Classes, Databases, and Prediction Methods of Pharmaceutically and Commercially Important Cystine-Stabilized Peptides
Abstract
1. Introduction
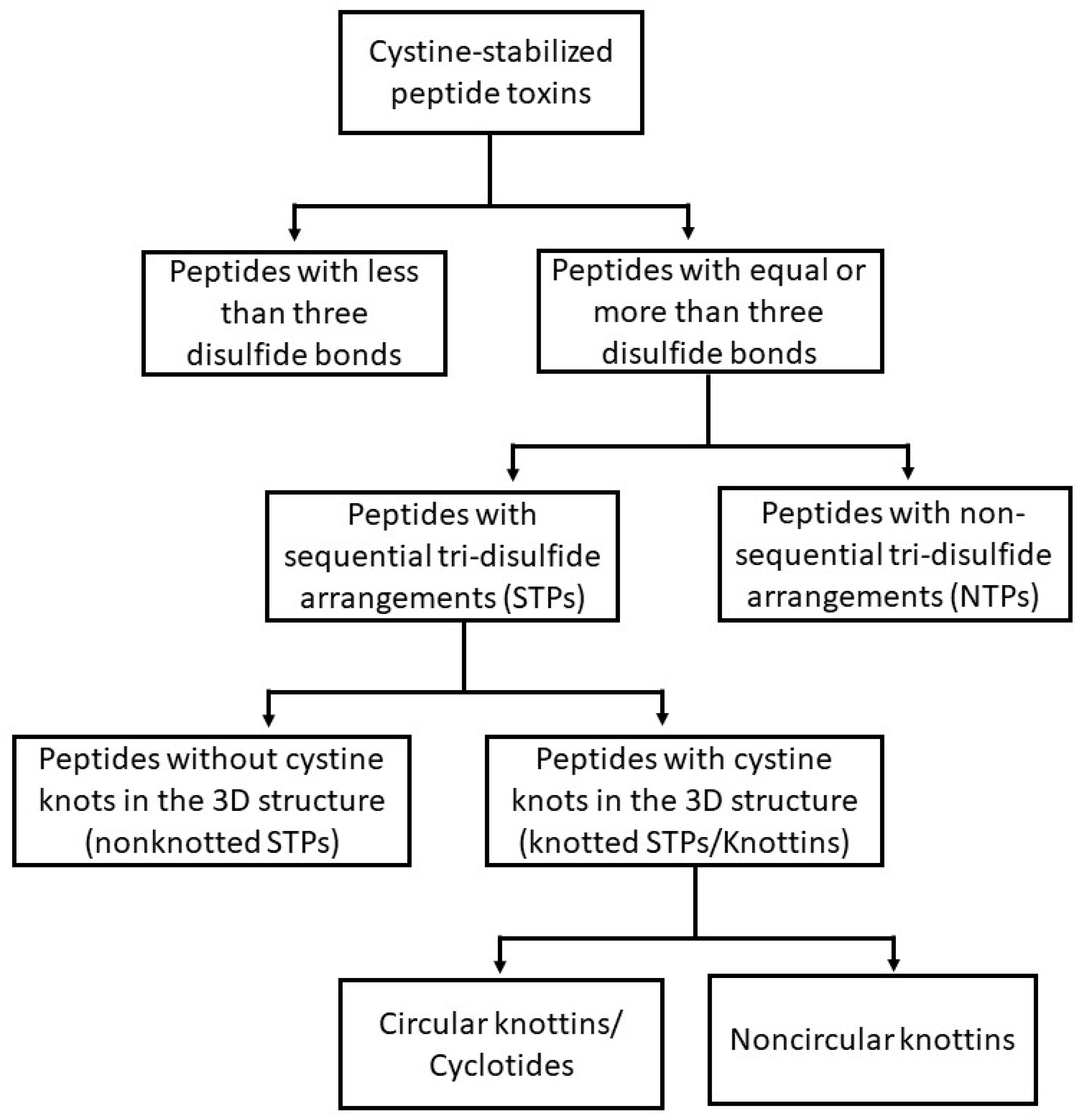
2. Cystine-Stabilized Peptide Families
2.1. Cystine-Stabilized Peptide Families Based on Disulfide Bonding Patterns
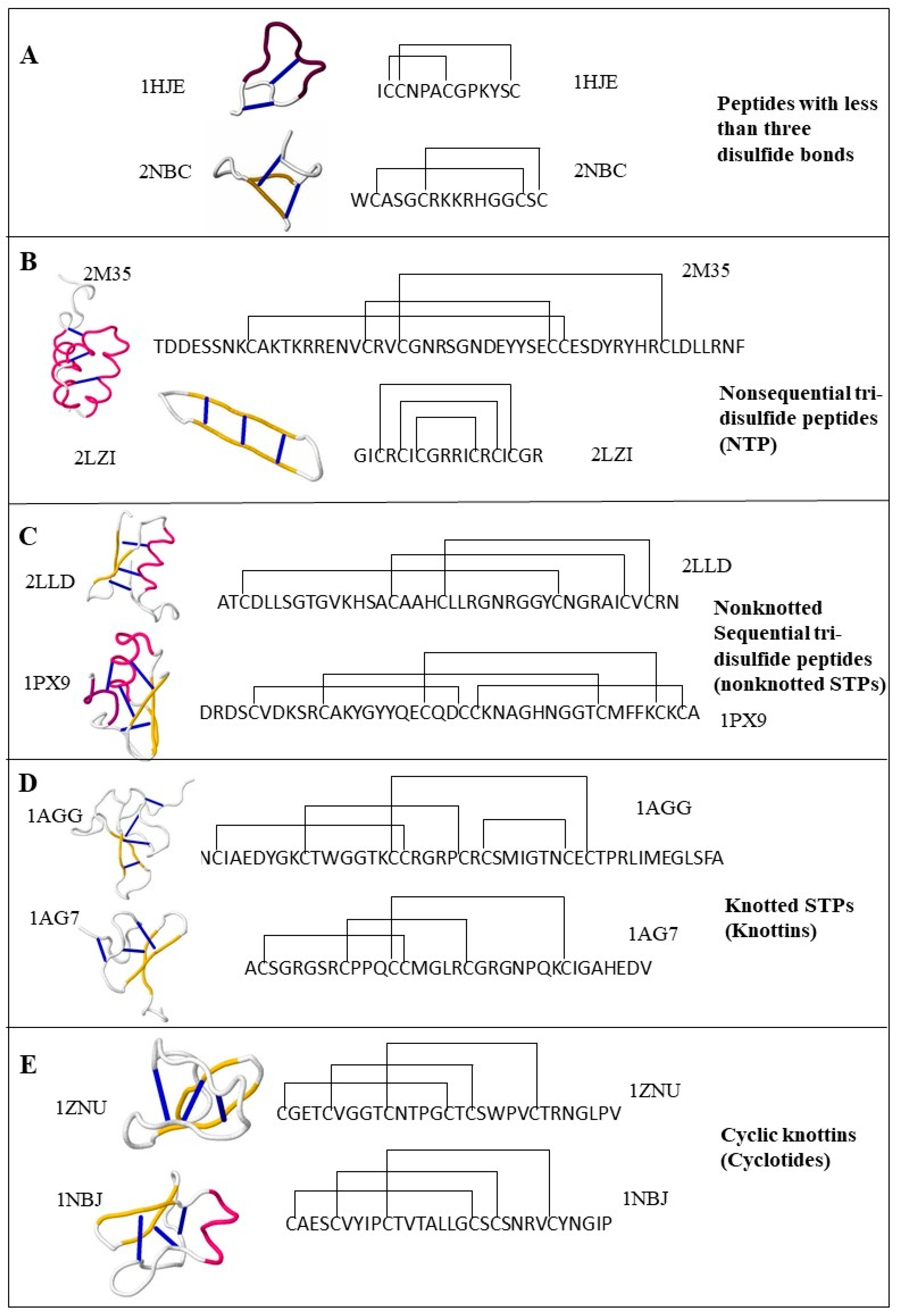
2.1.1. Peptides with Three or More Disulfide Bonds
2.1.2. Sequential Tri-Disulfide Peptides (STPs) and Nonsequential Tri-Disulfide Peptides (NTPs)
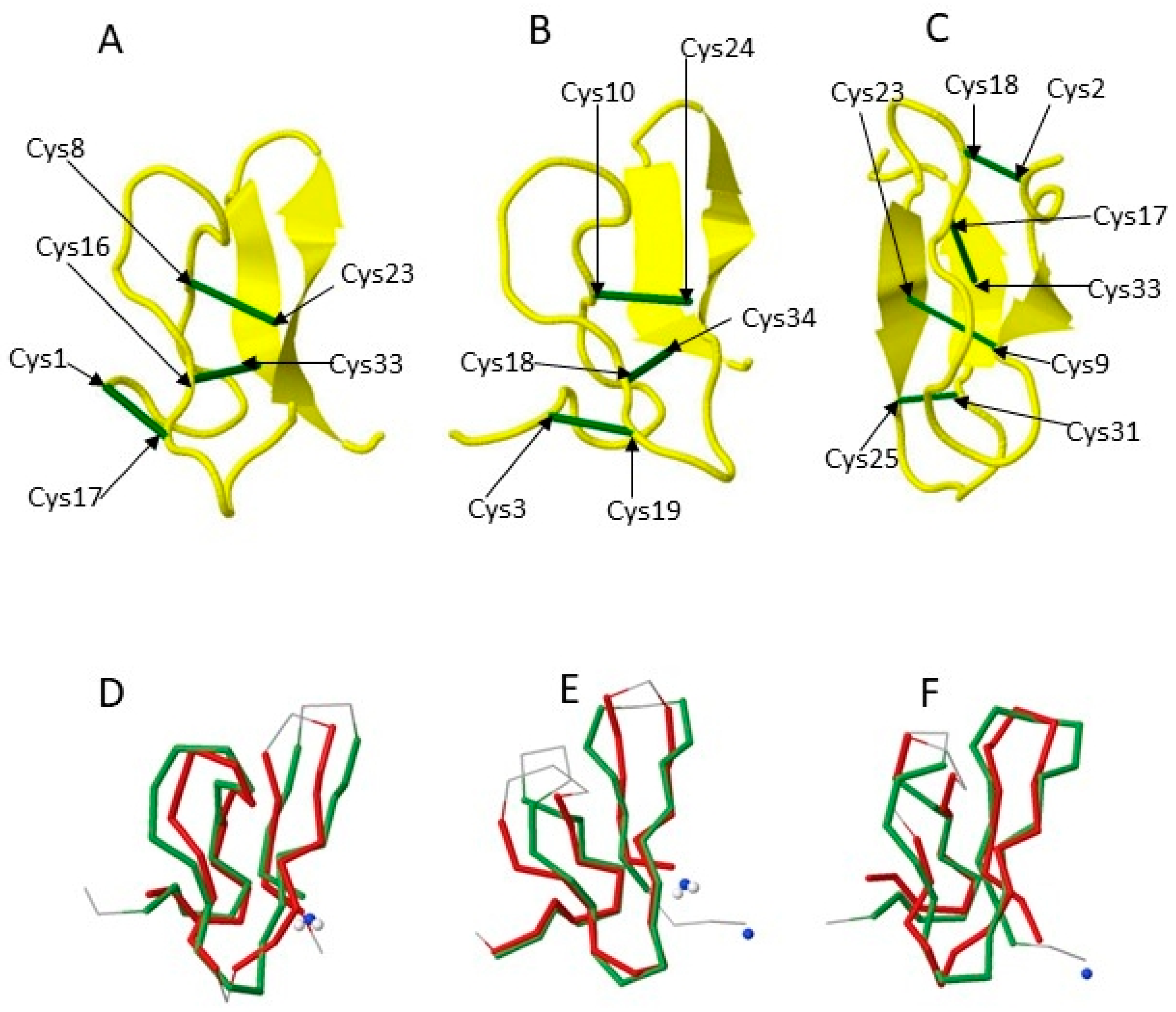
2.1.3. Knottins (Knotted STPs)
2.1.4. Cyclotides (Cyclic Knottins)
2.1.5. Peptides with Fewer than Three Disulfide Bonds
2.2. Source-Based Subfamilies of Cystine-Stabilized Peptides
2.2.1. Spider and Scorpion Toxins
2.2.2. Conotoxins
2.2.3. Plant Cysteine-Rich Peptides
2.2.4. Other Sources
3. Function-Based In Silico Classification of Cystine-Stabilized Peptides
4. Pharmaceutical and Commercial Applications
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Linial, M.; Rappoport, N.; Ofer, D. Overlooked Short Toxin-Like Proteins: A Shortcut to Drug Design. Toxins 2017, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Selitrennikoff, C.P. Antifungal Proteins. Appl. Environ. Microbiol. 2001, 67, 2883–2894. [Google Scholar] [CrossRef] [PubMed]
- Estrada, G.; Villegas, E.; Corzo, G. Spider venoms: A rich source of acylpolyamines and peptides as new leads for CNS drugs. Nat. Prod. Rep. 2007, 24, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Gruber, C.W.; Čemažar, M.; Anderson, M.A.; Craik, D.J. Insecticidal plant cyclotides and related cystine knot toxins. Toxicon 2007, 49, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Darbon, H.; Dyason, K.; Verdonck, F.; Tytgat, J. Evolutionary origin of inhibitor cystine knot peptides. FASEB J. 2003, 17, 1765–1767. [Google Scholar] [CrossRef] [PubMed]
- Gracy, J.; Le-Nguyen, D.; Gelly, J.-C.; Kaas, Q.; Heitz, A.; Chiche, L. KNOTTIN: The knottin or inhibitor cystine knot scaffold in 2007. Nucleic Acids Res. 2008, 36, D314–D319. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.J.; Herzig, V.; King, G.F.; Alewood, P.F. The insecticidal potential of venom peptides. Cell. Mol. Life Sci. 2013, 70, 3665–3693. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Teng, D.; Wang, X.; Xi, D.; Zhang, Y.; Hu, X.; Yang, Y.; Wang, J. Design, expression, and characterization of a novel targeted plectasin against methicillin-resistant Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2013, 97, 3991–4002. [Google Scholar] [CrossRef] [PubMed]
- King, G.F. Venoms as a platform for human drugs: Translating toxins into therapeutics. Expert Opin. Biol. Ther. 2011, 11, 1469–1484. [Google Scholar] [CrossRef] [PubMed]
- Thomma, B.; Cammue, B.; Thevissen, K. Plant defensins. Planta 2002, 216, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Olivera, B.; Gray, W.; Zeikus, R.; McIntosh, J.; Varga, J.; Rivier, J.; de Santos, V.; Cruz, L. Peptide neurotoxins from fish-hunting cone snails. Science 1985, 230, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.M.A.; Sajed, T.; Kearney, C.M.; Baker, E.J. PredSTP: A highly accurate SVM based model to predict sequential cystine stabilized peptides. BMC Bioinform. 2015, 16. [Google Scholar] [CrossRef] [PubMed]
- Gould, A.; Ji, Y.; Aboye, T.L.; Camarero, J.A. Cyclotides, a novel ultrastable polypeptide scaffold for drug discovery. Curr. Pharm. Des. 2011, 17, 4294–4307. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Garcia, M.L. Therapeutic potential of venom peptides. Nat. Rev. Drug Discov. 2003, 2, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Baindara, P.; Kapoor, A.; Korpole, S.; Grover, V. Cysteine-rich low molecular weight antimicrobial peptides from Brevibacillus and related genera for biotechnological applications. World J. Microbiol. Biotechnol. 2017, 33, 124. [Google Scholar] [CrossRef] [PubMed]
- Koehbach, J. Structure-Activity Relationships of Insect Defensins. Front. Chem. 2017, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Kellenberger, C.; Roussel, A. Structure-activity relationship within the serine protease inhibitors of the pacifastin family. Protein Peptide Lett. 2005, 12, 409–414. [Google Scholar] [CrossRef]
- Livett, B.G.; Sandall, D.W.; Keays, D.; Down, J.; Gayler, K.R.; Satkunanathan, N.; Khalil, Z. Therapeutic applications of conotoxins that target the neuronal nicotinic acetylcholine receptor. Toxicon 2006, 48, 810–829. [Google Scholar] [CrossRef] [PubMed]
- Henriques, S.T.; Huang, Y.H.; Rosengren, K.J.; Franquelim, H.G.; Carvalho, F.A.; Johnson, A.; Sonza, S.; Tachedjian, G.; Castanho, M.A.R.B.; Daly, N.L.; et al. Decoding the Membrane Activity of the Cyclotide Kalata B1: The importance of phosphatidylethanolamine phospholipids and lipid organization on hemolytic and Anti-HIV activities. J. Biol. Chem. 2011, 286, 24231–24241. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.M.A.; Heil, B.J.; Kearney, C.M.; Baker, E.J. Protein classification using modified n-grams and skip-grams. Bioinformatics 2017. [Google Scholar] [CrossRef] [PubMed]
- Postic, G.; Gracy, J.; Périn, C.; Chiche, L.; Gelly, J.-C. KNOTTIN: The database of inhibitor cystine knot scaffold after 10 years, toward a systematic structure modeling. Nucleic Acids Res. 2018, 46, D454–D458. [Google Scholar] [CrossRef] [PubMed]
- Saether, O.; Craik, D.J.; Campbell, I.D.; Sletten, K.; Juul, J.; Norman, D.G. Elucidation of the Primary and Three-Dimensional Structure of the Uterotonic Polypeptide Kalata B1. Biochemistry 1995, 34, 4147–4158. [Google Scholar] [CrossRef] [PubMed]
- Jennings, C.; West, J.; Waine, C.; Craik, D.; Anderson, M. Biosynthesis and insecticidal properties of plant cyclotides: The cyclic knotted proteins from Oldenlandia affinis. Proc. Natl. Acad. Sci. USA 2001, 98, 10614–10619. [Google Scholar] [CrossRef] [PubMed]
- Jennings, C.V.; Rosengren, K.J.; Daly, N.L.; Plan, M.; Stevens, J.; Scanlon, M.J.; Waine, C.; Norman, D.G.; Anderson, M.A.; Craik, D.J. Isolation, solution structure, and insecticidal activity of kalata B2, a circular protein with a twist: Do Möbius strips exist in nature? Biochemistry 2005, 44, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Colgrave, M.L.; Kotze, A.C.; Huang, Y.-H.; O’Grady, J.; Simonsen, S.M.; Craik, D.J. Cyclotides: Natural, circular plant peptides that possess significant activity against gastrointestinal nematode parasites of sheep. Biochemistry 2008, 47, 5581–5589. [Google Scholar] [CrossRef] [PubMed]
- Colgrave, M.L.; Kotze, A.C.; Ireland, D.C.; Wang, C.K.; Craik, D.J. The anthelmintic activity of the cyclotides: Natural variants with enhanced activity. ChemBioChem 2008, 9, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Colgrave, M.L.; Kotze, A.C.; Kopp, S.; McCarthy, J.S.; Coleman, G.T.; Craik, D.J. Anthelmintic activity of cyclotides: In vitro studies with canine and human hookworms. Acta Trop. 2009, 109, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Malagón, D.; Botterill, B.; Gray, D.J.; Lovas, E.; Duke, M.; Gray, C.; Kopp, S.R.; Knott, L.M.; McManus, D.P.; Daly, N.L.; et al. Anthelminthic activity of the cyclotides (kalata B1 and B2) against schistosome parasites. Biopolymers 2013, 100, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, K.J.; Daly, N.L.; Plan, M.R.; Waine, C.; Craik, D.J. Twists, knots, and rings in proteins. Structural definition of the cyclotide framework. J. Biol. Chem. 2003, 278, 8606–8616. [Google Scholar] [CrossRef] [PubMed]
- Koehbach, J.; Attah, A.F.; Berger, A.; Hellinger, R.; Kutchan, T.M.; Carpenter, E.J.; Rolf, M.; Sonibare, M.A.; Moody, J.O.; Wong, G.K.-S.; et al. Cyclotide discovery in Gentianales revisited-identification and characterization of cyclic cystine-knot peptides and their phylogenetic distribution in Rubiaceae plants: Cyclotide Discovery in Gentianales Revisited. Biopolymers 2013, 100, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Koehbach, J.; O’Brien, M.; Muttenthaler, M.; Miazzo, M.; Akcan, M.; Elliott, A.G.; Daly, N.L.; Harvey, P.J.; Arrowsmith, S.; Gunasekera, S.; et al. Oxytocic plant cyclotides as templates for peptide G protein-coupled receptor ligand design. Proc. Natl. Acad. Sci. USA 2013, 110, 21183–21188. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, S.M.; Sando, L.; Ireland, D.C.; Colgrave, M.L.; Bharathi, R.; Göransson, U.; Craik, D.J. A continent of plant defense peptide diversity: Cyclotides in Australian Hybanthus (Violaceae). Plant Cell 2005, 17, 3176–3189. [Google Scholar] [CrossRef] [PubMed]
- Avrutina, O.; Schmoldt, H.-U.; Gabrijelcic-Geiger, D.; Le Nguyen, D.; Sommerhoff, C.P.; Diederichsen, U.; Kolmar, H. Trypsin inhibition by macrocyclic and open-chain variants of the squash inhibitor MCoTI-II. Biol. Chem. 2005, 386, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Mylne, J.S.; Chan, L.Y.; Chanson, A.H.; Daly, N.L.; Schaefer, H.; Bailey, T.L.; Nguyencong, P.; Cascales, L.; Craik, D.J. Cyclic peptides arising by evolutionary parallelism via asparaginyl-endopeptidase-mediated biosynthesis. Plant Cell 2012, 24, 2765–2778. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.K.T.; Zhang, S.; Nguyen, N.T.K.; Nguyen, P.Q.T.; Chiu, M.S.; Hardjojo, A.; Tam, J.P. Discovery and characterization of novel cyclotides originated from chimeric precursors consisting of albumin-1 chain a and cyclotide domains in the Fabaceae family. J. Biol. Chem. 2011, 286, 24275–24287. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.K.T.; Lim, W.H.; Nguyen, P.Q.T.; Tam, J.P. Novel cyclotides and uncyclotides with highly shortened precursors from Chassalia chartacea and effects of methionine oxidation on bioactivities. J. Biol. Chem. 2012, 287, 17598–17607. [Google Scholar] [CrossRef] [PubMed]
- Poth, A.G.; Colgrave, M.L.; Lyons, R.E.; Daly, N.L.; Craik, D.J. Discovery of an unusual biosynthetic origin for circular proteins in legumes. Proc. Natl. Acad. Sci. USA 2011, 108, 10127–10132. [Google Scholar] [CrossRef] [PubMed]
- Poth, A.G.; Mylne, J.S.; Grassl, J.; Lyons, R.E.; Millar, A.H.; Colgrave, M.L.; Craik, D.J. Cyclotides associate with leaf vasculature and are the products of a novel precursor in petunia (Solanaceae). J. Biol. Chem. 2012, 287, 27033–27046. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.K.L.; Kaas, Q.; Chiche, L.; Craik, D.J. CyBase: A database of cyclic protein sequences and structures, with applications in protein discovery and engineering. Nucleic Acids Res. 2007, 36, D206–D210. [Google Scholar] [CrossRef] [PubMed]
- Conibear, A.C.; Rosengren, K.J.; Harvey, P.J.; Craik, D.J. Structural characterization of the cyclic cystine ladder motif of θ-defensins. Biochemistry 2012, 51, 9718–9726. [Google Scholar] [CrossRef] [PubMed]
- González, C.; Langdon, G.M.; Bruix, M.; Gálvez, A.; Valdivia, E.; Maqueda, M.; Rico, M. Bacteriocin AS-48, a microbial cyclic polypeptide structurally and functionally related to mammalian NK-lysin. Proc. Natl. Acad. Sci. USA 2000, 97, 11221–11226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hua, Z.; Huang, Z.; Chen, Q.; Long, Q.; Craik, D.J.; Baker, A.J.M.; Shu, W.; Liao, B. Two Blast-independent tools, CyPerl and CyExcel, for harvesting hundreds of novel cyclotides and analogues from plant genomes and protein databases. Planta 2015, 241, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Henz, M.E.; Gallagher, N.L.; Chai, S.; Gibbs, A.C.; Yan, L.Z.; Stiles, M.E.; Wishart, D.S.; Vederas, J.C. Solution structure of carnobacteriocin B2 and implications for structure-activity relationships among type IIa bacteriocins from lactic acid bacteria. Biochemistry 1999, 38, 15438–15447. [Google Scholar] [CrossRef] [PubMed]
- Fahrner, R.L.; Dieckmann, T.; Harwig, S.S.; Lehrer, R.I.; Eisenberg, D.; Feigon, J. Solution structure of protegrin-1, a broad-spectrum antimicrobial peptide from porcine leukocytes. Chem. Biol. 1996, 3, 543–550. [Google Scholar] [CrossRef]
- Rigby, A.C.; Lucas-Meunier, E.; Kalume, D.E.; Czerwiec, E.; Hambe, B.; Dahlqvist, I.; Fossier, P.; Baux, G.; Roepstorff, P.; Baleja, J.D.; et al. A conotoxin from Conus textile with unusual posttranslational modifications reduces presynaptic Ca2+ influx. Proc. Natl. Acad. Sci. USA 1999, 96, 5758–5763. [Google Scholar] [CrossRef] [PubMed]
- Saez, N.J.; Senff, S.; Jensen, J.E.; Er, S.Y.; Herzig, V.; Rash, L.D.; King, G.F. Spider-Venom Peptides as Therapeutics. Toxins 2010, 2, 2851–2871. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.-P.; Pan, X. A lectin-like peptide isolated from the venom of the chinese bird spider Selenocosmia huwena. Toxicon 1995, 33, 875–882. [Google Scholar] [CrossRef]
- Herzig, V.; Wood, D.L.A.; Newell, F.; Chaumeil, P.-A.; Kaas, Q.; Binford, G.J.; Nicholson, G.M.; Gorse, D.; King, G.F. ArachnoServer 2.0, an updated online resource for spider toxin sequences and structures. Nucleic Acids Res. 2011, 39, D653–D657. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H. Predicting Secretory Proteins with SignalP. Methods Mol. Biol. 2017, 1611, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.T.J.; Veeramani, A.; Srinivasan, K.N.; Ranganathan, S.; Brusic, V. SCORPION2: A database for structure-function analysis of scorpion toxins. Toxicon 2006, 47, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.T.J.; Srinivasan, K.N.; Seah, S.H.; Koh, J.L.Y.; Tan, T.W.; Ranganathan, S.; Brusic, V. Accurate prediction of scorpion toxin functional properties from primary structures. J. Mol. Gr. Model. 2005, 24, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Peng, C.; Yang, J.; Yi, Y.; Zhang, J.; Shi, Q. Cone Snails: A Big Store of Conotoxins for Novel Drug Discovery. Toxins 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Mir, R.; Karim, S.; Kamal, M.A.; Wilson, C.M.; Mirza, Z. Conotoxins: Structure, Therapeutic Potential and Pharmacological Applications. Curr. Pharm. Des. 2016, 22, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, C.J.A.; Cerutti, L.; de Castro, E.; Langendijk-Genevaux, P.S.; Bulliard, V.; Bairoch, A.; Hulo, N. PROSITE, a protein domain database for functional characterization and annotation. Nucleic Acids Res. 2010, 38, D161–D166. [Google Scholar] [CrossRef] [PubMed]
- Kaas, Q.; Yu, R.; Jin, A.-H.; Dutertre, S.; Craik, D.J. ConoServer: Updated content, knowledge, and discovery tools in the conopeptide database. Nucleic Acids Res. 2012, 40, D325–D330. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Bhavna, R.; Mohan Babu, R.; Ramakumar, S. Pseudo amino acid composition and multi-class support vector machines approach for conotoxin superfamily classification. J. Theor. Biol. 2006, 243, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Li, Q.-Z. Predicting conotoxin superfamily and family by using pseudo amino acid composition and modified Mahalanobis discriminant. Biochem. Biophys. Res. Commun. 2007, 354, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zheng, Y.; Tang, H. Identifying the Types of Ion Channel-Targeted Conotoxins by Incorporating New Properties of Residues into Pseudo Amino Acid Composition. BioMed Res. Int. 2016, 2016, 3981478. [Google Scholar] [CrossRef] [PubMed]
- Xianfang, W.; Junmei, W.; Xiaolei, W.; Yue, Z. Predicting the Types of Ion Channel-Targeted Conotoxins Based on AVC-SVM Model. BioMed Res. Int. 2017, 2017, 2929807. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.-F.; Ding, C.; Guo, S.-H.; Ding, H.; Chen, W.; Lin, H. Prediction of the types of ion channel-targeted conotoxins based on radial basis function network. Toxicol In Vitro 2013, 27, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Deng, E.-Z.; Yuan, L.-F.; Liu, L.; Lin, H.; Chen, W.; Chou, K.-C. iCTX-type: A sequence-based predictor for identifying the types of conotoxins in targeting ion channels. BioMed Res. Int. 2014, 2014, 286419. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.; Wang, S.; Wong, K.; Tan, W. Antimicrobial Peptides from Plants. Pharmaceuticals 2015, 8, 711–757. [Google Scholar] [CrossRef] [PubMed]
- Stotz, H.U.; Thomson, J.; Wang, Y. Plant defensins: Defense, development and application. Plant Signal. Behav. 2009, 4, 1010–1012. [Google Scholar] [CrossRef] [PubMed]
- Porto, W.F.; Souza, V.A.; Nolasco, D.O.; Franco, O.L. In silico identification of novel hevein-like peptide precursors. Peptides 2012, 38, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Teeter, M.M.; Mazer, J.A.; L’Italien, J.J. Primary structure of the hydrophobic plant protein crambin. Biochemistry 1981, 20, 5437–5443. [Google Scholar] [CrossRef] [PubMed]
- García-Olmedo, F.; Molina, A.; Segura, A.; Moreno, M. The defensive role of nonspecific lipid-transfer proteins in plants. Trends Microbiol. 1995, 3, 72–74. [Google Scholar] [CrossRef]
- Rees, D.C.; Lipscomb, W.N. Refined crystal structure of the potato inhibitor complex of carboxypeptidase A at 2.5 A resolution. J. Mol. Biol. 1982, 160, 475–498. [Google Scholar] [CrossRef]
- Segura, A.; Moreno, M.; Madueño, F.; Molina, A.; García-Olmedo, F. Snakin-1, a Peptide from Potato That Is Active Against Plant Pathogens. Mol. Plant-Microbe Interact. 1999, 12, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Sels, J.; Mathys, J.; De Coninck, B.M.A.; Cammue, B.P.A.; De Bolle, M.F.C. Plant pathogenesis-related (PR) proteins: A focus on PR peptides. Plant Physiol. Biochem. 2008, 46, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.; Singh, R.P.; Kushwaha, G.S.; Iqbal, N.; Singh, A.; Kaushik, S.; Kaur, P.; Sharma, S.; Singh, T.P. Current overview of allergens of plant pathogenesis related protein families. Sci. World J. 2014, 2014, 543195. [Google Scholar] [CrossRef] [PubMed]
- Stintzi, A.; Heitz, T.; Prasad, V.; Wiedemann-Merdinoglu, S.; Kauffmann, S.; Geoffroy, P.; Legrand, M.; Fritig, B. Plant “pathogenesis-related” proteins and their role in defense against pathogens. Biochimie 1993, 75, 687–706. [Google Scholar] [CrossRef]
- Silverstein, K.A.T.; Moskal, W.A.; Wu, H.C.; Underwood, B.A.; Graham, M.A.; Town, C.D.; VandenBosch, K.A. Small cysteine-rich peptides resembling antimicrobial peptides have been under-predicted in plants. Plant J. 2007, 51, 262–280. [Google Scholar] [CrossRef] [PubMed]
- Hammami, R.; Ben Hamida, J.; Vergoten, G.; Fliss, I. PhytAMP: A database dedicated to antimicrobial plant peptides. Nucleic Acids Res. 2009, 37, D963–D968. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.A.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genom. Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Solanki, V.; Sharma, S.; Thakur, K.G.; Krishnan, B.; Korpole, S. The intramolecular disulfide-stapled structure of laterosporulin, a class IId bacteriocin, conceals a human defensin-like structural module. FEBS J. 2015, 282, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Sit, C.S.; McKay, R.T.; Hill, C.; Ross, R.P.; Vederas, J.C. The 3D structure of thuricin CD, a two-component bacteriocin with cysteine sulfur to α-carbon cross-links. J. Am. Chem. Soc. 2011, 133, 7680–7683. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef]
- Hammami, R.; Zouhir, A.; Ben Hamida, J.; Fliss, I. BACTIBASE: A new web-accessible database for bacteriocin characterization. BMC Microbiol. 2007, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, R.I.; Ganz, T. Defensins of vertebrate animals. Curr. Opin. Immunol. 2002, 14, 96–102. [Google Scholar] [CrossRef]
- Rodriguezdelavega, R.; Possani, L. On the evolution of invertebrate defensins. Trends Genet. 2005, 21, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Mygind, P.H.; Fischer, R.L.; Schnorr, K.M.; Hansen, M.T.; Sönksen, C.P.; Ludvigsen, S.; Raventós, D.; Buskov, S.; Christensen, B.; De Maria, L.; et al. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature 2005, 437, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Oeemig, J.S.; Lynggaard, C.; Knudsen, D.H.; Hansen, F.T.; Nørgaard, K.D.; Schneider, T.; Vad, B.S.; Sandvang, D.H.; Nielsen, L.A.; Neve, S.; et al. Eurocin, a new fungal defensin: Structure, lipid binding, and its mode of action. J. Biol. Chem. 2012, 287, 42361–42372. [Google Scholar] [CrossRef] [PubMed]
- Seebah, S.; Suresh, A.; Zhuo, S.; Choong, Y.H.; Chua, H.; Chuon, D.; Beuerman, R.; Verma, C. Defensins knowledgebase: A manually curated database and information source focused on the defensins family of antimicrobial peptides. Nucleic Acids Res. 2007, 35, D265–D268. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.M.A.; Kearney, C.M.; Baker, E.J. CSPred: A machine-learning-based compound model to identify the functional activities of biologically-stable toxins. In Proceedings of the 2017 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Kansas City, MO, USA, 13–16 November 2017; pp. 2254–2255. [Google Scholar]
- Kedarisetti, P.; Mizianty, M.J.; Kaas, Q.; Craik, D.J.; Kurgan, L. Prediction and characterization of cyclic proteins from sequences in three domains of life. Biochim. Biophys. Acta 2014, 1844, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Herzig, V.; King, G. The Cystine Knot Is Responsible for the Exceptional Stability of the Insecticidal Spider Toxin ω-Hexatoxin-Hv1a. Toxins 2015, 7, 4366–4380. [Google Scholar] [CrossRef] [PubMed]
- Bonning, B.C.; Pal, N.; Liu, S.; Wang, Z.; Sivakumar, S.; Dixon, P.M.; King, G.F.; Miller, W.A. Toxin delivery by the coat protein of an aphid-vectored plant virus provides plant resistance to aphids. Nat. Biotechnol. 2013, 32, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Dutertre, S.; Nicke, A.; Tsetlin, V.I. Nicotinic acetylcholine receptor inhibitors derived from snake and snail venoms. Neuropharmacology 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhirnov, O.P.; Klenk, H.D.; Wright, P.F. Aprotinin and similar protease inhibitors as drugs against influenza. Antivir. Res. 2011, 92, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Bourinet, E.; Zamponi, G.W. Block of voltage-gated calcium channels by peptide toxins. Neuropharmacology 2016. [Google Scholar] [CrossRef] [PubMed]
- Dardevet, L.; Rani, D.; Aziz, T.; Bazin, I.; Sabatier, J.-M.; Fadl, M.; Brambilla, E.; De Waard, M. Chlorotoxin: A Helpful Natural Scorpion Peptide to Diagnose Glioma and Fight Tumor Invasion. Toxins 2015, 7, 1079–1101. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Inbar, O.; Zaaroor, M. Glioblastoma multiforme targeted therapy: The Chlorotoxin story. J. Clin. Neurosci. 2016, 33, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Layer, P.; Stanghellini, V. Review article: Linaclotide for the management of irritable bowel syndrome with constipation. Aliment. Pharmacol. Ther. 2014, 39, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Werle, M.; Kolmar, H.; Albrecht, R.; Bernkop-Schnürch, A. Characterisation of the barrier caused by luminally secreted gastro-intestinal proteolytic enzymes for two novel cystine-knot microproteins. Amino Acids 2008, 35, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Werle, M.; Kafedjiiski, K.; Kolmar, H.; Bernkop-Schnürch, A. Evaluation and improvement of the properties of the novel cystine-knot microprotein McoEeTI for oral administration. Int. J. Pharm. 2007, 332, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Troeira Henriques, S.; Craik, D.J. Cyclotide Structure and Function: The Role of Membrane Binding and Permeation. Biochemistry 2017, 56, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R.; He, J.; Yarbrough, D.K.; Qi, F.; Anderson, M.H.; Shi, W. Targeted Killing of Streptococcus mutans by a Pheromone-Guided “Smart” Antimicrobial Peptide. Antimicrob. Agents Chemother. 2006, 50, 3651–3657. [Google Scholar] [CrossRef] [PubMed]
- Peschen, D.; Li, H.-P.; Fischer, R.; Kreuzaler, F.; Liao, Y.-C. Fusion proteins comprising a Fusarium-specific antibody linked to antifungal peptides protect plants against a fungal pathogen. Nat. Biotechnol. 2004, 22, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Chougule, N.P.; Li, H.; Liu, S.; Linz, L.B.; Narva, K.E.; Meade, T.; Bonning, B.C. Retargeting of the Bacillus thuringiensis toxin Cyt2Aa against hemipteran insect pests. Proc. Natl. Acad. Sci. USA 2013, 110, 8465–8470. [Google Scholar] [CrossRef] [PubMed]
| Database Name | Target Protein | URL | Ref |
|---|---|---|---|
| Knottin | Knottins | http://www.dsimb.inserm.fr/KNOTTIN/ | [22] |
| Cybase | Cyclotides and other disulfide rich cyclic proteins | http://www.cybase.org.au/ | [40] |
| Arachnoserver | Spider toxins | http://www.arachnoserver.org | [49] |
| SCORPION2 * | Scorpion toxins | http://sdmc.i2r.a-star.edu.sg/scorpion/ | [51] |
| ConoServer | Conotoxins | http://www.conoserver.org | [56] |
| PhytAMP | Cystine-rich antimicrobial peptides from plants | http://phytamp.hammamilab.org | [74] |
| Bactibase | Bacteriocins | http://bactibase.hammamilab.org | [79] |
| Defensin Knowledgebase | Defensins | http://defensins.bii.a-star.edu.sg | [84] |
| Name of Software | Function | Web Link | Ref. |
|---|---|---|---|
| PredSTP | Predicts sequential tri-disulfide proteins (STP) from primary peptide sequence | http://crick.ecs.baylor.edu/ | [13] |
| CSPred | Classifies cystine-stabilized peptides based on functional characteristics of the primary peptide sequence | http://watson.ecs.baylor.edu/cspred | [85] |
| Knoter 1D | Predicts knottins from primary peptide sequence | http://www.dsimb.inserm.fr/KNOTTIN/knoter1d.php | [22] |
| Knoter 3D | Knottin prediction from 3D protein structure | http://www.dsimb.inserm.fr/KNOTTIN/knoter3d.php | [22] |
| Cypred | Cyclic structure prediction from primary peptide sequence | http://biomine.cs.vcu.edu/servers/CyPred | [86] |
| Cyclomode | Cyclotide 3D structure prediction from primary peptide sequence | http://www.cybase.org.au/?page=cyclomod | [40] |
| SpiderP | Predicts the subcellular localization of spider toxins from the primary peptide sequence | http://www.arachnoserver.org/spiderP.html | [50] |
| iCTX-Type | Predicts subclasses of ion-channel binding conotoxins from the primary peptide sequence | http://lin-group.cn/server/iCTX-Type | [62] |
| Peptide | Structure | Company | Stage | Use |
|---|---|---|---|---|
| HXTX-Hv1a spider toxin | STP (knottin) | Vestaron | Commercial crop spray (SPEAR™ product line) | Control of thrips, whiteflies, caterpillars, beetles |
| Plectasin NZ2114 | STP (defensin) | Novozyme (licensed to Sanofi-Aventis) | Phase I | Severe gram-positive bacterial infections |
| Brilacidin | Nonpeptide STP mimetic | Cellceutix | Phase II | Ulcerative proctitis |
| Linclotide | STP | Ironwood Pharmaceuticals | Phase III | Irritable bowel syndrome; chronic constipation |
| Ziconotide (Prialt) | STP (calcium channel blocker) | Azur Pharma | FDA approved; commercial | Analgesic |
| Alpha-bungaro-toxin | NTP (acetyl-choline receptor inhibitor) | Commercial | Diagnostics | |
| Aprotinin | NTP (trypsin inhibitor) | Nordic Group Pharmaceuticals | Commercial | Reduction of bleeding |
| Chlorotoxin | STP (chloride channel inhibitor) | Transmolecular | Phase III | Anti-glioma and imaging |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, S.M.A.; Kearney, C.M.; Baker, E. Classes, Databases, and Prediction Methods of Pharmaceutically and Commercially Important Cystine-Stabilized Peptides. Toxins 2018, 10, 251. https://doi.org/10.3390/toxins10060251
Islam SMA, Kearney CM, Baker E. Classes, Databases, and Prediction Methods of Pharmaceutically and Commercially Important Cystine-Stabilized Peptides. Toxins. 2018; 10(6):251. https://doi.org/10.3390/toxins10060251
Chicago/Turabian StyleIslam, S M Ashiqul, Christopher Michel Kearney, and Erich Baker. 2018. "Classes, Databases, and Prediction Methods of Pharmaceutically and Commercially Important Cystine-Stabilized Peptides" Toxins 10, no. 6: 251. https://doi.org/10.3390/toxins10060251
APA StyleIslam, S. M. A., Kearney, C. M., & Baker, E. (2018). Classes, Databases, and Prediction Methods of Pharmaceutically and Commercially Important Cystine-Stabilized Peptides. Toxins, 10(6), 251. https://doi.org/10.3390/toxins10060251





