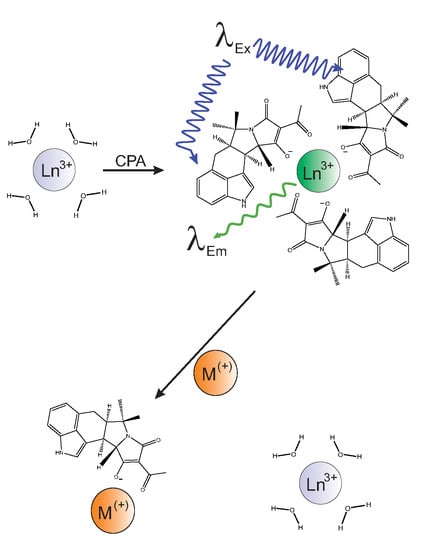
Complexation of the Mycotoxin Cyclopiazonic Acid with Lanthanides Yields Luminescent Products
Abstract
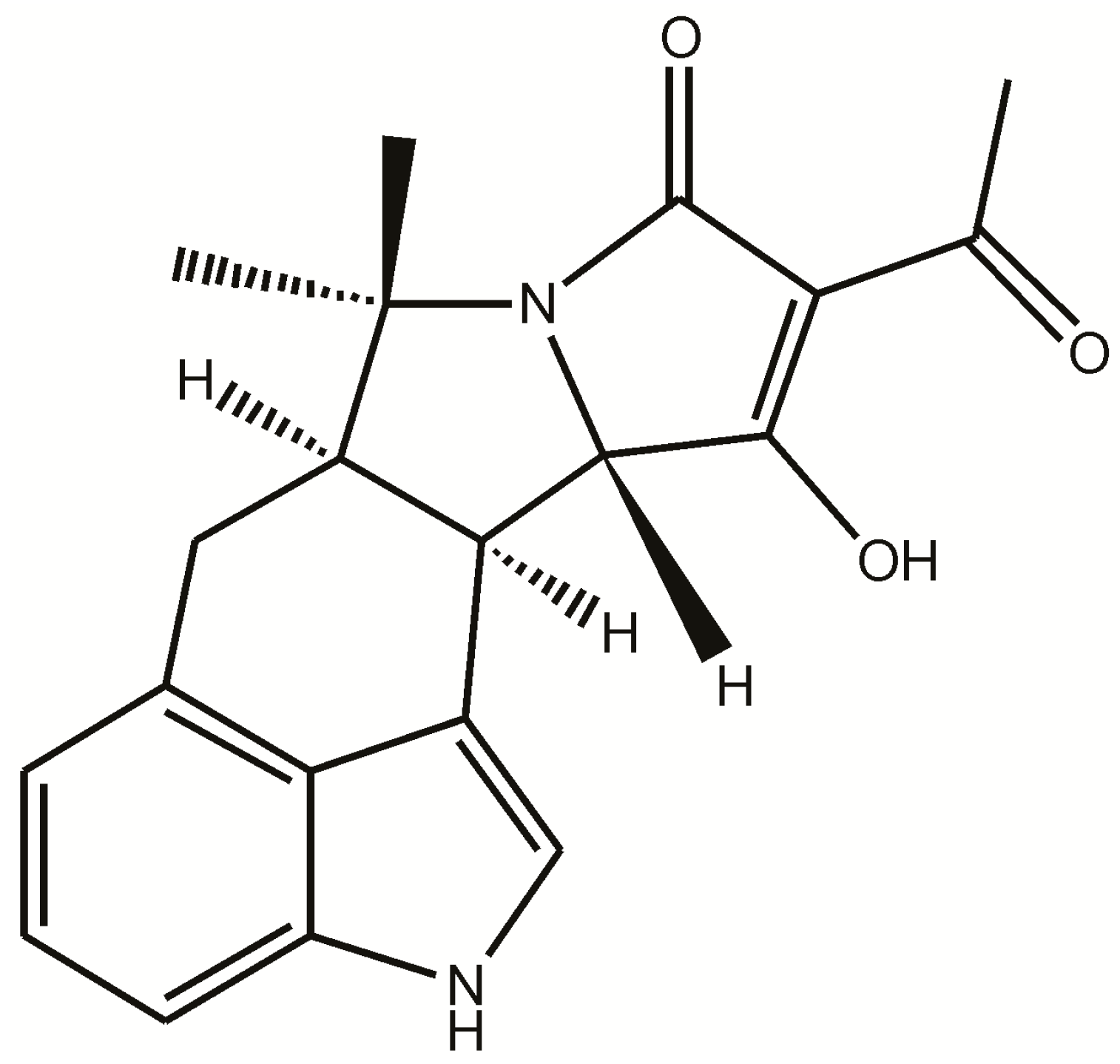
:1. Introduction
2. Results and Discussion
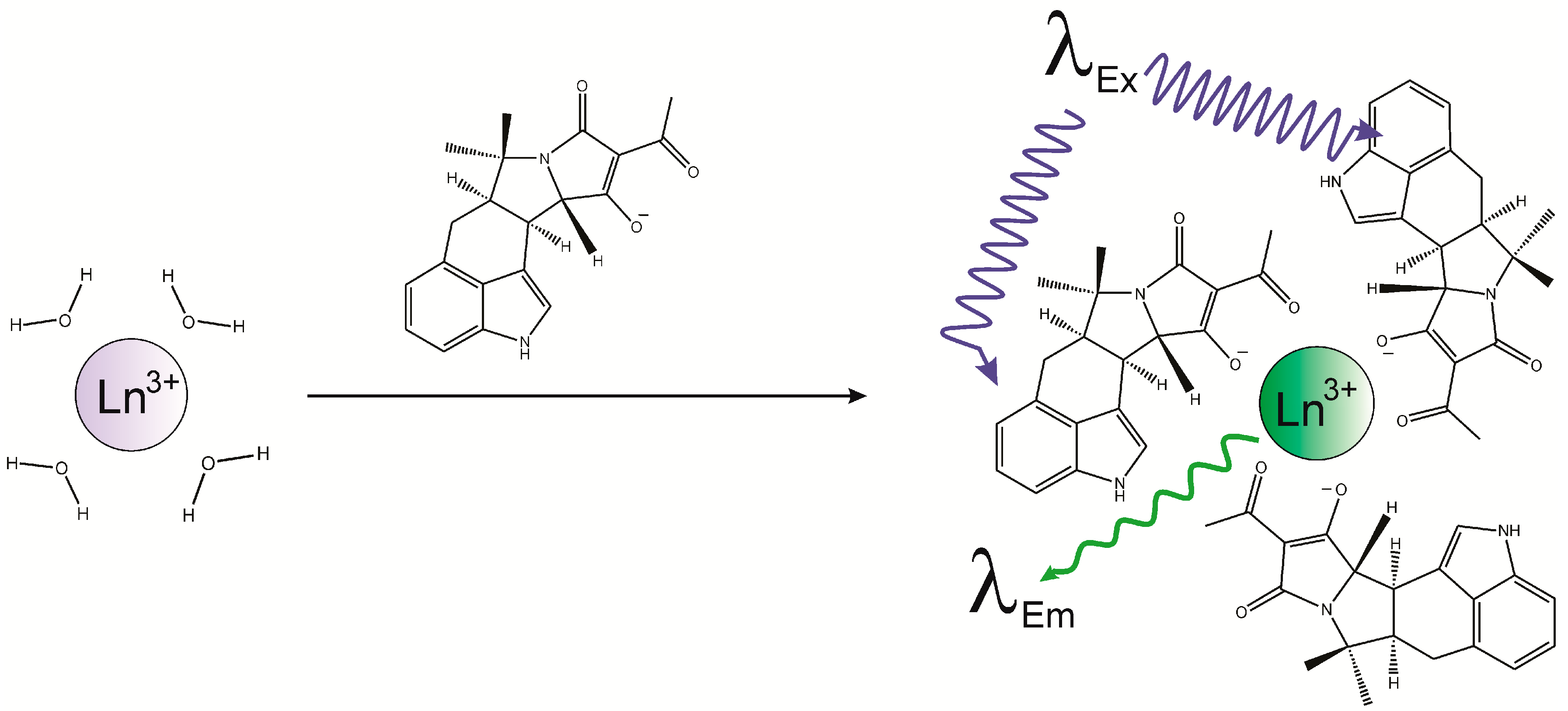
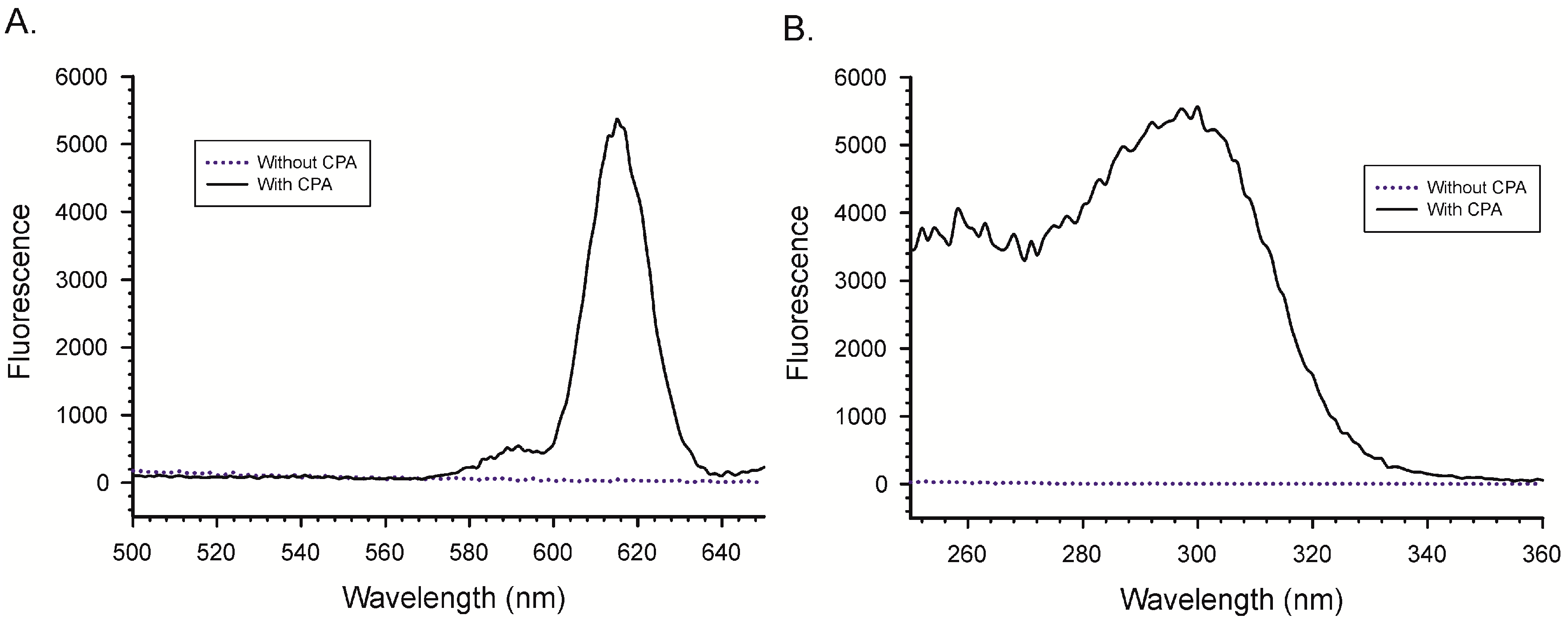
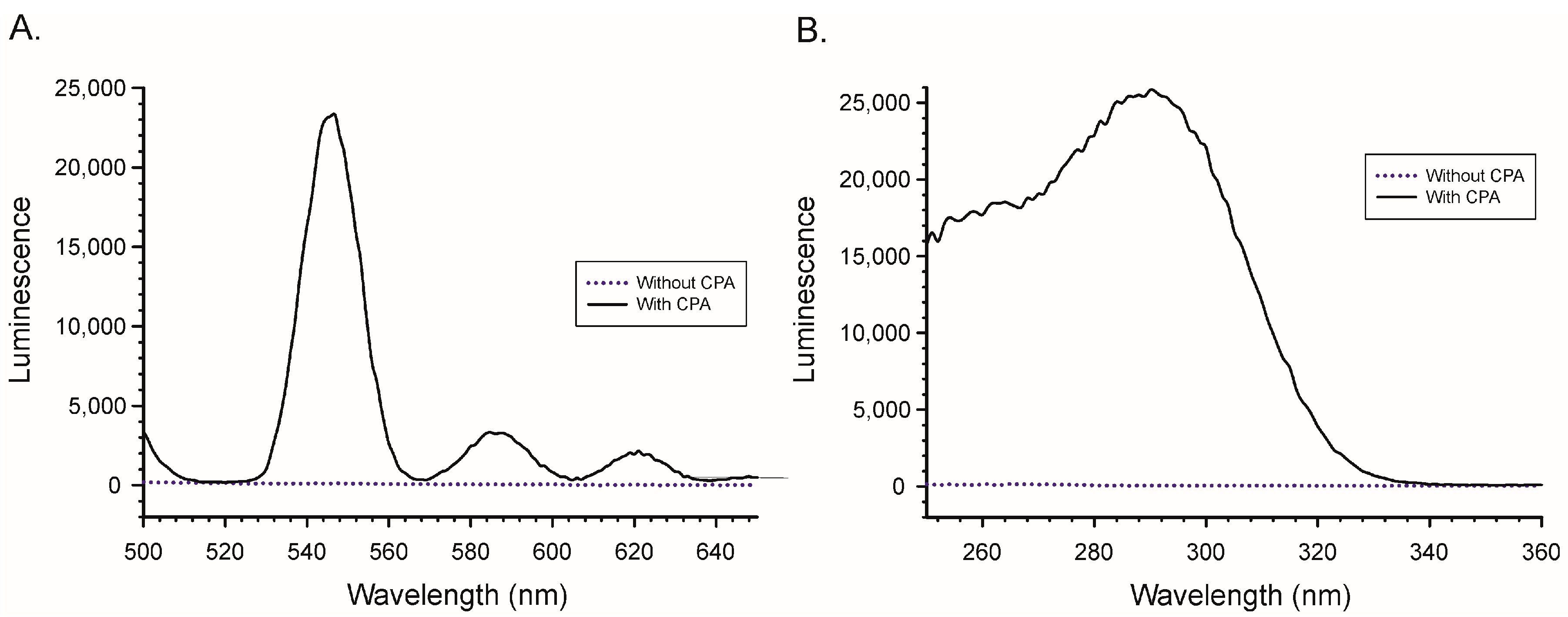
2.1. Binding of Lanthanides by CPA and Induction of Luminescence
2.2. Importance of the Lanthanide
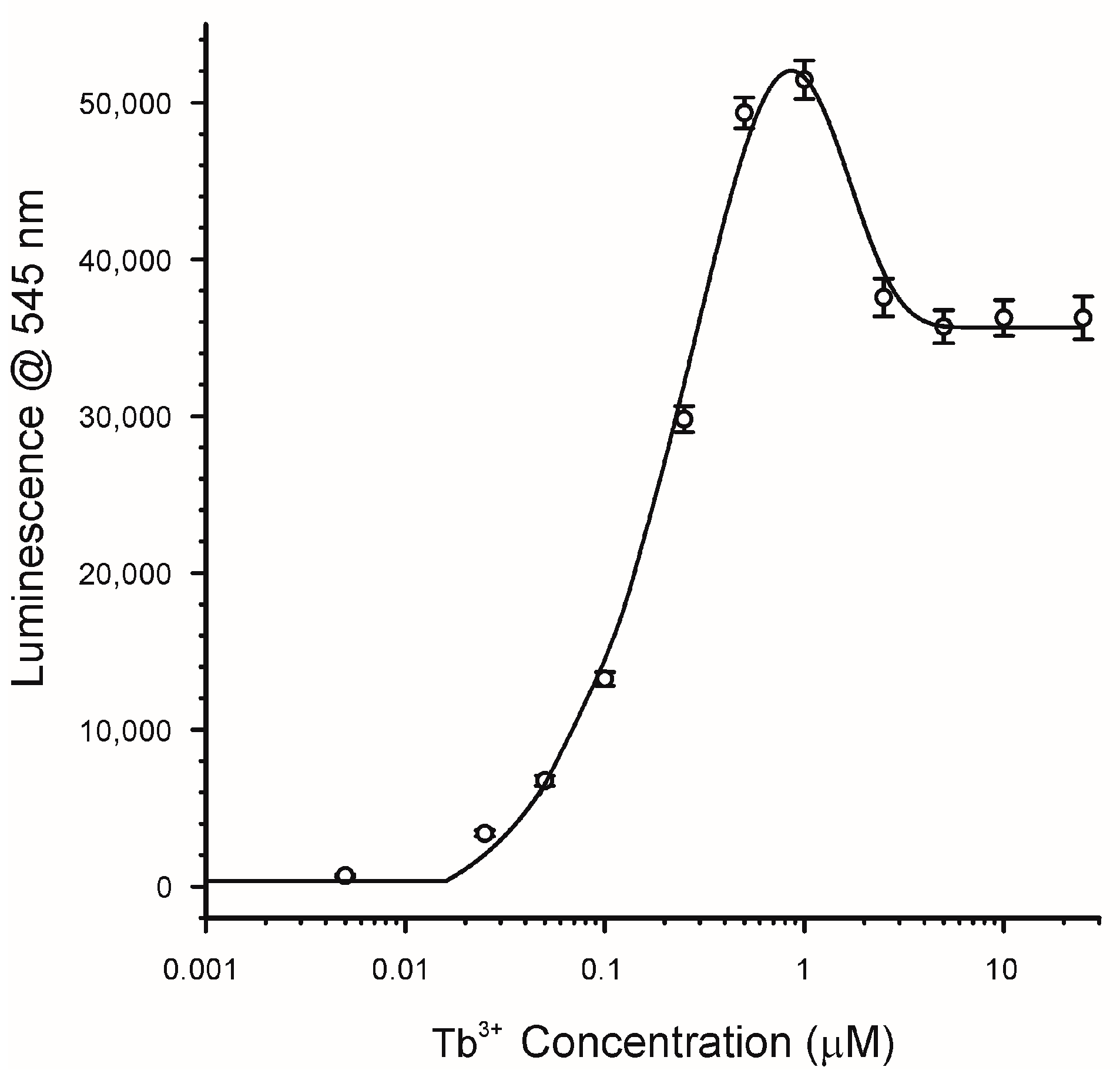
2.3. Effects of Lanthanide Concentration
2.4. Effects of Environment
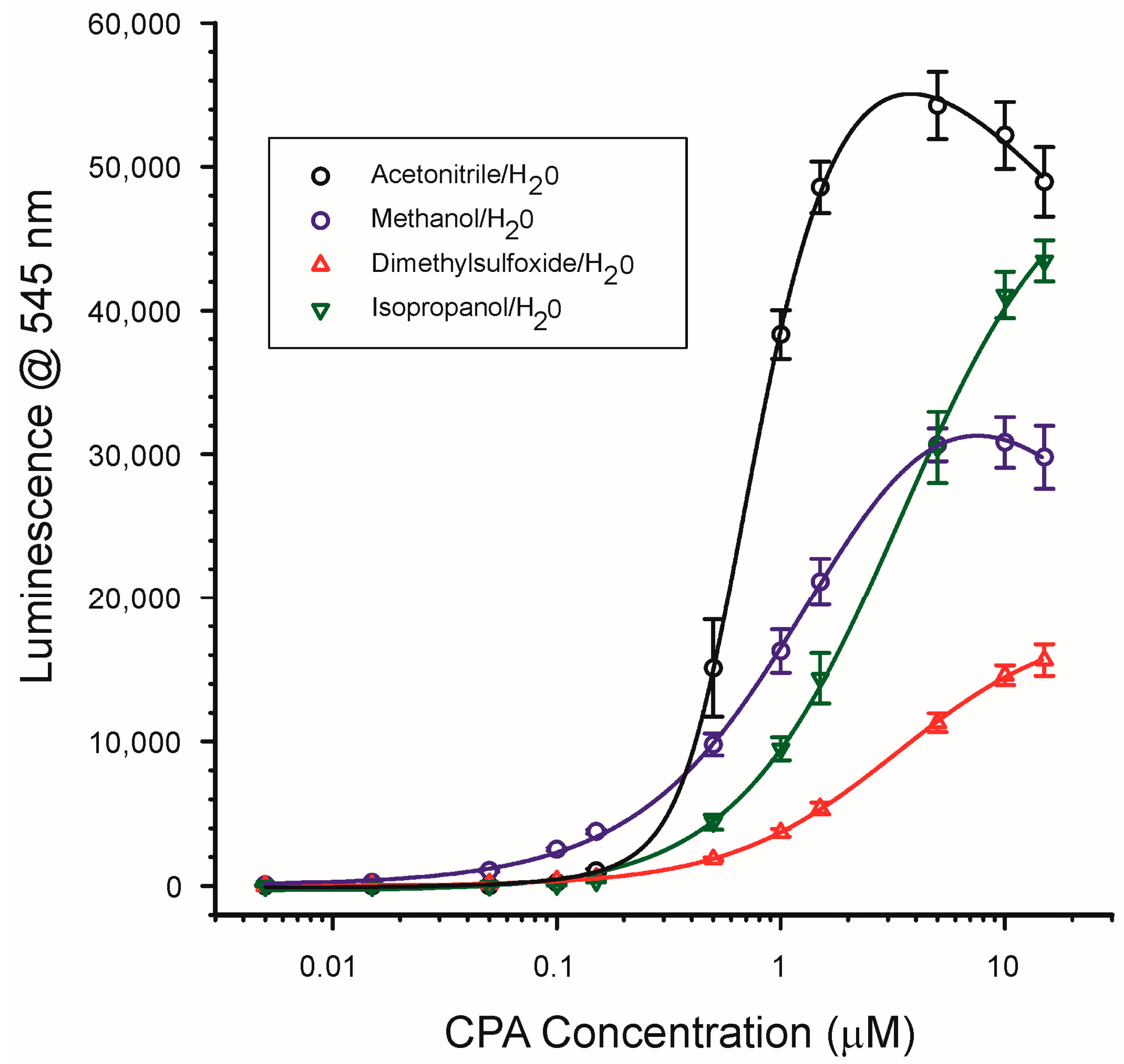
2.4.1. Solvent Type
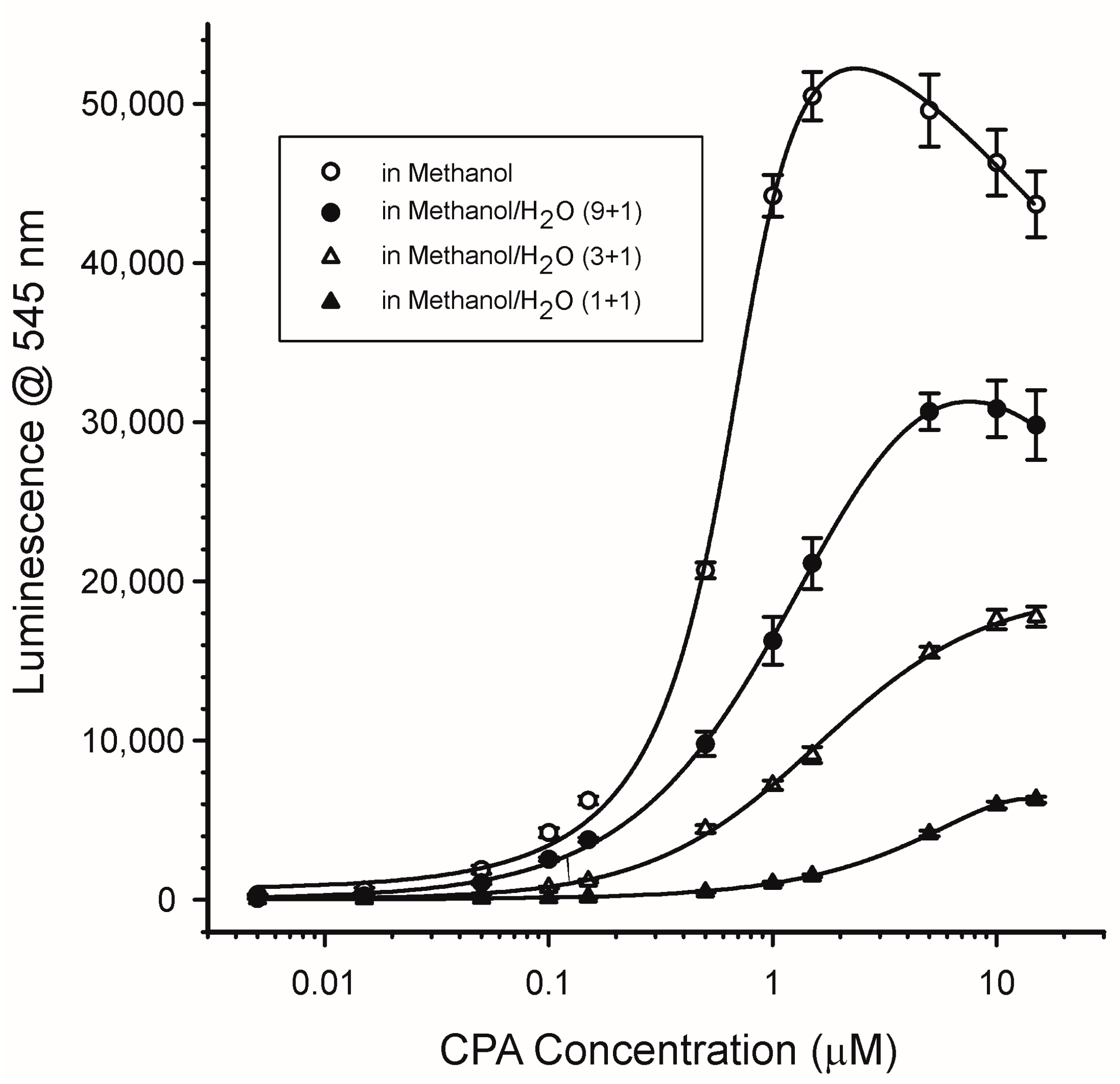
2.4.2. Water Content and pH
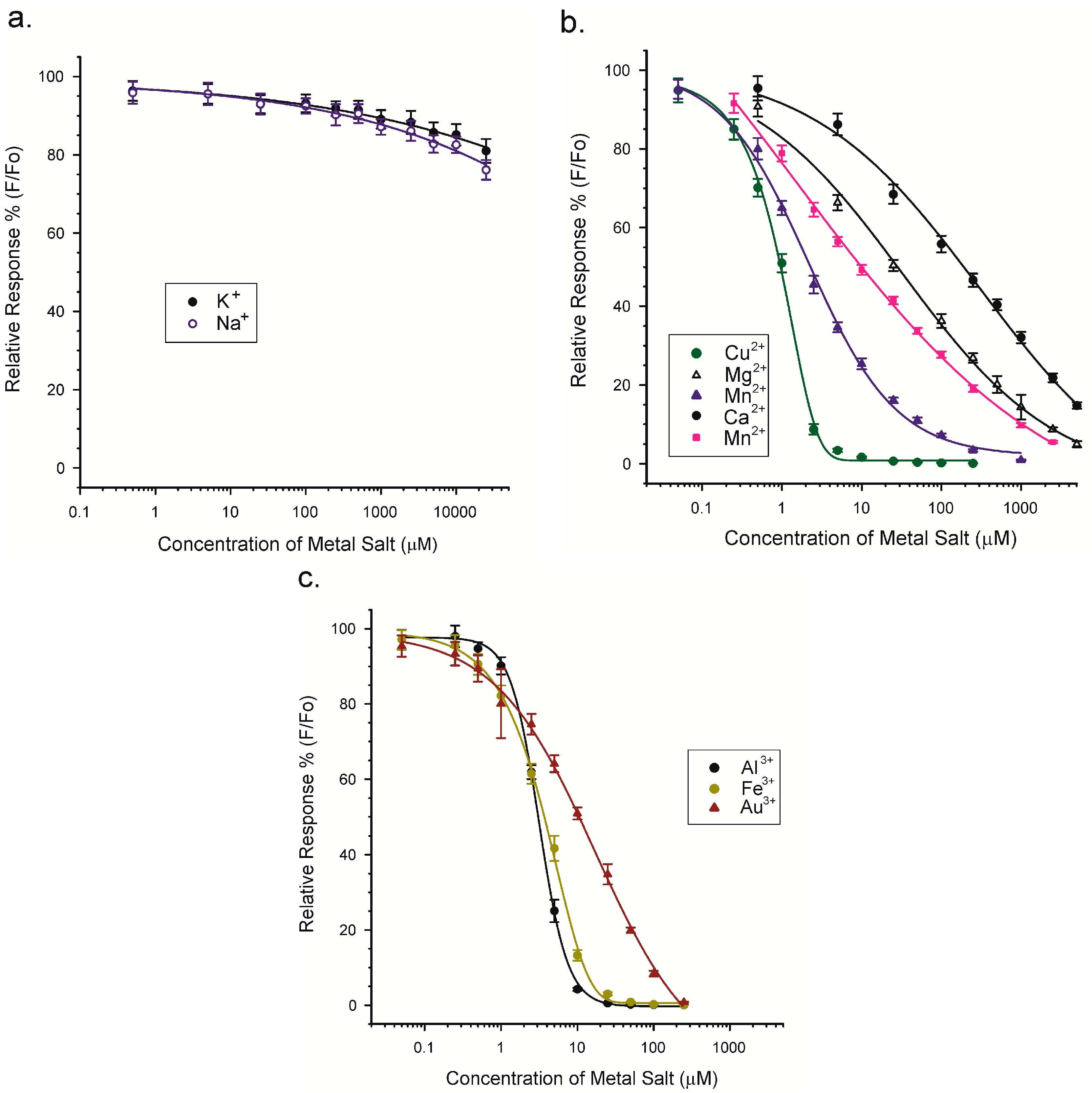
2.5. Competitive Luminescence Assay and Effects of Metal Cations
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Excitation and Emission Spectra
4.3. Effects of Lanthanide Concentration
4.4. Environmental Effects
4.4.1. Effects of Solvent Type
4.4.2. Effects of Water Content and pH
4.5. Competitive Luminescence Assay
4.6. Data Analysis and Curve Fitting
Funding
Conflicts of Interest
Disclaimer
References
- Holzapfel, C.W. The isolation and structure of cyclopiazonic acid, a toxic metabolite of Penicillium cyclopium Westling. Tetrahedron 1968, 24, 2101–2119. [Google Scholar] [CrossRef]
- Ostry, V.; Toman, J.; Grosse, Y.; Malir, F. Cyclopiazonic acid: 50th anniversary of its discovery. World Mycotoxin J. 2018, 11, 135–148. [Google Scholar] [CrossRef]
- Dorner, J.W. Recent advances in analytical methodology for cyclopiazonic acid. In Mycotoxins in Food Safety; DeVries, J., Trucksess, M.W., Jackson, L.S., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; pp. 107–116. [Google Scholar]
- Trucksess, M.W.; Mislivec, P.P.; Young, K.; Bruce, V.R.; Page, S.W. Cyclopiazonic acid production by cultures of Aspergillus and Penicillium species isolated from dried beans, corn meal, macaroni, and pecans. J. AOAC Int. 1987, 70, 123–126. [Google Scholar]
- Goto, T.; Wicklow, D.T.; Ito, Y. Aflatoxin and cyclopiazonic acid production by a sclerotium-producing Aspergillus tamarii strain. Appl. Environ. Microbiol. 1996, 62, 4036–4038. [Google Scholar] [PubMed]
- Horn, B.W.; Dorner, J.W. Regional differences in production of aflatoxin B1 and cyclopiazonic acid by soil isolates of Aspergillus flavus along a transect within the United States. Appl. Environ. Microbiol. 1999, 65, 1444–1449. [Google Scholar] [PubMed]
- Gallagher, R.T.; Richard, J.L.; Stahr, H.M.; Cole, R.J. Cyclopiazonic acid production by aflatoxigenic and non-aflatoxigenic strains of Aspergillus flavus. Mycopathologia 1978, 66, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Lansden, J.A.; Davidson, J.I. Occurrence of cyclopiazonic acid in peanuts. Appl. Environ. Microbiol. 1983, 45, 766–769. [Google Scholar] [PubMed]
- Urano, T.; Trucksess, M.W.; Matuskik, J.; Dorner, J.W. Liquid chromatographic determination of cyclopiazonic acid in corn and peanuts. J. AOAC Int. 1992, 75, 319–322. [Google Scholar]
- Burdock, G.A.; Flamm, W.G. Review article: Safety assessment of the mycotoxin cyclopiazonic acid. Int. J. Toxicol. 2000, 19, 195–218. [Google Scholar] [CrossRef]
- Hayashi, Y.; Yoshizawa, T. Analysis of cyclopiazonic acid in corn and rice by a newly developed method. Food Chem. 2005, 93, 215–221. [Google Scholar] [CrossRef]
- Heperkan, D.; Somuncuoglu, S.; Karbancioglu-Güler, F.; Mecik, N. Natural contamination of cyclopiazonic acid in dried figs and co-occurrence of aflatoxin. Food Control 2012, 23, 82–86. [Google Scholar] [CrossRef]
- Miller, C.D.; Richard, J.L.; Osweiler, G.D. Cyclopiazonic acid toxicosis in young turkeys: Clinical, physiological, and serological observations. Toxin Rev. 2011, 30, 42–46. [Google Scholar] [CrossRef]
- Cole, R.J. Etiology of turkey X disease in retrospect: A case for the involvement of cyclopiazonic acid. Mycotoxin Res. 1986, 2, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Voss, K.A. In vivo and in vitro toxicity of cyclopiazonic acid (CPA). In Mycotoxins, Biotoxins, Wood Decay, Air Quality, Cultural Properties, General Biodeterioration, and Degradation; Llewellyn, G.C., O’Rear, C.E., Eds.; Springer Science: New York, NY, USA, 1990; Volume 3, pp. 67–84. [Google Scholar]
- Akbari, P.; Malekinejad, H.; Rahmani, F.; Rezabakhsh, A.; Fink-Gremmels, J. Cyclopiazonic acid attenuates the divalent cations and augments the mRNA level of iNOS in the liver and kidneys of chickens. World Mycotoxin J. 2012, 5, 153–161. [Google Scholar] [CrossRef]
- Goeger, D.E.; Riley, R.T.; Dorner, J.W.; Cole, R.J. Cyclopiazonic acid inhibition of the Ca2+-transport ATPase in rat skeletal muscle sarcoplasmic reticulum vesicles. Biochem. Pharmacol. 1988, 37, 978–981. [Google Scholar] [CrossRef]
- Takahashi, S.; Kato, Y.; Adachi, M.; Agata, N.; Tanaka, H.; Shigenobu, K. Effects of cyclopiazonic acid on rat myocardium: Inhibition of calcium uptake into sarcoplasmic reticulum. J. Pharmacol. Exp. Ther. 1995, 272, 1095–1100. [Google Scholar] [PubMed]
- Riley, R.T.; Goerger, D.E.; Norred, W.P. Disruption of calcium homeostasis: The cellular mechanism of cyclopiazonic acid toxicity in laboratory animals. In Molecular Approaches to Food Safety Issues Involving Toxic Microorganisms; Eklund, M., Richard, J.L., Mise, K., Eds.; Alaken, Inc.: Fort Collins, CO, USA, 1995; pp. 461–480. [Google Scholar]
- Moncoq, K.; Trieber, C.A.; Young, H.S. The molecular basis for cyclopiazonic acid inhibition of the sarcoplasmic reticulum calcium pump. J. Biol. Chem. 2007, 282, 9748–9757. [Google Scholar] [CrossRef] [PubMed]
- Laursen, M.; Bublitz, M.; Moncoq, K.; Olesen, C.; Møller, J.V.; Young, H.S.; Nissen, P.; Morth, J.P. Cyclopiazonic acid is complexed to a divalent metal ion when bound to the sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 2009, 284. [Google Scholar] [CrossRef] [PubMed]
- Di Marino, D.; Ilda, D.A.; Andrea, C.; Allegra, V.; Anna, T. Characterization of the differences in the cyclopiazonic acid binding mode to mammalian and P. falciparum Ca2+ pumps: A computational study. Prot. Struct. Funct. Bioinform. 2015, 83, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Maragos, C.M.; Sieve, K.K.; Bobell, J. Detection of cyclopiazonic acid (CPA) in maize by immunoassay. Mycotoxin Res. 2017, 33, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Da Motta, S.; Valente Soares, L.M. Simultaneous determination of tenuazonic and cyclopiazonic acids in tomato products. Food Chem. 2000, 71, 111–116. [Google Scholar] [CrossRef]
- Maragos, C.M. Photolysis of cyclopiazonic acid to fluorescent products. World Mycotoxin J. 2009, 2, 77–84. [Google Scholar] [CrossRef]
- Moldes-Anaya, A.S.; Asp, T.N.; Eriksen, G.S.; Skaar, I.; Rundberget, T. Determination of cyclopiazonic acid in food and feeds by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 3812–3818. [Google Scholar] [CrossRef] [PubMed]
- Diaz, G.J.; Thompson, W.; Martos, P.A. Stability of cyclopiazonic acid in solution. World Mycotoxin J. 2010, 3, 25–33. [Google Scholar] [CrossRef]
- Ansari, P.; Haeubl, G. Determination of cyclopiazonic acid in white mould cheese by liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) using a novel internal standard. Food Chem. 2016, 211, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Hahnau, S.; Weiler, E.W. Monoclonal antibodies for the enzyme immunoassay of the mycotoxin cyclopiazonic acid. J. Agric. Food Chem. 1993, 41, 1076–1080. [Google Scholar] [CrossRef]
- Yu, W.; Chu, F.S. Improved direct competitive enzyme-linked immunosorbent assay for cyclopiazonic acid in corn, peanuts, and mixed feed. J. Agric. Food Chem. 1998, 46, 1012–1017. [Google Scholar] [CrossRef]
- Aulsebrook, M.L.; Graham, B.; Grace, M.R.; Tuck, K.L. Lanthanide complexes for luminescence-based sensing of low molecular weight analytes. Coord. Chem. Rev. 2017. [Google Scholar] [CrossRef]
- Vazquez, B.I.; Fente, C.; Franco, C.; Cepeda, A.; Prognon, P.; Mahuzier, G. Simultaneous high-performance liquid chromatographic determination of ochratoxin A and citrinin in cheese by time-resolved luminescence using terbium. J. Chromatogr. A 1996, 727, 185–193. [Google Scholar] [CrossRef]
- De Girolamo, A.; Le, L.; Penner, G.; Schena, R.; Visconti, A. Analytical performances of a DNA-ligand system using time-resolved fluorescence for the determination of ochratoxin A in wheat. Anal. Bioanal. Chem. 2012, 403, 2627–2634. [Google Scholar] [CrossRef] [PubMed]
- Heine, J.; Műller-Buschbaum, K. Engineering metal-based luminescence in coordination polymers and metal-organic frameworks. Chem. Soc. Rev. 2013, 42, 9232–9242. [Google Scholar] [CrossRef] [PubMed]
- Nesheim, S.; Stack, M.E. Preparation of mycotoxin standards. In Mycotoxin Protocols; Trucksess, M.W., Pohland, A.E., Eds.; Humana Press: Totowa, NJ, USA, 2001; Volume 157, pp. 31–36. [Google Scholar]
- Ricci, R.W.; Kilichowski, K.B. Fluorescence quenching of the indole ring system by lanthanide ions. J. Phys. Chem. 1974, 78, 1953–1956. [Google Scholar] [CrossRef]
- Aletti, A.B.; Gillen, D.M.; Gunnlaugsson, T. Luminescent/colorimetric probes and (chemo-) sensors for detecting anions based on transition and lanthanide ion receptor/binding complexes. Coord. Chem. Rev. 2018, 354, 98–120. [Google Scholar] [CrossRef]
- Systat Software, Inc. TableCurve 2D User’s Manual Version 5.01 for Windows; Systat Software Inc.: Richmond, CA, USA, 2002; 661p, ISBN 81-88341-07-X. [Google Scholar]

| pH | Luminescence a |
|---|---|
| 3.0 | 42,250 ± 1590 |
| 4.0 | 44,170 ± 2540 |
| 5.0 | 23,270 ± 1850 |
| 6.0 | 12,200 ± 940 |
| 7.0 | 1560 ± 140 |
| Cation | IC50 (µM) a | Molar Ratio of Cation: CPA at the IC50 b | Equation c |
|---|---|---|---|
| Cu2+ | 0.98 ± 0.06 | 0.65 | 8187 |
| Co2+ | 2.29 ± 0.14 | 1.52 | 8013 |
| Al3+ | 3.08 ± 0.13 | 2.05 | 8013 |
| Fe3+ | 3.71 ± 0.33 | 2.47 | 8007 |
| Mn2+ | 9.98 ± 0.45 | 6.65 | 8013 |
| Au3+ | 10.7 ± 0.07 | 7.1 | 8013 |
| Mg2+ | 28.3 ± 1.0 | 18.8 | 8013 |
| Ca2+ | 191 ± 19 | 127 | 8013 |
| Na+ | >25,000 | >16,667 | NA d |
| K+ | >25,000 | >16,667 | NA |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maragos, C.M. Complexation of the Mycotoxin Cyclopiazonic Acid with Lanthanides Yields Luminescent Products. Toxins 2018, 10, 285. https://doi.org/10.3390/toxins10070285
Maragos CM. Complexation of the Mycotoxin Cyclopiazonic Acid with Lanthanides Yields Luminescent Products. Toxins. 2018; 10(7):285. https://doi.org/10.3390/toxins10070285
Chicago/Turabian StyleMaragos, Chris M. 2018. "Complexation of the Mycotoxin Cyclopiazonic Acid with Lanthanides Yields Luminescent Products" Toxins 10, no. 7: 285. https://doi.org/10.3390/toxins10070285
APA StyleMaragos, C. M. (2018). Complexation of the Mycotoxin Cyclopiazonic Acid with Lanthanides Yields Luminescent Products. Toxins, 10(7), 285. https://doi.org/10.3390/toxins10070285