Validation of a Method for Cylindrospermopsin Determination in Vegetables: Application to Real Samples Such as Lettuce (Lactuca sativa L.)
Abstract
:1. Introduction
2. Results and Discussion
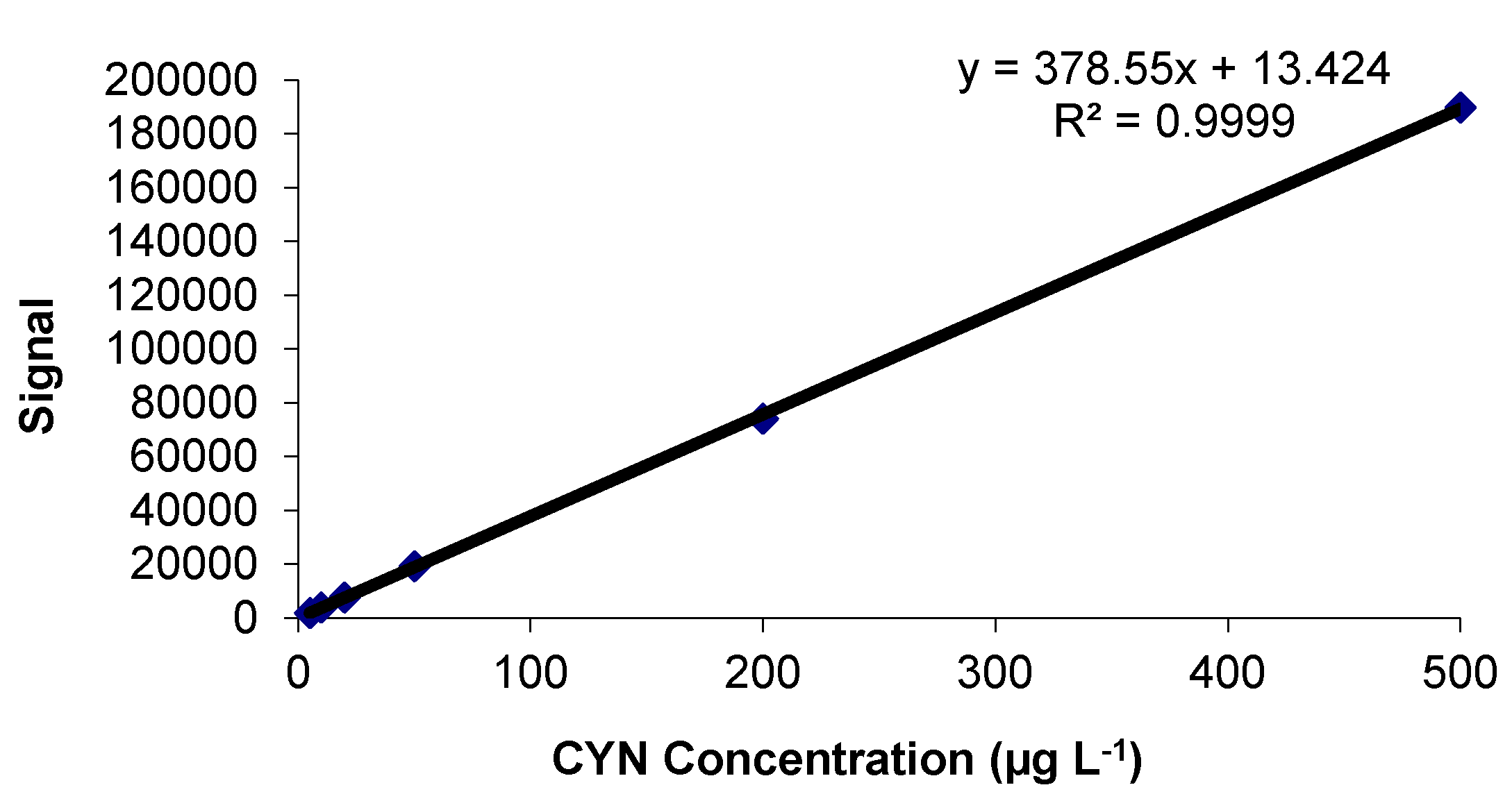
2.1. Calibration Study
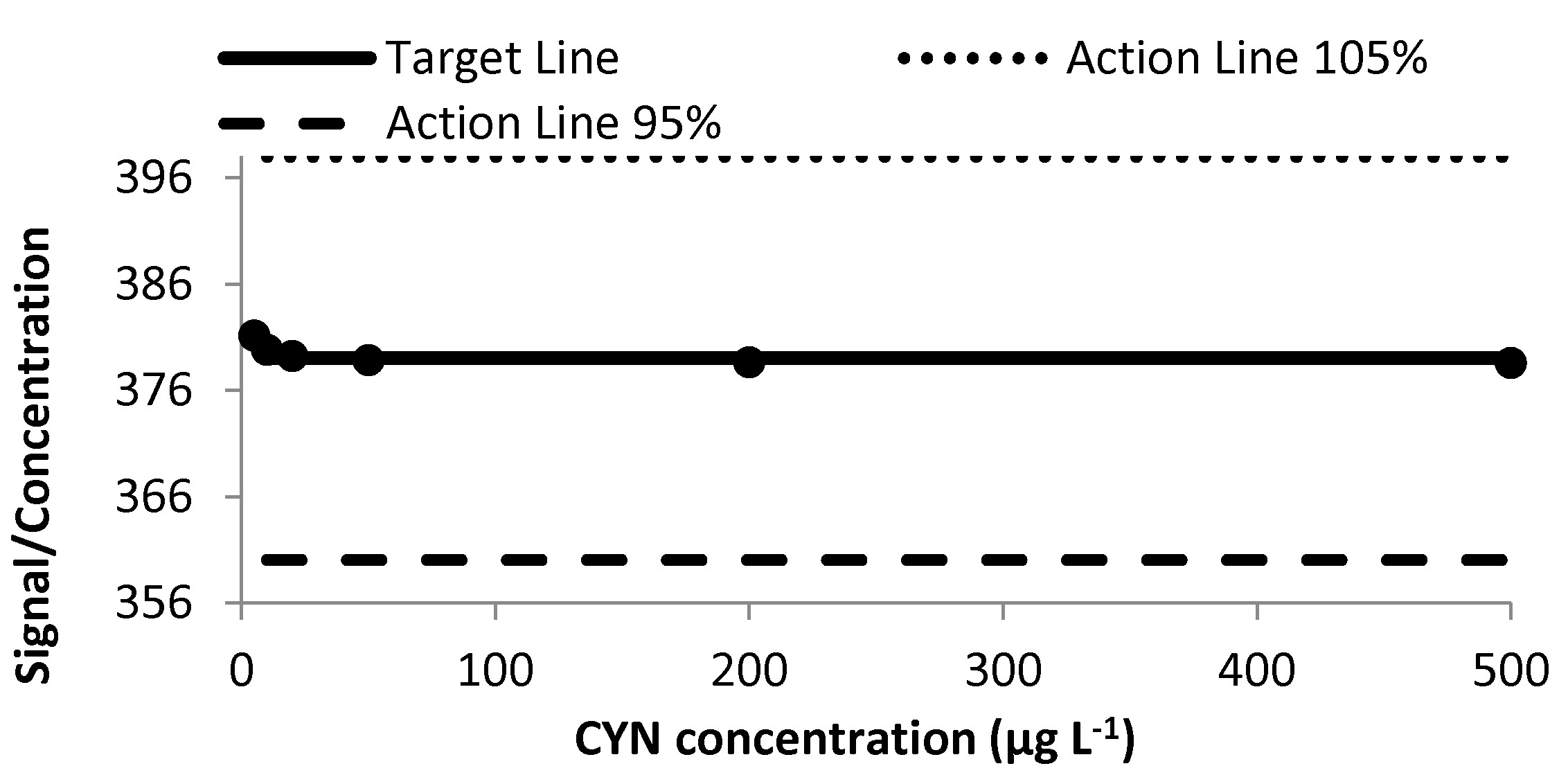
2.1.1. Linearity and Goodness of the Fit
2.1.2. Limits of Detection and Quantitation
2.1.3. Precision
2.1.4. Trueness and Recovery
2.1.5. Ruggedness Study
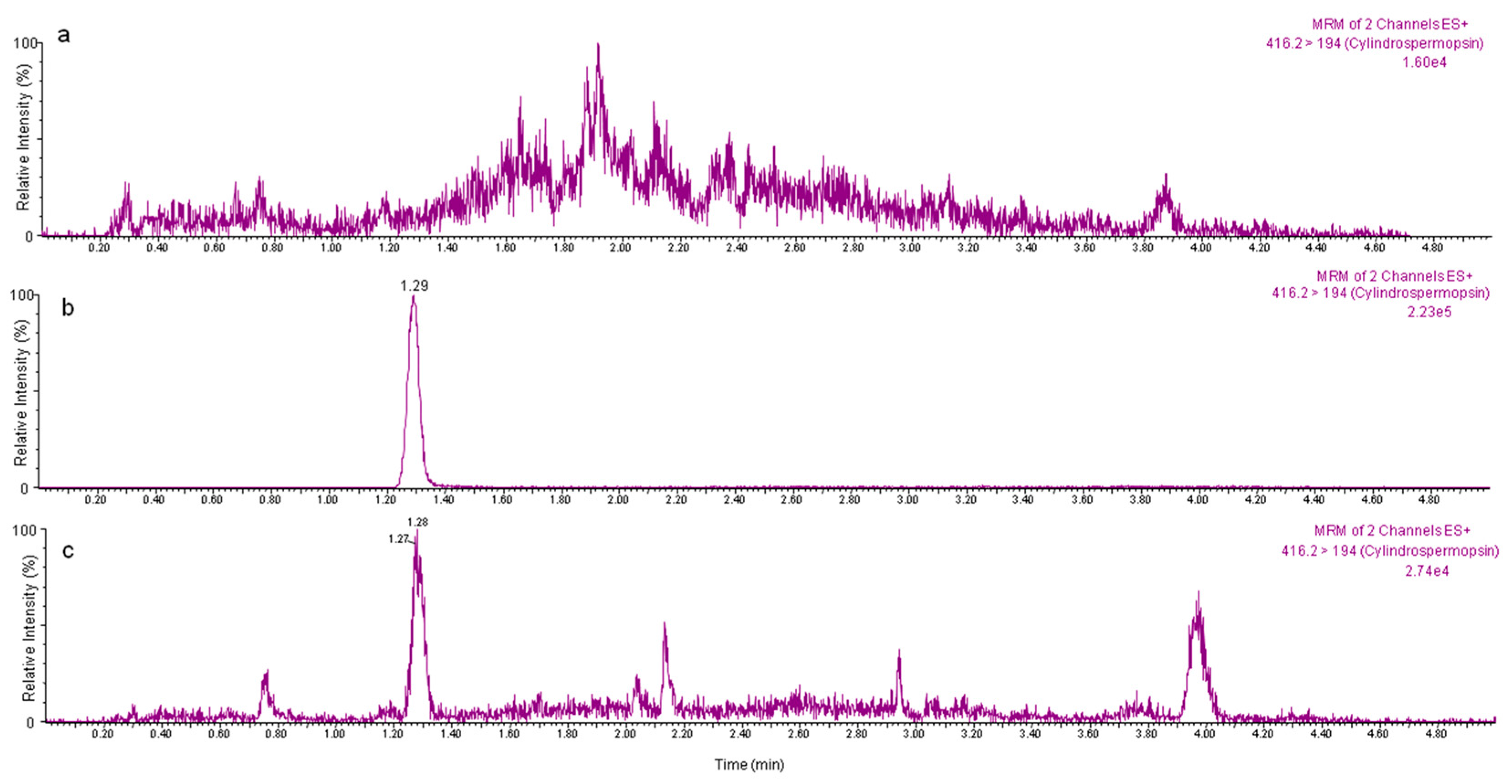
2.2. Application of the Proposed Method to Edible Vegetables Exposed to CYN
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Solvent Extraction and Purification Procedures
4.3. Chromatographic Conditions
4.4. Experimental Exposure of Vegetables and Application of the Validated Method
4.5. Method Validation
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.; Azevedo, J.; Freitas, M.; Pinto, E.; Almeida, A.; Vasconcelos, V.; Campos, A. Analysis of the use of microcystin-contaminated water in the growth and nutritional quality of the root-vegetable, Daucus carota. Environ. Sci. Pollut. Res. 2017, 24, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Kinnear, S. Cylindrospermopsin: A decade of progress on bioaccumulation research. Mar. Drugs 2010, 8, 542–564. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, I.; Moore, R.E.; Runnegar, M.T.C. Cylindrospermopsin: A potent hepatotoxin from the blue-green alga Cylindrospermopsis raciborskii. J. Am. Chem. Soc. 1992, 114, 7941–7942. [Google Scholar] [CrossRef]
- Harada, K.I.; Ohtani, I.; Iwamoto, K.; Suzuki, M.; Watanabe, M.F.; Watanabe, M.; Terao, K. Isolation of cylindrospermopsin from a cyanobacterium Umezekia natans and its screening method. Toxicon 1994, 32, 73–84. [Google Scholar] [CrossRef]
- Banker, R.; Carmeli, S.; Hadas, O.; Teltsch, B.; Porat, R.; Sukenik, A. Identification of cylindrospermopsin in Aphanizomenon ovalisporum (cyanophyceae) isolated from Lake Kinneret, Israel. J. Phycol. 1997, 33, 613–616. [Google Scholar] [CrossRef]
- Spoof, L.; Berg, K.A.; Rapala, J.; Lahti, K.; Lepistö, L.; Metcalf, J.S.; Codd, G.A.; Meriluoto, J. First observation of cylindrospermopsin in Anabaena lapponica isolated from the Boreal Environment (Finland). Environ. Toxicol. 2006, 21, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Seifert, M.; McGregor, G.; Eaglesham, G.; Wickramasinghe, W.; Shaw, G. First evidence for the production of cylindrospermopsin and deoxy-cylindrospermopsin by the freshwater benthic cyanobacterium, Lyngbya wollei (Farlow ex Gornont) Speziale and Dyck. Harmful Algae 2007, 6, 73–80. [Google Scholar] [CrossRef]
- Runnegar, M.T.; Kong, S.M.; Zhong, Y.Z.; Lu, S.C. Inhibition of reduced glutathione synthesis by cyanobacterial alkaloid cylindrospermopsin in cultured rat hepatocytes. Biochem. Pharmacol. 1995, 49, 219–225. [Google Scholar] [CrossRef]
- Froscio, S.; Humpage, A.R.; Wickramasinghe, W.; Shaw, G.; Falconer, I.R. Interaction of the cyanobacterial toxin cylindrospermopsin with the eukaryotic protein synthesis system. Toxicon 2008, 51, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Humpage, A. Toxin types, toxicokinetics and toxicodynamics. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs. Advances in Experimental Medicine and Biology; Hudnel, H.K., Ed.; Springer Press: New York, NY, USA, 2008; Volume 619, pp. 383–415. ISBN 9780387758640. [Google Scholar]
- Bazin, E.; Mourot, A.; Humpage, A.R.; Fessard, V. Genotoxicity of a freshwater cyanotoxin, cylindrospermopsin, in two human cell lines: Caco-2 and HepaRG. Environ. Mol. Mutagen. 2010, 51, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Žegura, B.; Gajski, G.; Štraser, A.; Garaj-Vrhovac, V. Cylindrospermopsin induced DNA damage and alteration in the expression of genes involved in the response to DNA damage, apoptosis and oxidative stress. Toxicon 2011, 58, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Humpage, A.R.; Fontaine, F.; Froscio, S.; Burcham, P.; Falconer, I.R. Cylindrospermopsin genotoxicity and cytotoxicity: Role of cytochrome P-450 and oxidative stress. J. Toxicol. Environ. Health A 2005, 68, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Praena, D.; Pichardo, S.; Jos, A.; Moreno, F.J.; Cameán, A.M. Biochemical and pathological toxic effects induced by the cyanotoxin Cylindrospermopsin on the human cell line Caco-2. Water Res. 2012, 46, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Praena, D.; Pichardo, S.; Jos, A.; Cameán, A.M. Toxicity and glutathione implication in the effects observed by exposure of the liver fish cell line PLHC-1 to pure Cylindrospermopsin. Ecotoxicol. Environ. Saf. 2012, 74, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Puerto, M.; Jos, A.; Pichardo, S.; Gutierrez-Praena, D.; Cameán, A.M. Acute effects of pure cylindrospermopsin on the activity and transcription of antioxidant enzymes in tilapia (Oreochromis niloticus) exposed by gavage. Ecotoxicology 2011, 20, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Praena, D.; Jos, A.; Pichardo, S.; Cameán, A.M. Oxidative stress responses in tilapia (Oreochromis niloticus) exposed to a single dose of pure cylindrospermopsin under laboratory conditions: Influence of exposure route and time of sacrifice. Aquat. Toxicol. 2011, 105, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Guillén, R.; Prieto, A.I.; Vasconcelos, V.M.; Cameán, A.M. Cyanobacterium producing cylindrospermopsin cause oxidative stress at environmentally relevant concentrations in sub-chronically exposed tilapia (Oreochromis niloticus). Chemosphere 2013, 90, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Humpage, A.R.; Falconer, I.R. Oral toxicity of the cyanobacterial toxin cylindrospermopsin in male swiss albino mice: Determination of no observed adverse effect level for deriving a drinking water guideline value. Environ. Toxicol. 2003, 18, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Corbel, S.; Mougin, C.; Bouaïcha, N. Cyanobacterial toxins: Modes of actions, fate in aquatic and soil ecosystems, phytotoxicity and bioaccumulation in agricultural crops. Chemosphere 2014, 96, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vasas, G.; Gáspár, A.; Surányi, G.; Batta, G.; Gyémánt, G.; M-Hamvas, M.; Máthé, C.; Grigorszky, I.; Molnár, E.; Borbély, G. Capillary electrophoretic assay and purification of cylindrospermopsin, a cyanobacterial toxin from Aphanizomenon ovalisporum, by plant test (blue-green sinapis test). Anal. Biochem. 2002, 302, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Beyer, D.; Surányi, G.; Vasas, G.; Roszik, J.; Erdodi, F.; M-Hamvas, M.; Bácsi, I.; Bátori, R.; Serfozo, Z.; Szigeti, Z.M.; et al. Cylindrospermopsin induces alterations of root histology and microtubule organization in common reed (Phragmites australis) plantlets cultured in vitro. Toxicon 2009, 54, 440–449. [Google Scholar] [CrossRef] [PubMed]
- M-Hamvas, M.; Máthé, C.; Vasas, G.; Jámbrik, K.; Papp, M.; Beyer, D.; Mészáros, I.; Borbély, G. Cylindrospermopsin and microcystin-LR alter the growth, development and peroxidase enzyme activity of white mustard (Sinapis alba L.) seedlings, a comparative analysis. Acta Biol. Hung. 2010, 61, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Guillén, R.; Campos, A.; Machado, J.; Freitas, M.; Azevedo, J.; Pinto, E.; Almeida, A.; Cameán, A.M.; Vasconcelos, V. Effects of Chrysosporum (Aphanizomenon) ovalisporum extracts containing cylindrospermopsin on growth, photosynthetic capacity, and mineral content of carrots (Daucus carota). Ecotoxicology 2017, 26, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.S.; Barakate, A.; Codd, G.A. Inhibition of plant protein synthesis by the cyanobacterial hepatotoxin, cylindrospermopsin. FEMS Microbiol. Lett. 2004, 235, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Vasconcelos, V. Allelopathic effect of Cylindrospermopsis raciborskii extracts on the germination and growth of several plant species. Chem. Ecol. 2010, 26, 263–271. [Google Scholar] [CrossRef]
- Prieto, A.; Campos, A.; Cameán, A.; Vasconcelos, V. Effects on growth and oxidative stress status of rice plants (Oryza sativa) exposed to two extracts of toxin-producing cyanobacteria (Aphanizomenon ovalisporum and Microcystis aeruginosa). Ecotoxicol. Environ. Saf. 2011, 74, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.; Azevedo, J.; Pinto, E.; Neves, J.; Campos, A.; Vasconcelos, V. Effects of microcystin-LR, cylindrospermopsin and a microcystin-LR/cylindrospermopsin mixture on growth, oxidative stress and mineral content in lettuce plants (Lactuca sativa L.). Ecotoxicol. Environ. Saf. 2015, 116, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Kinnear, S.; Fabbro, L.; Duivenvoorden, L. Variable growth responses of water thyme (Hydrilla verticillata) to whole-cell extracts of Cylindrospermopsis raciborskii. Arch. Environ. Contam. Toxicol. 2008, 54, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Garda, T.; Riba, M.; Vasas, G.; Beyer, D.; M-Hamvas, M.; Hajdu, G.; Tándor, I.; Máthé, C. Cytotoxic effects of cylindrospermopsin in mitotic and non-mitotic Vicia faba cells. Chemosphere 2015, 120, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Praena, D.; Campos, A.; Azevedo, J.; Neves, J.; Freitas, M.; Guzmán-Guillén, R.; Caméan, A.M.; Renaut, J.; Vasconcelos, V. Exposure of Lycopersicon Esculentum to microcystin-LR: Effects in the leaf preoteome and toxin translocation from water to leaves and fruits. Toxins 2014, 6, 1837–1854. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Praena, D.; Jos, A.; Pichardo, S.; Moreno, I.M.; Cameán, A.M. Presence and bioaccumulation of microcystins and cylindrospermopsin in food and the effectiveness of some cooking techniques at decreasing their concentrations: A review. Food Chem. Toxicol. 2013, 53, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Hereman, T.C.; Bittencourt-Oliveira, M.C. Bioaccumulation of Microcystins in lettuce. J. Phycol. 2012, 48, 1535–1537. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt-Oliveira, M.C.; Cordeiro-Araújo, M.K.; Chia, M.A.; Arruda-Neto, J.D.; de Oliveira, E.T.; dos Santos, F. Lettuce irrigated with contaminated water: Photosynthetic effects, antioxidative response and bioaccumulation of microcystin congeners. Ecotoxicol. Environ. Saf. 2016, 128, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro-Araújo, M.K.; Chia, M.A.; Arruda-Neto, J.D.T.; Tornisielo, V.L.; Vilca, F.Z.; Bittencourt-Oliveira, M.C. Microcystin-LR bioaccumulation and depuration kinetics in lettuce and arugula: Human health risk assessment. Sci. Total Environ. 2016, 566–567, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro-Araújo, M.K.; Chia, M.A.; Bittencourt-Oliveira, M.C. Potential human health risk assessment of cylindrospermopsin accumulation and depuration in lettuce and arugula. Harmful Algae 2017, 68, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Azevedo, J.; Campos, A.; Vasconcelos, V.; Pereira, A.L. Biochemical and growth performance of the aquatic macrophyte Azolla filiculoides to sub-chronic exposure to cylindrospermopsin. Ecotoxicology 2015, 24, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- White, S.H.; Duivenvoorden, L.J.; Fabbro, L.D. Absence of free-cylindrospermopsin bioconcentration in water thyme (Hydrilla verticillata). Bull. Environ. Contam. Toxicol. 2005, 75, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Chiswell, R.K.; Shaw, G.R.; Eaglesham, G.; Smith, M.J.; Norris, R.L.; Seawright, A.A.; Moore, M.R. Stability of cylindrospermopsin, the toxin from the cyanobacterium, Cylindrospermopsis raciborskii: Effect of pH, temperature, and sunlight on decomposition. Environ. Toxicol. 1999, 14, 155–161. [Google Scholar] [CrossRef]
- Rucker, J.; Stuken, A.; Nixdorf, B.; Fastner, J.; Chorus, I.; Wiedner, C. Concentration of particulate and dissolved cylindrospermopsin in 21 Aphanizomenun-dominated temperate lakes. Toxicon 2007, 50, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Kittler, K.; Schreiner, M.; Krumbein, A.; Manzei, S.; Koch, M.; Rohn, S.; Maul, R. Uptake of the cyanobacterial toxin cylindrospermopsin in Brassica vegetables. Food Chem. 2012, 133, 875–879. [Google Scholar] [CrossRef]
- Ngnitcho, P.-F.K.; Khan, I.; Tango, C.N.; Hussain, M.S.; Oh, D.H. Inactivation of bacterial pathogens on lettuce, sprouts, and spinach using hurdle technology. Innov. Food Sci. Emerg. Technol. 2017, 43, 68–76. [Google Scholar] [CrossRef]
- Testai, E.; Buratti, F.M.; Funari, E.; Manganelli, M.; Vichi, S.; Arnich, N.; Biré, R.; Fessard, V.; Sialehaamoa, A. Review and analysis of occurrence, exposure and toxicity of cyanobacteria toxins in food. EFSA Support. Publ. 2016, 13, 1–309. [Google Scholar] [CrossRef]
- Cameán, A.; Moreno, I.M.; Ruiz, M.J.; Picó, Y. Determination of microcystins in natural blooms and cyanobacterial strains cultures by matrix solid-phase dispersion and liquid chromatography-mass spectrometry. Anal. Bioanal. Chem. 2004, 380, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.J.; Cameán, A.M.; Moreno, I.M.; Picó, Y. Determination of microcystins in biological samples by matrix solid-phase dispersion (MSDP) and liquid chromatography-mass spectrometry (LC-MS). J. Chromatogr. A 2005, 1073, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Bláhová, L.; Oravec, M.; Marsálek, B.; Sejnohová, L.; Simek, Z.; Bláha, L. The first occurrence of the cyanobacterial alkaloid toxin cylindrospermopsin in the Czech Republic as determined by immunochemical and LC/MS methods. Toxicon 2009, 53, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Eaglesham, G.; Norris, K.R.; Shaw, G.R.; Smith, M.J.; Chiswell, R.K.; Davis, B.C.; Neville, G.R.; Seawright, A.A.; Moore, M.R. Use of HPLC-MS/MS to monitor cylindrospermopsin, a blue–green algal toxin, for public health purposes. Environ. Toxicol. 1999, 14, 151–155. [Google Scholar] [CrossRef]
- Bogialli, S.; Bruno, M.; Curini, R.; Corcia, A.D.; Fanali, C.; Lagana, A. Monitoring algal toxins in lake water by liquid chromatography tandem mass spectrometry. Environ. Sci. Technol. 2006, 40, 2917–2923. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Guillén, R.; Prieto Ortega, A.I.; Moreno, I.; González, G.; Soria-Díaz, M.E.; Vasconcelos, V.; Cameán, A.M. Development and optimization of a method for the determination of Cylindrospermopsin from strains of Aphanizomenon cultures: Intra-laboratory assessment of its accuracy by using validation standards. Talanta 2012, 100, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Guillén, R.; Prieto, A.I.; Gónzalez, A.G.; Soria-Díaz, M.E.; Cameán, A.M. Cylindrospermopsin determination in water by LC-MS/MS: Optimization and validation the method and application to real samples. Environ. Toxicol. Chem. 2012, 31, 2233–2238. [Google Scholar] [CrossRef] [PubMed]
- Caixach, J.; Flores, C.; Spoof, L.; Meriluoto, J.; Schmidt, W.; Mazur-Marzec, H.; Hiskia, A.; Kaloudis, T.; Furey, A. Liquid Chromatography-Mass Spectrometry. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; Wiley & Sons: Chichester, UK, 2007; pp. 218–257. ISBN 9781119068686. [Google Scholar]
- Guzmán-Guillén, R.; Moreno, I.M.; Prieto, A.I.; Soria-Díaz, M.E.; Vasconcelos, V.M.; Cameán, A.M. CYN determination in tissues from fresh water fish by LC–MS/MS: Validation and application in tissues from subchronically exposed tilapia (Oreochromis niloticus). Talanta 2015, 131, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Pekar, H.; Westerberga, E.; Brunoa, O.; Laanec, A.; Perssond, K.M.; Sundstromf, L.F.; Thim, A.M. Fast, rugged and sensitive ultra-high pressure liquid chromatography tandem mass spectrometry method for analysis of cyanotoxins in raw water and drinking water- First findings of anatoxins, cylindrospermopsins and microcystin variants in Swedish source waters and infiltration ponds. J. Chromatogr. A 2016, 1429, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Guillén, R.; Maisanaba, S.; Prieto Ortega, A.I.; Valderrama-Fernández, R.; Jos, A.; Cameán, A.M. Changes on cylindrospermopsin concentration and characterization of decomposition products in fish muscle (Oreochromis niloticus) by boiling and steaming. Food Control 2017, 77, 210–220. [Google Scholar] [CrossRef]
- Prieto, A.I.; Guzmán-Guillén, R.; Valderrama-Fernández, R.; Jos, A.; Cameán, A.M. Influence of Cooking (Microwaving and Broiling) on Cylindrospermopsin Concentration in Muscle of Nile Tilapia (Oreochromis niloticus) and Characterization of Decomposition Products. Toxins 2017, 9, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Barwick, V.J. (Ed.) Eurachem/CITAC Guide: Guide to Quality in Analytical Chemistry: An Aid to Accreditation, 3rd ed.; Eurachem: London, UK, 2016; ISBN 978-0-948926-32-7. Available online: www.eurachem.org (accessed on 15 December 2017).
- AOAC International; AOAC Official Methods of Analysis. Guidelines for Standard Method Performance Requirements; Appendix F; AOAC International: Rockville, MD, USA, 2016.
- Dell’Aversano, C.; Eaglesham, G.K.; Quilliam, M.A. Analysis of cyanobacterial toxins by hydrophilic interaction liquid chromatography–mass spectrometry. J. Chromatogr. A 2004, 1028, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Barwick, V.J.; Prichard, E. (Eds.) Eurachem Guide: Terminology in Analytical Measurement—Introduction to VIM 3; Eurachem: London, UK, 2011; ISBN 978-0-948926-29-7. Available online: www.eurachem.org (accessed on 15 December 2017).
- Huber, L. Validation and Qualification in Analytical Laboratories; Interpharm: East Englewood, CO, USA, 1998; pp. 1–288. ISBN 9780849382673. [Google Scholar]
- ICH Harmonised Tripartite Guideline, Validation of Analytical Procedures: Text and Methodology, ICH Working Group, November 2005. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf (accessed on 15 December 2017).
- González, A.G.; Herrador, M.A. A practical guide to analytical method validation, including measurement uncertainty and accuracy profiles. Trends Anal. Chem. 2007, 26, 227–238. [Google Scholar] [CrossRef]
- González, A.G.; Herrador, M.A.; Asuero, A.G. Intra-laboratory assessment of method accuracy (trueness and precision) by using validation standards. Talanta 2010, 82, 1995–1998. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Ellison, S.L.R.; Wood, R. Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Caires, A.O.; Borges, T.; Lima, A.M.S.D.S.; Silva, L.O.B.; do Santos, W.N.L. Robustness evaluation in analytical methods optimized using experimental designs. Microchem. J. 2017, 131, 163–169. [Google Scholar] [CrossRef]
- Youden, W.Y. Statistical Techniques for Collaborative Tests; Association of Official Analytical Chemists: Washington, DC, USA, 1967; pp. 1–64. [Google Scholar]
- Crush, J.R.; Briggs, L.R.; Sprosen, J.M.; Nichols, S.N. Effect of irrigation with lake water containing Microcystins on Microcystin content and growth of ryegrass, clover, rape, and lettuce. Environ. Toxicol. 2008, 23, 246–252. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. The EFSA Comprehensive European Food Consumption Database. 2015. Available online: https://www.efsa.europa.eu/en/food-consumption/comprehensive-database (accessed on 15 December 2017).
- Jensen, M.H.; Malter, A.J. Chapter 7: Water Supply, Water Quality and Mineral Nutrition. In Protected Agriculture: A Global Review; The World Bank: Washington, DC, USA, 1995; pp. 65–69. ISBN 0253-7494. [Google Scholar]
- Guzmán-Guillén, R.; Prieto, A.I.; Moreno, I.; Rios, V.; Vasconcelos, V.M.; Cameán, A.M. Effects of depuration on oxidative biomarkers in Tilapia (Oreochromis niloticus) after subchronic exposure to cyanobacterium producing cylindrospermopsin. Aquat. Toxicol. 2014, 149, 40–49. [Google Scholar] [CrossRef] [PubMed]
| Analytical Parameters | |||||||
|---|---|---|---|---|---|---|---|
| CYN Concentration Level (µg L−1) | SW (µg L−1) | SB (µg L−1) | SIP (µg L−1) | RSDIP (%) | Recoveries (%) | LOD (ng g−1 fw a) | LOQ (ng g−1 fw a) |
| 20 | 1.88 | 3.72 | 2.64 | 12.72 | 104 | 0.22 | 0.42 |
| 200 | 19.25 | 36.96 | 26.50 | 14.70 | 90 | ||
| 500 | 48.79 | 78.21 | 60.22 | 14.20 | 85 | ||
| Combined Variables | t Values | ||
|---|---|---|---|
| F1 | High (+) | 1 min and 15 s | 1.36 |
| Low (−) | 1 min | ||
| F2 | High (+) | 10 mL | 0.297 |
| Low (−) | 9.5 mL | ||
| F3 | High (+) | 10 mL | 0.208 |
| Low (−) | 9.5 mL | ||
| Population Class | Age Range (Years) | Lettuce Consumption (g kg−1 b.w. Day) | Potential CYN Exposure (ng CYN kg−1 b.w. Day) | Estimated % TDI | |||
|---|---|---|---|---|---|---|---|
| Mean Range | 95th Percentile Range | Mean Range | 95th Percentile Range | Mean Range | 95th Percentile Range | ||
| Children | 3–10 | 0.05–1.15 | 0.05–3.40 | 0.12–2.72 | 0.12–8.06 | 0.39–9.08 | 0.39–26.86 |
| Adolescents | 10–18 | 0.07–0.92 | 0.16–2.78 | 0.17–2.18 | 0.38–6.59 | 0.55–7.27 | 1.26–24.23 |
| Adults | 18–65 | 0.03–0.65 | 0.11–1.56 | 0.07–1.54 | 0.26–3.70 | 0.24–5.13 | 0.87–12.32 |
| Elderly | 65–75 | 0.07–5.39 | 0.24–29.76 | 0.17–12.77 | 0.57–70.53 | 0.55–42.58 | 1.90–235.10 |
| Very elderly | >75 | 0.06–0.75 | 0.10–1.21 | 0.14–1.78 | 0.24–2.87 | 0.47–5.92 | 0.79–9.56 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prieto, A.I.; Guzmán-Guillén, R.; Díez-Quijada, L.; Campos, A.; Vasconcelos, V.; Jos, Á.; Cameán, A.M. Validation of a Method for Cylindrospermopsin Determination in Vegetables: Application to Real Samples Such as Lettuce (Lactuca sativa L.). Toxins 2018, 10, 63. https://doi.org/10.3390/toxins10020063
Prieto AI, Guzmán-Guillén R, Díez-Quijada L, Campos A, Vasconcelos V, Jos Á, Cameán AM. Validation of a Method for Cylindrospermopsin Determination in Vegetables: Application to Real Samples Such as Lettuce (Lactuca sativa L.). Toxins. 2018; 10(2):63. https://doi.org/10.3390/toxins10020063
Chicago/Turabian StylePrieto, Ana I., Remedios Guzmán-Guillén, Leticia Díez-Quijada, Alexandre Campos, Vitor Vasconcelos, Ángeles Jos, and Ana M. Cameán. 2018. "Validation of a Method for Cylindrospermopsin Determination in Vegetables: Application to Real Samples Such as Lettuce (Lactuca sativa L.)" Toxins 10, no. 2: 63. https://doi.org/10.3390/toxins10020063
APA StylePrieto, A. I., Guzmán-Guillén, R., Díez-Quijada, L., Campos, A., Vasconcelos, V., Jos, Á., & Cameán, A. M. (2018). Validation of a Method for Cylindrospermopsin Determination in Vegetables: Application to Real Samples Such as Lettuce (Lactuca sativa L.). Toxins, 10(2), 63. https://doi.org/10.3390/toxins10020063










