ong, C.N. Critical role of reactive oxygen species formation in microcystin-induced cytoskeleton disruption in primary cultured hepatocytes. J. Toxicol. Environ. Health Part A 2001, 64, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.E.; Paatero, G.I.; Meriluoto, J.A.; Codd, G.A.; Kass, G.E.; Nicotera, P.; Orrenius, S. Rapid microfilament reorganization induced in isolated rat hepatocytes by microcystin-LR, a cyclic peptide toxin. Exp. Cell Res. 1989, 185, 86–100. [Google Scholar] [CrossRef]
- Espina, B.; Louzao, M.C.; Cagide, E.; Alfonso, A.; Vieytes, M.R.; Yasumoto, T.; Botana, L.M. The methyl ester of okadaic acid is more potent than okadaic acid in disrupting the actin cytoskeleton and metabolism of primary cultured hepatocytes. Br. J. Pharmacol. 2010, 159, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Tu, W.-W.; Lazar, L.; Chen, D.-N.; Zhao, J.-S.; Xu, J. Hyperphosphorylation of microfilament-associated proteins is involved in microcystin-LR-induced toxicity in HL7702 cells. Environ. Toxicol. 2015, 30, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Frangez, R.; Zuzek, M.C.; Mrkun, J.; Suput, D.; Sedmak, B.; Kosec, M. Microcystin-LR affects cytoskeleton and morphology of rabbit primary whole embryo cultured cells in vitro. Toxicon 2003, 41, 999–1005. [Google Scholar] [CrossRef]
- Khan, S.A.; Wickstrom, M.L.; Haschek, W.M.; Schaeffer, D.J.; Ghosh, S.; Beasley, V.R. Microcystin-LR and kinetics of cytoskeletal reorganization in hepatocytes, kidney cells, and fibroblasts. Nat. Toxins 1996, 4, 206–214. [Google Scholar] [CrossRef]
- Wickstrom, M.L.; Khan, S.A.; Haschek, W.M.; Wyman, J.F.; Eriksson, J.E.; Schaeffer, D.J.; Beasley, V.R. Alterations in microtubules, intermediate filaments, and microfilaments induced by microcystin-LR in cultured cells. Toxicol. Pathol. 1995, 23, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Kozdeba, M.; Borowczyk, J.; Zimolag, E.; Wasylewski, M.; Dziga, D.; Madeja, Z.; Drukala, J. Microcystin-LR affects properties of human epidermal skin cells crucial for regenerative processes. Toxicon 2014, 80, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Rymuszka, A. Microcystin-LR induces cytotoxicity and affects carp immune cells by impairment of their phagocytosis and the organization of the cytoskeleton. J. Appl. Toxicol. 2013, 33, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, X.; Zhou, W.; Qiao, Q.; Liang, H.; Li, G.; Wang, J.; Cai, F. The interactive effects of cytoskeleton disruption and mitochondria dysfunction lead to reproductive toxicity induced by microcystin-LR. PLoS ONE 2013, 8, e53949. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Tu, W.W.; Xu, J. Mechanisms of microcystin-LR-induced cytoskeletal disruption in animal cells. Toxicon 2015, 101, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Gosner, K.L. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 1960, 16, 183–190. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues Pires Júnior, O.; De Oliveira, N.B.; Bosque, R.J.; Nice Ferreira, M.F.; Morais Aurélio da Silva, V.; Martins Magalhães, A.C.; Correia de Santana, C.J.; De Souza Castro, M. Histopathological Evaluation of the Exposure by Cyanobacteria Cultive Containing [d-Leu1]Microcystin-LR on Lithobates catesbeianus Tadpoles. Toxins 2018, 10, 318. https://doi.org/10.3390/toxins10080318
Rodrigues Pires Júnior O, De Oliveira NB, Bosque RJ, Nice Ferreira MF, Morais Aurélio da Silva V, Martins Magalhães AC, Correia de Santana CJ, De Souza Castro M. Histopathological Evaluation of the Exposure by Cyanobacteria Cultive Containing [d-Leu1]Microcystin-LR on Lithobates catesbeianus Tadpoles. Toxins. 2018; 10(8):318. https://doi.org/10.3390/toxins10080318
Chicago/Turabian StyleRodrigues Pires Júnior, Osmindo, Natiela Beatriz De Oliveira, Renan J. Bosque, Maria Fernanda Nice Ferreira, Veronica Morais Aurélio da Silva, Ana Carolina Martins Magalhães, Carlos José Correia de Santana, and Mariana De Souza Castro. 2018. "Histopathological Evaluation of the Exposure by Cyanobacteria Cultive Containing [d-Leu1]Microcystin-LR on Lithobates catesbeianus Tadpoles" Toxins 10, no. 8: 318. https://doi.org/10.3390/toxins10080318
APA StyleRodrigues Pires Júnior, O., De Oliveira, N. B., Bosque, R. J., Nice Ferreira, M. F., Morais Aurélio da Silva, V., Martins Magalhães, A. C., Correia de Santana, C. J., & De Souza Castro, M. (2018). Histopathological Evaluation of the Exposure by Cyanobacteria Cultive Containing [d-Leu1]Microcystin-LR on Lithobates catesbeianus Tadpoles. Toxins, 10(8), 318. https://doi.org/10.3390/toxins10080318




