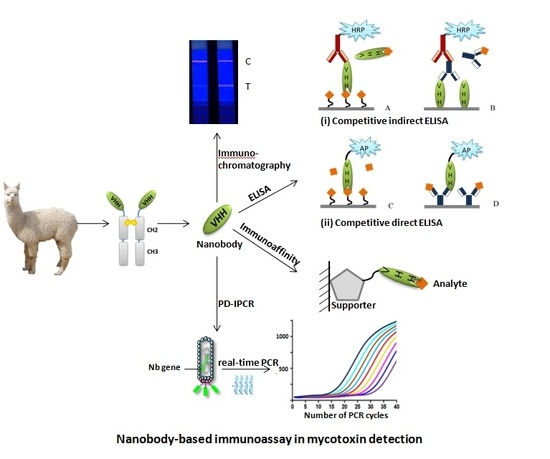
Nanobody Technology for Mycotoxin Detection: Current Status and Prospects
Abstract
1. Introduction
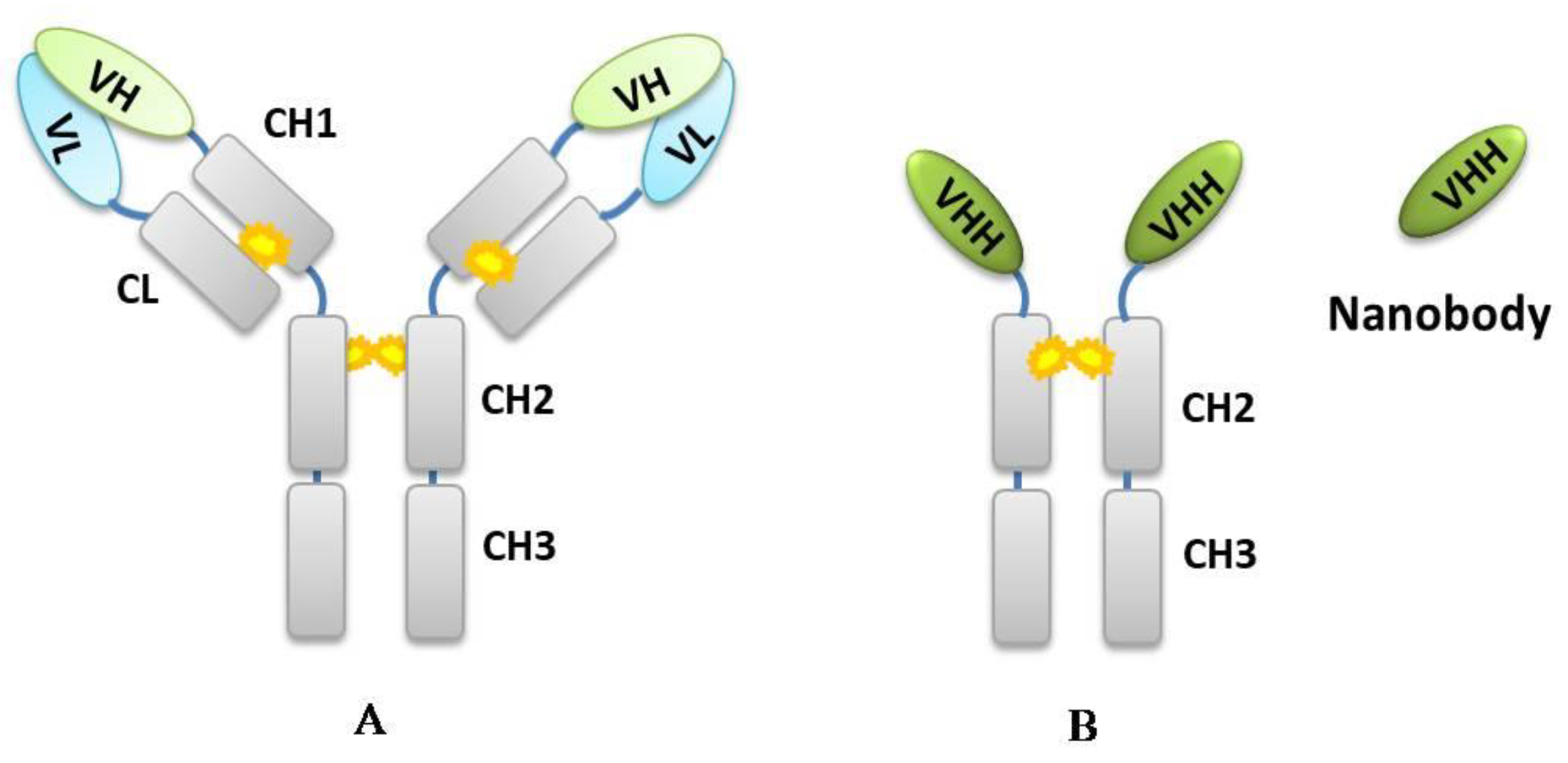
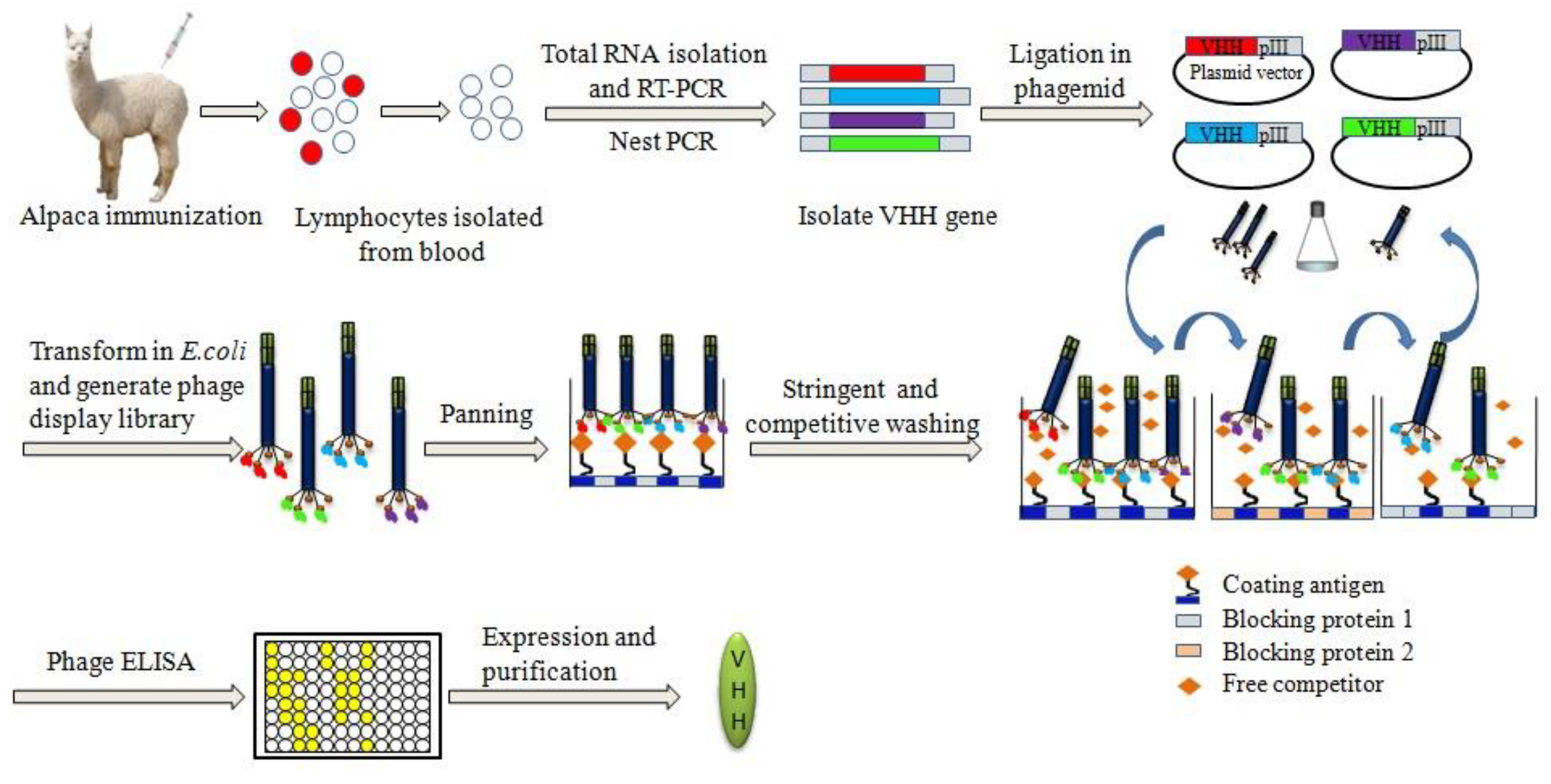
2. Principle of Developing Chemical-Specific Nb
3. Performance of Nbs in the Field of Chemical Analysis
3.1. Affinity (Sensitivity)
3.2. Selectivity
3.3. Stability
3.4. Solubility
4. Application of Nbs in the Area of Mycotoxin Detection
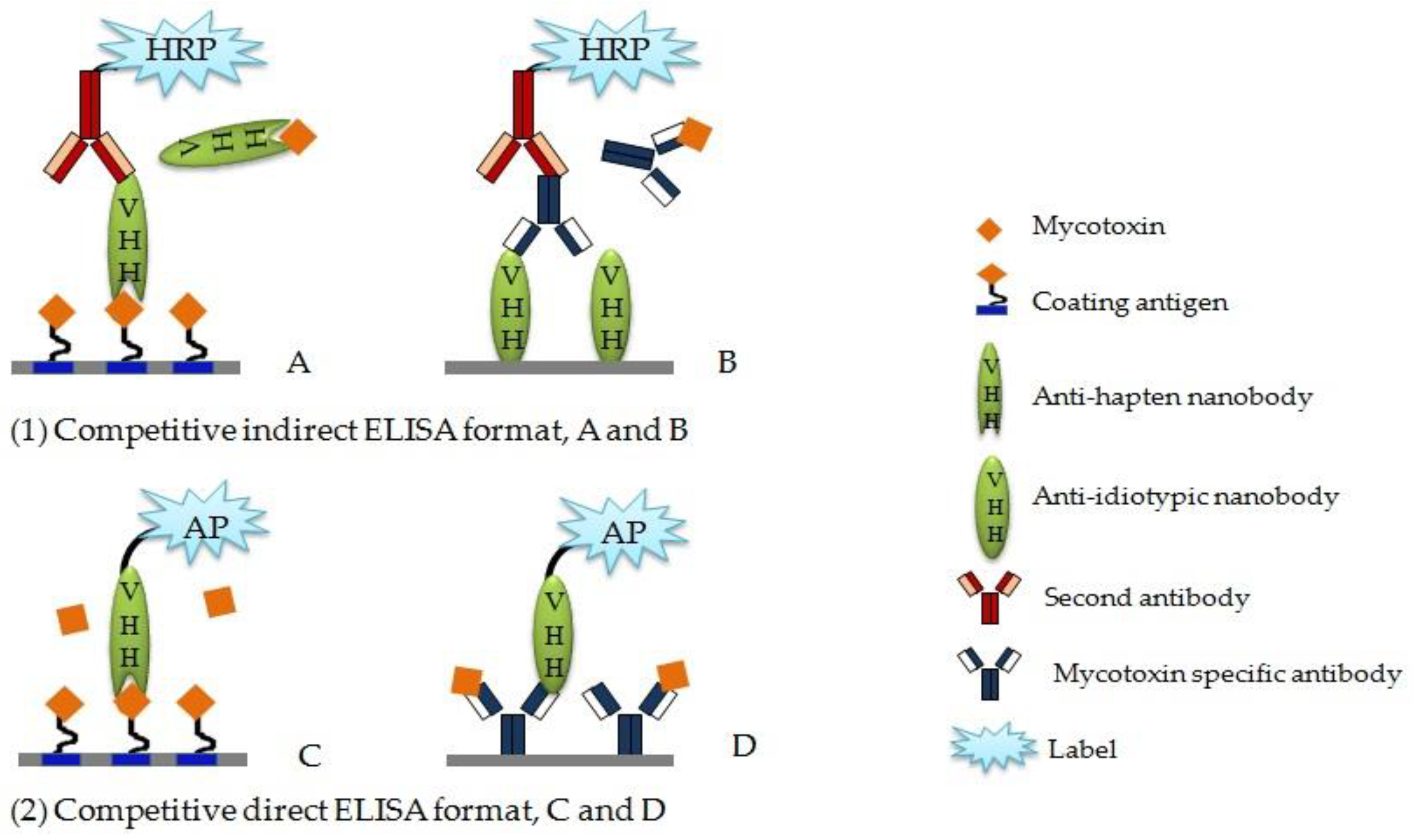
4.1. Competitive Enzyme-Linked Immunosorbent Aassay
4.2. Anti-Idiotypic Nb-based ELISA
4.3. Phage Display Mediated Immuno-Polymerase Chain Reaction
4.4. Nb-Based Fluorescence Polarization Immunoassay
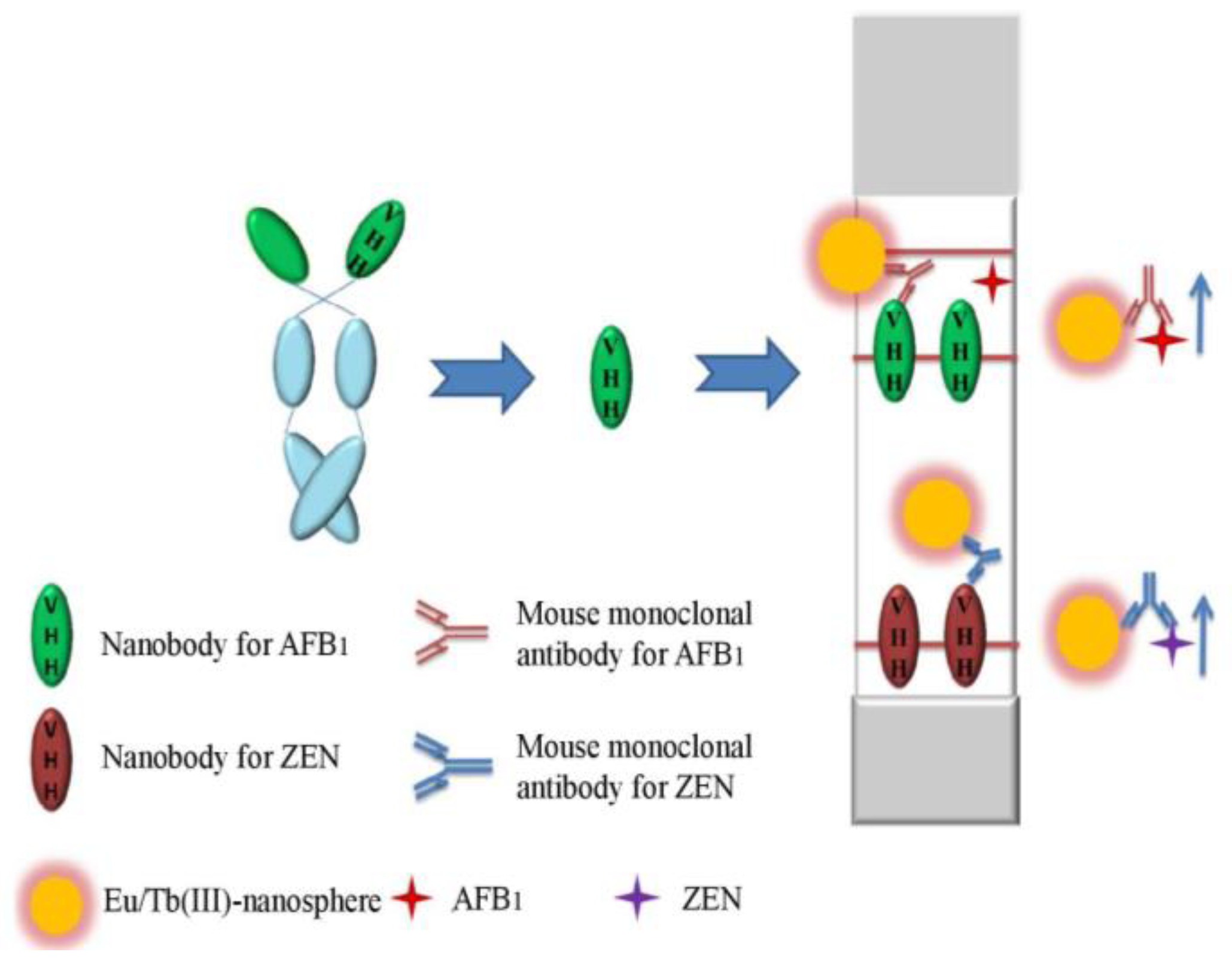
4.5. Nb-based Lateral-Flow Immunoassays
4.6. Nb-Based Immunoaffinity Method
5. Challenge of Nb Application in Analytical Chemistry
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Stoev, S.D. Food safety and increasing hazard of mycotoxin occurrence in foods and feeds. Crit. Rev. Food Sci. 2013, 53, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.S.; Brasel, J.M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef]
- Sweeney, M.J.; Dobson, A.D.W. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int. J. Food Microbiol. 1998, 43, 141–158. [Google Scholar] [CrossRef]
- Shuib, N.S.; Makahleh, A.; Salhimi, S.M.; Saad, B. Determination of aflatoxin M1 in milk and dairy products using high performance liquid chromatography-fluorescence with post column photochemical derivatization. J. Chromatogr. A 2017, 1510, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Hong, S.Y.; Kang, J.W.; Cho, S.M.; Lee, K.R.; An, T.K.; Lee, C.; Chung, S.H. Simultaneous determination of multi-mycotoxins in cereal grains collected from South Korea by LC/MS/MS. Toxins 2017, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Urusov, A.E.; Zherdev, A.V.; Dzantiev, B.B. Immunochemical methods of mycotoxin analysis (review). Appl. Biochem. Microbiol. 2010, 46, 253–266. [Google Scholar] [CrossRef]
- Tripathi, P.; Upadhyay, N.; Nara, S. Recent advancements in lateral flow immunoassays: A journey for toxin detection in food. Crit. Rev. Food Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.Q.; Li, X.; Li, P.W.; Zhang, Q.; Li, R.; Zhang, W.; Ding, X.X.; Lei, J.W.; Zhang, Z.W. Development and application of an immunoaffinity column enzyme immunoassay for mycotoxin zearalenone in complicated samples. PLoS ONE 2014, 9, e85606. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Li, H.P.; Zhang, J.B.; Liu, J.L.; Hu, Z.Q.; Gong, A.D.; Huang, T.; Liao, Y.C. Chicken single-chain antibody fused to alkaline phosphatase detects aspergillus pathogens and their presence in natural samples by direct sandwich enzyme-linked immunosorbent assay. Anal. Chem. 2013, 85, 10992–10999. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, P.W.; Zhang, Q.; Zhang, W.; Zhang, Z.W.; Wang, T.; He, T. Determination of aspergillus pathogens in agricultural products by a specific nanobody-polyclonal antibody sandwich ELISA. Sci. Rep. 2017, 7, 4348. [Google Scholar] [CrossRef] [PubMed]
- Li, P.W.; Zhang, Q.; Zhang, W. Immunoassays for aflatoxins. Trac-Trends Anal. Chem. 2009, 28, 1115–1126. [Google Scholar] [CrossRef]
- EU No. 165/2010. Commission Regulation (EU) No. 165/2010 of 26 February 2010, Amending Regulation (EC) No. 1881/2006, Setting Maximum Levels for Certain Contaminants in Foodstuffs as Regards Aflatoxins (Text with EEA relevance); European Commission: Brussels, Belgium, 2010. [Google Scholar]
- EC No. 1126/2007. Commission Regulation (EC) No. 1126/2007 of 28 September 2007, Amending Regulation (EC) No. 1881/2006, Setting Maximum Levels for Certain Contaminants in Foodstuffs as Regards Fusarium Toxins in Maize and Maize Products (Text with EEA relevance); European Commission: Brussels, Belgium, 2007. [Google Scholar]
- EU No 212/2014. Commission Regulation (EU) No. 212/2014 of 6 March 2014, Amending Regulation (EC) No. 1881/2006 as Regards Maximum Levels of the Contaminant Citrinin in Food Supplements Based on Rice Fermented with Red Yeast Monascuspurpureus (Text with EEA Relevance); European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Shiu, C.M.; Wang, J.J.; Yu, F.Y. Sensitive enzyme-linked immunosorbent assay and rapid one-step immunochromatographic strip for fumonisin B1 in grain-based food and feed samples. J. Sci. Food Agric. 2010, 90, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.H.; Que, S.J.; Gao, Y.M.; Yuan, J.; Ye, Z.; Du, M.; Lin, G.M.; Liu, L.C.; Wang, S.H. Artificial antigen synthesis and the development of polyclonal antibody-based immunoassay for citreoviridin determination. World J. Microbiol. Biotechnol. 2014, 30, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.H.; Li, P.W.; Zhang, Q.; Zhang, W.; Huang, Y.L.; Ding, X.X.; Jiang, J. Production of ultrasensitive generic monoclonal antibodies against major aflatoxins using a modified two-step screening procedure. Anal. Chim. Acta 2009, 636, 63–69. [Google Scholar] [CrossRef] [PubMed]
- KalayuYirga, S.; Ling, S.; Yang, Y.; Yuan, J.; Wang, S. The preparation and identification of a monoclonal antibody against citrinin and the development of detection via indirect competitive ELISA. Toxins 2017, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, P.W.; Lei, J.W.; Zhang, W.; Li, C.M. A simple strategy to obtain ultra-sensitive single-chain fragment variable antibodies for aflatoxin detection. Rsc Adv. 2013, 3, 22367–22372. [Google Scholar] [CrossRef]
- Arola, H.O.; Tullila, A.; Nathanail, A.V.; Nevanen, T.K. A simple and specific noncompetitive ELISA method for HT-2 toxin detection. Toxins 2017, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hammers, C.; Songa, E.B.; Bendahman, N.; Hammers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Narciso, J.E.T.; Uy, I.D.C.; Cabang, A.B.; Chavez, J.F.C.; Pablo, J.L.B.; Padilla-Concepcion, G.P.; Padlan, E.A. Analysis of the antibody structure based on high-resolution crystallographic studies. New Biotechnol. 2011, 28, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Arbabi Ghahroudi, M.; Desmyter, A.; Wyns, L.; Hamers, R.; Muyldermans, S. Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Lett. 1997, 414, 521–526. [Google Scholar] [CrossRef]
- Cortez-Retamozo, V.; Lauwereys, M.; Gh, G.H.; Gobert, M.; Conrath, K.; Muyldermans, S.; Baetselier, P.D.; Revets, H. Efficient tumor targeting by single-domain antibody fragments of camels. Int. J. Cancer 2002, 98, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Hudson, P.J.; Souriau, C. Engineered antibodies. Nat. Med. 2003, 9, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Yau, K.Y.; Lee, H.; Hall, J.C. Emerging trends in the synthesis and improvement of hapten-specific recombinant antibodies. Biotechnol. Adv. 2003, 21, 599–637. [Google Scholar] [CrossRef]
- Le Gall, F.; Reusch, U.; Moldenhauer, G.; Little, M.; Kipriyanov, S.M. Immunosuppressive properties of anti-CD3 single-chain Fv and diabody. J. Immunol. Methods 2004, 285, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Vu, K.B.; Ghahroudi, M.A.; Wyns, L.; Muyldermans, S. Comparison of llama VH sequences from conventional and heavy chain antibodies. Mol. Immunol. 1997, 34, 1121–1131. [Google Scholar] [CrossRef]
- Chan, P.H.; Pardon, E.; Menzer, L.; De Genst, E.; Kumita, J.R.; Christodoulou, J.; Saerens, D.; Brans, A.; Bouillenne, F.; Archer, D.B.; et al. Engineering a camelid antibody fragment that binds to the active site of human lysozyme and inhibits its conversion into amyloid fibrils. Biochemistry 2008, 47, 11041–11054. [Google Scholar] [CrossRef] [PubMed]
- LutjeHulsik, D.; Liu, Y.Y.; Strokappe, N.M.; Battella, S.; El Khattabi, M.; Mccoy, L.E.; Sabin, C.; Hinz, A.; Hock, M.; Macheboeuf, P.; et al. A gp41 MPER-specific llama VHH requires a hydrophobic CDR3 for neutralization but not for antigen recognition. PLoS Pathog. 2013, 9, e1003202. [Google Scholar] [CrossRef]
- Muyldermans, S.; Baral, T.; Retamozzo, V.C.; De Baetselier, P.; De Genst, E.; Kinne, J.; Leonhardt, H.; Magez, S.; Nguyen, V.; Revets, H. Camelid immunoglobulins and nanobody technology. Vet. Immunol. Immunopathol. 2009, 128, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Saerens, D.; Frederix, F.; Reekmans, G.; Conrath, K.; Jans, K.; Brys, L.; Huang, L.; Bosmans, E.; Maes, G.; Borghs, G.; et al. Engineering camel single-domain antibodies and immobilization chemistry for human prostate-specific antigen sensing. Anal. Chem. 2005, 77, 7547–7555. [Google Scholar] [CrossRef] [PubMed]
- Franco, E.J.; Sonneson, G.J.; Delegge, T.J.; Hofstetter, H.; Horn, J.R.; Hofstetter, O. Production and characterization of a genetically engineered anti-caffeine camelid antibody and its use in immunoaffinity chromatography. J. Chromatogr. B 2010, 878, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Majkova, Z.; Bever, C.R.S.; Kim, H.J.; Zhang, Q.; Dechant, J.E.; Gee, S.J.; Hammock, B.D.A. Chem, Isolation of alpaca anti-idiotypic heavy-chain single-domain antibody for the aflatoxin immunoassay. Anal. Chem. 2013, 85, 8298–8303. [Google Scholar] [CrossRef] [PubMed]
- Conrath, K.E.; Lauwereys, M.; Wyns, L.; Muyldermans, S. Camel single-domain antibodies as modular building units in bispecific and bivalent antibody constructs. J. Biol. Chem. 2001, 276, 7346–7350. [Google Scholar] [CrossRef] [PubMed]
- De Groeve, K.; Deschacht, N.; De Koninck, C.; Caveliers, V.; Lahoutte, T.; Devoogdt, N.; Muyldermans, S.; De Baetselier, P.; Raes, G. Nanobodies as tools for in vivo imaging of specific immune cell types. J. Nucl. Med. 2010, 51, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Schoonooghe, S.; Laoui, D.; Van Ginderachter, J.A.; Devoogdt, N.; Lahoutte, T.; De Baetselier, P.; Raes, G. Novel applications of nanobodies for in vivo bio-imaging of inflamed tissues in inflammatory diseases and cancer. Immunobiology 2012, 217, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.; Heukers, R.; Sornkom, J.; Kok, R.J.; van Bergen EnHenegouwen, P.M. Targeting tumors with nanobodies for cancer imaging and therapy. J. Control. Release 2013, 172, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Yau, K.Y.; Groves, M.A.; Li, S.; Sheedy, C.; Lee, H.; Tanha, J.; Mackenzie, C.R.; Jermutus, L.; Hall, J.C. Selection of hapten-specific single-domain antibodies from a non-immunized llama ribosome display library. J. Immunol. Methods 2003, 281, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Sheedy, C.; Yau, K.Y.; Hirama, T.; Mackenzie, C.R.; Hall, J.C. Selection, characterization, and CDR shuffling of naive llama single-domain antibodies selected against auxin and their cross-reactivity with auxinic herbicides from four chemical families. J. Agric. Food Chem. 2006, 54, 3668–3678. [Google Scholar] [CrossRef] [PubMed]
- Ladenson, R.C.; Crimmins, D.L.; Landt, Y.; Ladenson, J.H. Isolation and characterization of a thermally stable recombinant anti-caffeine heavy-chain antibody fragment. Anal. Chem. 2006, 78, 4501–4508. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rueda, N.; Behar, G.; Ferré, V.; Pugniere, M.; Roquet, F.; Gastinel, L.; Jacquot, C.; Aubry, J.; Baty, D.; Barbet, J. Generation of llama single-domain antibodies against methotrexate, a prototypical hapten. Mol. Immunol. 2007, 44, 1680–1690. [Google Scholar] [CrossRef] [PubMed]
- Bever, C.S.; Dong, J.X.; Vasylieva, N.; Barnych, B.; Cui, Y.; Xu, Z.L.; Hammock, B.D.; Gee, S.J. VHH antibodies: Emerging reagents for the analysis of environmental chemicals. Anal. Bioanal. Chem. 2016, 408, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Zhang, Q.; Hu, X.; Zhang, W. A toxin-free enzyme-linked immunosorbent assay for the analysis of aflatoxins based on a VHH surrogate standard. Anal. Bioanal. Chem. 2016, 408, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xiong, L.; Li, Y.; Xiong, Y.; Tu, Z.; Fu, J.; Tang, X. Citrinin detection using phage-displayed anti-idiotypic single-domain antibody for antigen mimicry. Food Chem. 2015, 177, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, Q.; Xu, Y.; Liu, X.; Shu, M.; Tu, Z.; Li, Y.; Wang, W.; Cao, D. Anti-idiotypic VHH phage display-mediated immuno-PCR for ultrasensitive determination of mycotoxin zearalenone in cereals. Talanta 2016, 147, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; He, Q.; Xu, Y.; Tu, Z.; Yang, H.; Qiu, Y.; Wang, X.; Liu, Y. Phage displayed anti-idiotypic nanobody mediated immuno-PCR for sensitive and environmentally friendly detection of mycotoxin ochratoxin A. Anal. Methods 2016, 8, 7824–7831. [Google Scholar] [CrossRef]
- Qiu, Y.L.; He, Q.H.; Xu, Y.; Bhunia, A.K.; Tu, Z.; Chen, B.; Liu, Y.Y. Deoxynivalenol-mimic nanobody isolated from a naïve phage display nanobody library and its application in immunoassay. Anal. Chim. Acta 2015, 887, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; Xu, Y.; Wang, D.; Liu, X.; Li, Y.; He, Q.; Tu, Z.; Qiu, Q.; Ji, Y.; Wang, X. Anti-idiotypic nanobody: A strategy for development of sensitive and green immunoassay for fumonisin B1. Talanta 2015, 143, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xiong, L.; Li, Y.; Xiong, Y.; Tu, Z.; Fu, J.; Chen, B. Anti-idiotypic nanobody as citrinin mimotope from a naive alpaca heavy chain single domain antibody library. Anal. Bioanal. Chem. 2015, 407, 5333–5341. [Google Scholar] [CrossRef] [PubMed]
- Pardon, E.; Laeremans, T.; Triest, S.; Rasmussen, S.G.; Wohlkönig, A.; Ruf, A.; Muyldermans, S.; Hol, W.G.; Kobilka, B.K.; Steyaert, J. A general protocol for the generation of Nanobodies for structural biology. Nat. Protoc. 2014, 9, 674–693. [Google Scholar] [CrossRef] [PubMed]
- Desmyter, A.; Spinelli, S.; Roussel, A.; Cambillau, C. Camelid nanobodies: Killing two birds with one stone. Curr. Opin. Struct. Biol. 2015, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; McCoy, M.R.; Majkova, Z.; Dechant, J.E.; Gee, S.J.; Tabares-da Rosa, S.; González-Sapienza, G.G.; Hammock, B.D. Isolation of alpaca anti-hapten heavy chain single domain antibodies for development of sensitive immunoassay. Anal. Chem. 2011, 84, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Tabares-da Rosa, S.; Rossotti, M.; Carleiza, C.; Carrión, F.; Pritsch, O.; Ahn, K.C.; Last, J.A.; Hammock, B.D.; González-Sapienza, G. Competitive selection from single domain antibody libraries allows isolation of high-affinity antihapten antibodies that are not favored in the llama immune response. Anal. Chem. 2011, 83, 7213–7220. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Wang, Y.; Li, P.; Zhang, Q.; Lei, J.; Zhang, Z.; Ding, X.; Zhou, H.; Zhang, W. Nanobody-based enzyme immunoassay for aflatoxin in agro-products with high tolerance to cosolvent methanol. Anal. Chem. 2014, 86, 8873–8880. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Li, P.; Zhang, Q.; Kim, H.J.; Gee, S.J.; Hammock, B.D. Phage-displayed peptide that mimics aflatoxins and its application in immunoassay. J. Agric. Food Chem. 2013, 61, 2426–2433. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bever, C.R.; Majkova, Z.; Dechant, J.E.; Yang, J.; Gee, S.J.; Xu, T.; Hammock, B.D. Heterologous antigen selection of camelid heavy chain single domain antibodies against tetrabromobisphenol A. Anal. Chem. 2014, 86, 8296–8302. [Google Scholar] [CrossRef] [PubMed]
- Pírez-Schirmer, M.; Rossotti, M.; Badagian, N.; Leizagoyen, C.; Brena, B.M.; Gonzalez-Sapienza, G. Comparison of three anti-hapten VHH selection strategies for the development of highly sensitive immunoassays for microcystins. Anal. Chem. 2017, 89, 6800–6806. [Google Scholar] [CrossRef] [PubMed]
- Doyle, P.J.; Arbabi-Ghahroudi, M.; Gaudette, N.; Furzer, G.; Savard, M.E.; Gleddie, S.; Mclean, M.D.; Mackenzie, C.R.; Hall, J.C. Cloning, expression, and characterization of a single-domain antibody fragment with affinity for 15-acetyl-deoxynivalenol. Mol. Immunol. 2008, 45, 3703–3713. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.P.; Goldman, E.R. TNT detection using llama antibodies and a two-step competitive fluid array immunoassay. J. Immunol. Methods 2008, 339, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sapienza, G.; Rossotti, M.A.; Rosa, S.T. Single-domain antibodies as versatile affinity reagents for analytical and diagnostic applications. Front. Immunol. 2017, 8, 977. [Google Scholar] [CrossRef] [PubMed]
- Bever, C.R.; Majkova, Z.; Radhakrishnan, R.; Suni, I.; Mccoy, M.; Wang, Y.; Dechant, J.; Gee, S.; Hammock, B.D. Development and utilization of camelid VHH antibodies from alpaca for 2,2′,4,4′-tetrabrominated diphenyl ether detection. Anal. Chem. 2014, 86, 7875–7882. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.C.; Gee, S.J.; Tsai, H.J.; Bennett, D.; Nishioka, M.G.; Blum, A.; Fishman, E.; Hammock, B.D. Immunoassay for monitoring environmental and human exposure to the polybrominated diphenyl ether BDE-47. Environ. Sci. Technol. 2009, 43, 7784–7790. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, C.; Ou, J.; Cui, Y.; Wang, L.; Lv, C.; Liu, K.; Wang, B.; Xu, T.; Li, Q.; Liu, S. Development of a monoclonal antibody-based enzyme-linked immunosorbent assay for tetrabromobisphenol A. Monoclon. Antib. Immunodiagn. Immunother. 2013, 32, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wang, J.; Liu, S.; Lv, C.; Shelver, W.L.; Li, Q.; Li, J. A highly sensitive and selective immunoassay for the detection of tetrabromobisphenol A in soil and sediment. Anal. Chim. Acta 2012, 751, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.V.; Mayi, D.K.; Reddy, M.U.; Thirumala-Devi, K.; Reddy, D.V. Aflatoxins B1 in different grades of chillies (Capsicum annum L.) in India as determined by indirect competitive-ELISA. Food Addit. Contam. 2001, 18, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tang, Z.; Duan, Z.; He, Z.; Mei, S.; Wang, X.; Gee, S.J.; Hammock, B.D.; Xu, Y. Nanobody-based enzyme immunoassay for ochratoxin A in cereal with high resistance to matrix interference. Talanta 2017, 164, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, P.; Zhang, Q.; Zhang, Z.; Li, R.; Zhang, W.; Ding, X.; Chen, X.; Tang, X. A sensitive immunoaffinity column-linked indirect competitive ELISA for ochratoxin A in cereal and oil products based on a new monoclonal antibody. Food Anal. Methods 2013, 6, 1433–1440. [Google Scholar] [CrossRef]
- Thirumala-Devi, K.; Mayo, M.A.; Reddy, G.; Reddy, S.V.; Delfosse, P.; Reddy, D.V.R. Production of polyclonal antibodies against ochratoxin A and its detection in chilies by ELISA. J. Agric. Food Chem. 2000, 48, 5079–5082. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.C.; Savard, M.E.; Lau, R. Production of monoclonal antibodies for the specific detection of deoxynivalenol and 15-acetyldeoxynivalenol by ELISA. J. Agric. Food Chem. 1995, 43, 1740–1744. [Google Scholar] [CrossRef]
- Usleber, E.; Schneider, E.; Martlbauer, E.; Terplan, G. Two formats of enzyme immunoassay for 15-acetyldeoxynivalenol applied to wheat. J. Agric. Food Chem. 1993, 41, 2019–2023. [Google Scholar] [CrossRef]
- Wesolowski, J.; Alzogaray, V.; Reyelt, J.; Unger, M.; Juarez, K.; Urrutia, M.; Cauerhff, A.; Danquah, W.; Rissiek, B.; Scheuplein, F. Single domain antibodies: Promising experimental and therapeutic tools in infection and immunity. Med. Microbiol. Immunol. 2009, 198, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, Y.; Wang, L.; Hu, B.; Li, P.; Liu, F. Effect of hapten structures on specific and sensitive enzyme-linked immunosorbent assays for N-methylcarbamate insecticide metolcarb. Anal. Chim. Acta 2008, 625, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Fodey, T.L.; Greer, N.M.; Crooks, S.R. Antibody production: Low dose immunogen vs. low incorporation hapten using salmeterol as a model. Anal. Chim. Acta 2009, 637, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, P.; Yang, Y.; Zhang, Q.; Zhang, W.; Xiao, Z.; Ding, X. A high selective immunochromatographic assay for rapid detection of aflatoxin B1. Talanta 2011, 85, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, J.; Zhang, L.; Fan, Z.; Yu, T.; Jiang, F.; Tang, X.; Zhang, Z.; Zhang, W.; Zhang, Q. Doses of immunogen contribute to specificity spectrums of antibodies against aflatoxin. Toxins 2017, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- van Houwelingen, A.; De Saeger, S.; Rusanova, T.; Waalwijk, C.; Beekwilder, J. Generation of recombinant alpaca VHH antibody fragments for the detection of the mycotoxin ochratoxin A. World Mycotoxin J. 2008, 1, 407–417. [Google Scholar] [CrossRef]
- Pérez, J.M.; Renisio, J.G.; Prompers, J.J.; van Platerink, C.J.; Cambillau, C.; Darbon, H.; Frenken, L.G. Thermal unfolding of a llama antibody fragment: A two-state reversible process. Biochemistry 2001, 40, 74–83. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, T.; Muyldermans, S.; Depicker, A. Nanobody-based products as research and diagnostic tools. Trends Biotechnol. 2014, 32, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Muyldermans, S. Single domain camel antibodies: Current status. Rev. Mol. Biotechnol. 2001, 74, 277–302. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Wan, D.; Xiong, Y.; He, Z.; Wang, X.; Gee, S.J.; Ryu, D.; Hammock, B.D. Development of a nanobody-alkaline phosphatase fusion protein and its application in a highly sensitive direct competitive fluorescence enzyme immunoassay for detection of ochratoxin A in cereal. Anal. Chem. 2015, 87, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, Y.; Xiong, Y.; Tu, Z.; Li, Y.; He, Z.; Qiu, Y.; Fu, J.; Gee, S.J.; Hammock, B.D. VHH phage-based competitive real-time immuno-polymerase chain reaction for ultrasensitive detection of ochratoxin A in cereal. Anal. Chem. 2014, 86, 7471–7477. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Li, P.; Zhang, Q.; Wang, Y.; Zhang, Z.; Ding, X.; Zhang, W. Anti-idiotypic nanobody-phage based real-time immuno-PCR for detection of hepatocarcinogen aflatoxin in grains and feedstuffs. Anal. Chem. 2014, 86, 10841–10846. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, P.; Zhang, Q.; Zhang, Z.; Zhang, W.; Jiang, J. Time-resolved fluorescence immunochromatographic assay developed using two idiotypic nanobodies for rapid, quantitative, and simultaneous detection of aflatoxin and zearalenone in maize and its products. Anal. Chem. 2017, 89, 11520–11528. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.L.; He, Q.H.; Xu, Y.; Wang, W.; Liu, Y.Y. Modification of a deoxynivalenol-antigen-mimicking nanobody to improve immunoassay sensitivity by site-saturation mutagenesis. Anal. Bioanal. Chem. 2016, 408, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Zarebski, L.M.; Urrutia, M.; Goldbaum, F.A. Llama single domain antibodies as a tool for molecular mimicry. J. Mol. Biol. 2005, 349, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Bazin, I.; Tria, S.A.; Hayat, A.; Marty, J.L. New biorecognition molecules in biosensors for the detection of toxins. Biosens. Bioelectron. 2017, 87, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Tu, Z.; Huang, X.; Xie, B.; Xiong, Y.H.; Xu, Y. Magnetic beads carrying poly(acrylic acid) brushes as “nanobody containers” for immunoaffinity purification of aflatoxin B1 from corn samples. RSC Adv. 2015, 5, 77380–77387. [Google Scholar] [CrossRef]
- Li, X.; Li, P.; Zhang, Q.; Li, Y.; Zhang, W.; Ding, X. Molecular characterization of monoclonal antibodies against aflatoxins: A possible explanation for the highest sensitivity. Anal. Chem. 2012, 84, 5229–5235. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, S.; Frenken, L.G.; Hermans, P.; Verrips, T.; Brown, K.; Tegoni, M.; Cambillau, C. Camelid heavy-chain variable domains provide efficient combining sites to haptens. Biochemistry 2000, 39, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, S.; Tegoni, M.; Frenken, L.; Van, V.C.; Cambillau, C. Lateral recognition of a dye hapten by a llama VHH domain. J. Mol. Biol. 2001, 311, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Fanning, S.W.; Horn, J.R. An anti-hapten camelid antibody reveals a cryptic binding site with significant energetic contributions from a nonhypervariable loop. Protein Sci. 2011, 20, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Pasha, S.K.; Manickam, P.; Bhansali, S. Single-domain antibody thermally stable electrochemical immunosensor. Biosens. Bioelectron. 2016, 83, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, Z.W.; Zhang, Q.; Li, P.W. Mycotoxin determination in foods using advanced sensors based on antibodies or aptamers. Toxins 2016, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Wang, R.; Bever, C.R.S.; Xing, S.; Hammock, B.D.; Pan, T. Smartphone-interfaced lab-on-a-chip devices for field-deployable enzyme-linked immunosorbent assay. Biomicrofluidics 2014, 8, 3187–3194. [Google Scholar] [CrossRef] [PubMed]

| Mycotoxins | Major Producers | Major Host | Toxin Effects | Maximum Level |
|---|---|---|---|---|
| Aflatoxins (AFB1, AFB2, AFG1, AFG2) | Aspergillus flavus, A. parasiticus | maize, nuts, cottonseed, peanuts | hepatotoxicity, cancer, immunosuppression | 8.0 μg/kg (AFB1); 15 μg/kg (total aflatoxins) (Commission Regulation, EU No.165/2010) [12] |
| Fumonisins (FB1, FB2) | Fusarium verticillioides, F. proliferatum | maize | hepatotoxicity, cancer, pulmonary edema, leukoencephalomalacia | 4000 μg/kg in maize; 1000 μg/kg in maize-based foods (EC No.126/2007) [13] |
| Deoxynivalenol (DON) | F.graminearum, F. culmorum | maize, wheat, barley | gastrointestinal toxicity, inflammation of central nervous system | 1250 μg/kg in cereals (EC No.1126/2007) |
| Zearalenone (ZEN) | F. culmorum, F.graminearum | maize, barley, wheat, rice | infertility, abortion | 350 μg/kg in maize; 100 μg/kg in other cereals (EC No.1126/2007) |
| OchratoxinA (OTA) | Penicillium verrucosum, A.ochraceus | cereal-derived products, spices, wine | nephrotoxic, carcinogenic, teratogenic, immunotoxic effects | 5.0 μg/kg in maize (EC No.1881/2006) |
| Citrinin (CIT) | P.citrinum, P. verruscosum, P.expansum, A.terreus, Monascus ruber | corn, wheat, barley, rice | nephrotoxic, hepatotoxic, immunotoxic, carcinogenic effects | 2000 μg/kg (EU No.212/2014) [14] |
| Mycotoxin | Antibody | Assay Format | Sensitivity, IC50 |
|---|---|---|---|
| AFB1 | Nb | icELISA [55] | 0.754 ng/mL |
| mAb | icELISA [17] | 0.0002 ng/mL | |
| pAb | icELISA [66] | 2.0 ng/mL | |
| OTA | Nb | icELISA [67] | 0.64 ng/mL |
| mAb | icELISA [68] | 0.058 ng/mL | |
| pAb | icELISA [69] | 5.0 ng/mL | |
| 15-acetyl-DON | Nb | fluorescence polarization [59] | 419 ng/mL |
| mAb | dcELISA [70] | 1000 ng/mL | |
| pAb | dcELISA [71] | 1.9 ng/mL |
| Targets (MW). | NB TYPE | Assay Formats, (Ref.) | Sensitivity, IC50; Linear Range, IC20–IC80; LOD, IC10 | Specificity (CR) | Thermal Stability | Solvent/Matrix Stability |
|---|---|---|---|---|---|---|
| OTA (403.813) | Anti-hapten Nb | icELISA [67] | IC50: 0.64 ng/mL; LR: 0.27–1.47 ng/mL; LOD: 0.16 ng/mL | not tested | 95 °C for 5min, retained45% of binding activity; 90 °C for 75 min, retained25%of binding activity | dilution factor ×2.5 eliminate the matrix interference |
| Nb-AP | dcELISA [81] | IC50: 0.13 ng/mL; LR: 0.06–0.43 ng/mL; LOD: 0.04 ng/mL, | CR: 0.1% with OTB, ZEN, DON, and AFB1 | not tested | Susceptive to methanol, ionic strength and low pH (≤6.0) | |
| phage-Nb | Nb-IPCR [82] | LR: 10−5–1.0 ng/mL; LOD: 3.7 × 10−6 ng/mL; | CR: 3.5% with OTB; 0.1% with FB1, ZEN, DON, and AFB1 | not tested | not tested | |
| phage-AI-Nb | AI-Nb-IPCR [47] | LR: 0.01–10 ng/mL; LOD: 0.004 ng/mL | CR: 0.1% with FB1, ZEN, DON, and AFB1 | not tested | susceptive to methanol over 10% | |
| AFB1 (312.27) | Anti-hapten Nb | icELISA [55] | IC50: 0.754 ng/mL; LR: 0.117–5.676 ng/mL; LOD: 0.05 ng/mL | CR: 10% with AFB2, AFG1, and AFG2 | 85 °C for 60 min, retained40% of binding activity | stable in 40% methanol and 40% acetone |
| phage-AI-Nb | AI-Nb-IPCR [83] | LOD: 0.02 ng/mL | CR: 50% with AFB2 and AFG1; 13.5% with AFG2 | not tested | susceptive to methanol over 10% | |
| AI-Nb | AI-ELISA [34] | IC50: 0.16 ng/mL; LR: 0.09–0.82 ng/mL; LOD: 0.06 ng/mL | CR: 50% with AFB2 and AFG1; 30% with AFG2 and AFM1 | 80 °C for 60 min, retained 20% of bindingactivity | stable in methanol below 40% | |
| AI-Nb | TRFICA [84] | IC50: 0.46 ng/mL; LR: 0.13–4.54 ng/mL; LOD: 0.05 ng/mL | CR: 50% with AFB2 and AFG1; 31.1% with AFG2; 19.4% with AFM1 | not tested | not tested | |
| ZEN (318.37) | phage-AI-Nb | AI-Nb-IPCR [46] | LR: 0.01–10 ng/mL; LOD: 0.0065 ng/mL | CR: 0.1% with AFB1, DON, and OTA | not tested | susceptive to methanol over 5% |
| AI-Nb | TRFICA [84] | IC50: 0.86 ng/mL; LR: 0.20–2.77 ng/mL; LOD: 0.07 ng/mL; | CR: 78.1% with β-zearalenol | not tested | not tested | |
| 15-acetyl-DON (338.35) | Anti-hapten Nb | fluorescence polarization [59] | IC50: 419.5 ng/mL (monomer) 167.175 ng/mL (pentamer) | CR: 0.1% with neosolaniol, diacetoxyscirpenol, and T-2 toxin | not tested | not tested |
| DON (296.32) | AI-Nb | AI-ELISA [48] | IC50: 8.77 ng/mL; LR: 2.18–62.25 ng/mL; LOD: 1.16 ng/mL | CR: 0.1% with FB1, ZEN, AFB1, and OTA | 95 °C for 5 min, retained60% of binding activity | not tested |
| phage-AI-Nb | AI-PELISA [85] | IC50: 24.49 ng/mL; LR: 9.51–180.15 ng/mL | CR: 0.1%with FB1, ZEN, AFB1, and OTA | 95 °C for 5 min, retained55% of binding activity | stable in ionic strength below 50 mM and pH between 5.0 and 8.0 | |
| FB1 (721.84) | AI-Nb | AI-ELISA [49] | IC50: 0.95 ng/mL; LR: 0.27–5.92 ng/mL; LOD: 0.15 ng/mL | CR: 4.93% with FB2 | not tested | stable in ionic strength below 50 mM and pH between 5.0 and 8.0 |
| CIT (250.25) | AI-Nb | AI-ELISA [50] | IC50: 44.6 ng/mL; LR: 5.0–300.0 ng/mL; | CR: 0.1% with AFB1, ZEN, DON, and OTA | not tested | susceptive to methanol over 20% |
| phage-AI-Nb | AI-PELISA [45] | IC50: 10.9 ng/mL; LR: 2.5–100.0 ng/mL | CR: 0.1% with AFB1, ZEN, DON, and OTA | not tested | stable in methanol below 25%, ionic strength below 55 mM and pH between 5.4 and 9.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, T.; Zhu, J.; Nie, Y.; Hu, R.; Wang, T.; Li, P.; Zhang, Q.; Yang, Y. Nanobody Technology for Mycotoxin Detection: Current Status and Prospects. Toxins 2018, 10, 180. https://doi.org/10.3390/toxins10050180
He T, Zhu J, Nie Y, Hu R, Wang T, Li P, Zhang Q, Yang Y. Nanobody Technology for Mycotoxin Detection: Current Status and Prospects. Toxins. 2018; 10(5):180. https://doi.org/10.3390/toxins10050180
Chicago/Turabian StyleHe, Ting, Jiang Zhu, Yao Nie, Rui Hu, Ting Wang, Peiwu Li, Qi Zhang, and Yunhuang Yang. 2018. "Nanobody Technology for Mycotoxin Detection: Current Status and Prospects" Toxins 10, no. 5: 180. https://doi.org/10.3390/toxins10050180
APA StyleHe, T., Zhu, J., Nie, Y., Hu, R., Wang, T., Li, P., Zhang, Q., & Yang, Y. (2018). Nanobody Technology for Mycotoxin Detection: Current Status and Prospects. Toxins, 10(5), 180. https://doi.org/10.3390/toxins10050180