Fibroblast Growth Factor Receptor, a Novel Receptor for Vegetative Insecticidal Protein Vip3Aa
Abstract
1. Introduction
2. Results
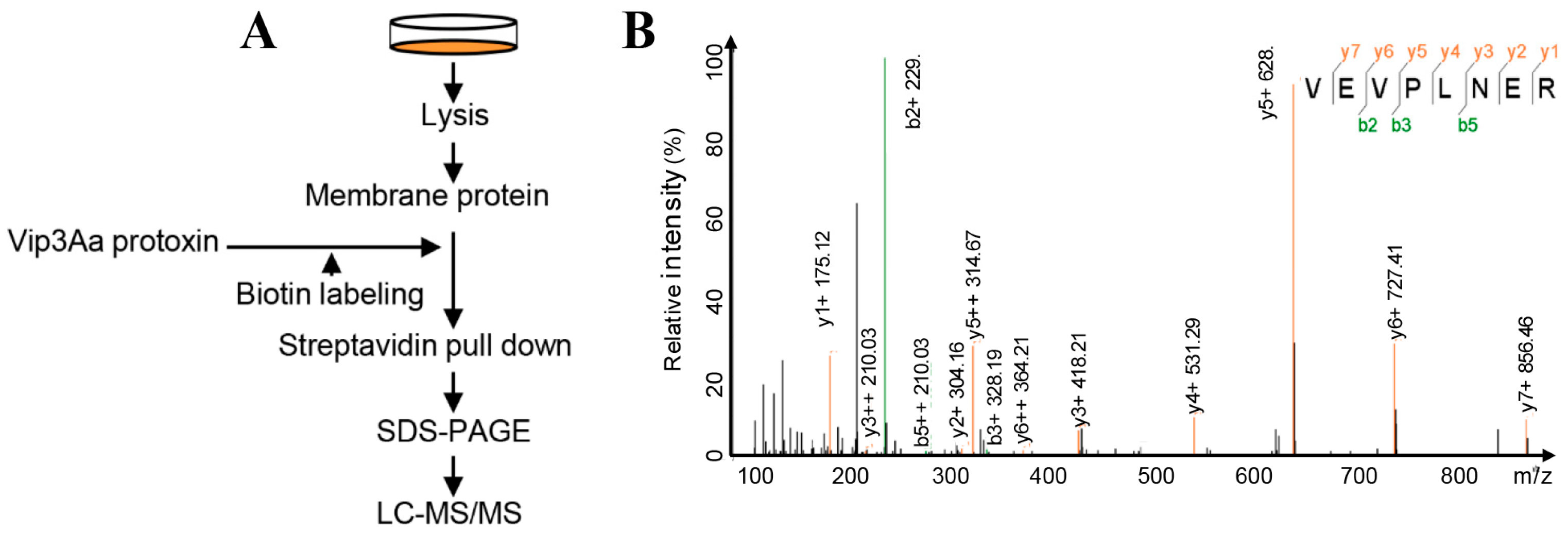
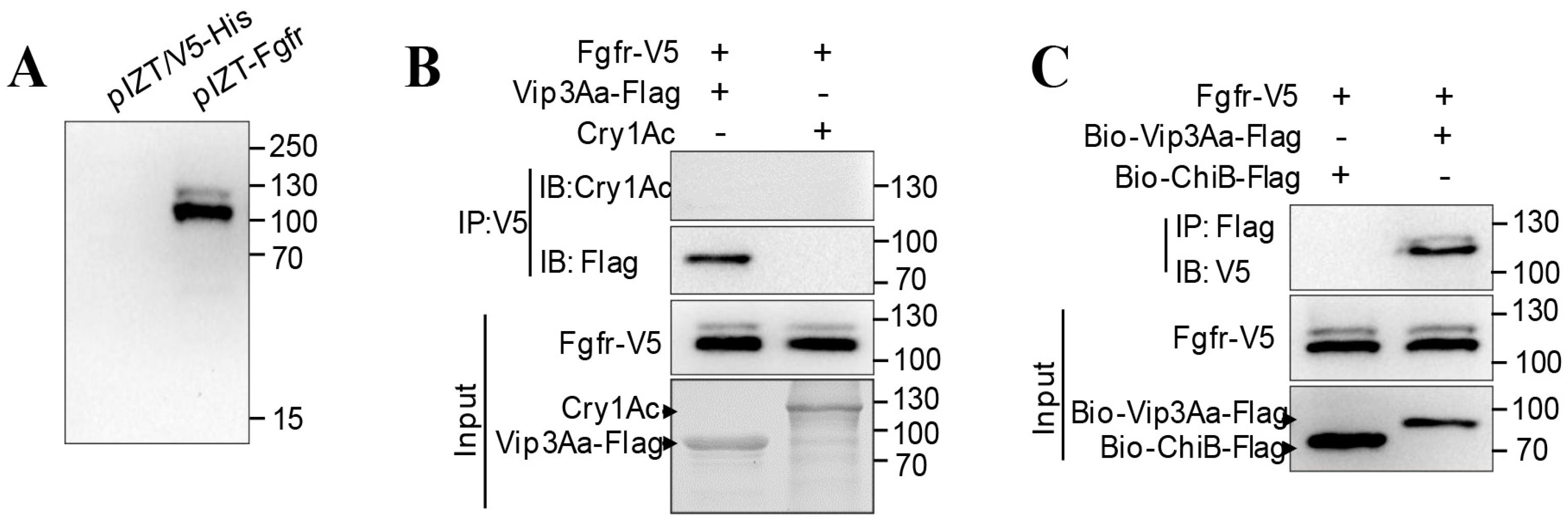
2.1. Interacting Partners to Vip3Aa Include Sf-FGFR
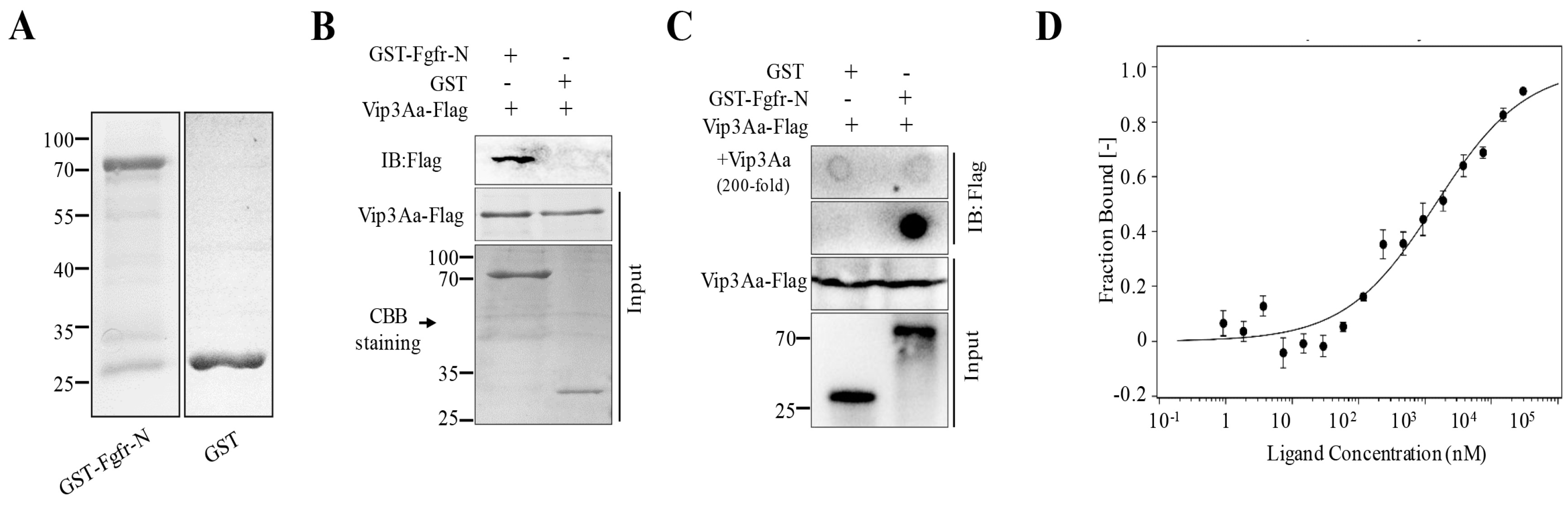
2.2. The Extracellular Regions of Sf-FGFR Binds to Vip3Aa
2.3. Ex vivo Binding Study of Sf-FGFR and Vip3Aa
2.4. Vip3Aa-RFP could Co-localize with Sf-FGFR on the Suface of Sf9 Cells
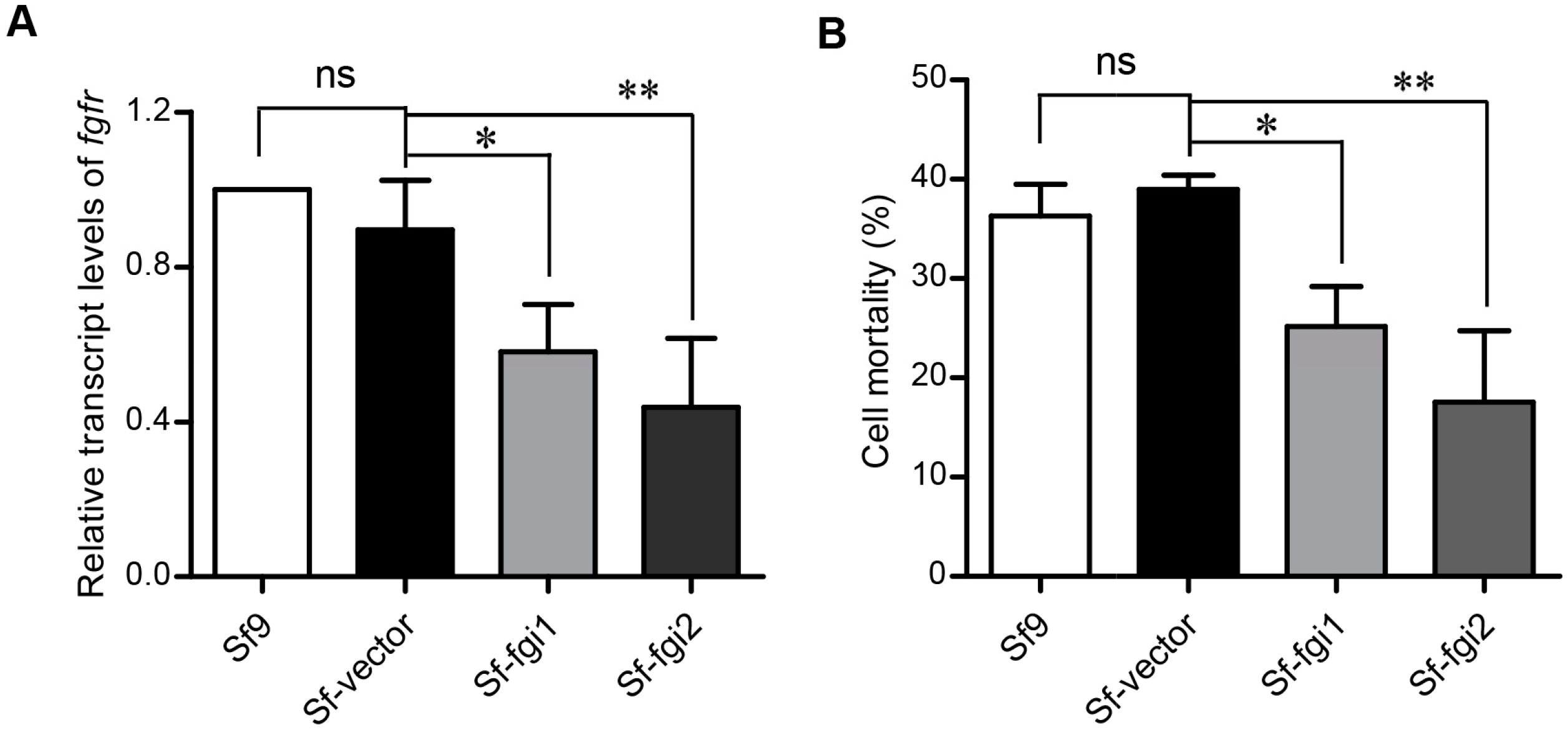
2.5. Reducing the Expression of Sf-FGFR Gene in Sf9 Cells Decreases the Cell Sensitivity to Vip3Aa
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Bacterial Strains and Cell Lines
5.2. Chemicals
5.3. Protein Purification
5.4. Microscale Thermophoresis (MST) Assay
5.5. Plasmid Construction, Preparation, and Transfection
5.6. Mass Spectrometry
5.7. Western Blotting and Immunoprecipitation
5.8. Dot Blotting and Pull-down Assay
5.9. Immunostaining and Confocal Microscopy
5.10. Cytotoxicity Assays
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; Caballero, P. Bacillus thuringiensis toxins: An overview of their biocidal activity. Toxins 2014, 6, 3296–3325. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Lopez, L.; Soberon, M.; Bravo, A. Bacillus thuringiensis insecticidal three-domain Cry toxins: Mode of action, insect resistance and consequences for crop protection. FEMS Microbiol. Rev. 2013, 37, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.L.; Soccol, V.T.; Soccol, C.R. Bacillus thuringiensis: Mechanism of action, resistance, and new applications: A review. Crit. Rev. Biotechnol. 2016, 36, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Carrière, Y. Surge in insect resistance to transgenic crops and prospects for sustainability. Nat. Biotechnol. 2017, 35, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Pigott, C.R.; Ellar, D.J. Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol. Mol. Biol. Rev. 2007, 71, 255–281. [Google Scholar] [CrossRef] [PubMed]
- Adang, M.J.; Crickmore, N.; Jurat-Fuentes, J.L. Diversity of Bacillus thuringiensis crystal toxins and mechanism of action. Adv. Insect Physiol. 2014, 47, 39–87. [Google Scholar]
- Estruch, J.J.; Warren, G.W.; Mullins, M.A.; Nye, G.J.; Craig, J.A.; Koziel, M.G. Vip3A, a novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectrum of activities against lepidopteran insects. Proc. Natl. Acad. Sci. USA 1996, 93, 5389–5394. [Google Scholar] [CrossRef] [PubMed]
- Chakroun, M.; Banyuls, N.; Bel, Y.; Escriche, B.; Ferré, J. Bacterial vegetative insecticidal proteins (Vip) from entomopathogenic bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 329–350. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Hou, X.Y.; Tan, T.T.; Cao, Z.L.; Mei, S.Q.; Yan, B.; Chang, J.; Han, L.; Zhao, D.; Cai, J. Scavenger receptor-C acts as a receptor for Bacillus thuringiensis vegetative insecticidal protein Vip3Aa and mediates the internalization of Vip3Aa via endocytosis. PLoS Pathog. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, V. From microbial sprays to insect-resistant transgenic plants: History of the biospesticide Bacillus thuringiensis. A review. Agron. Sustain. Dev. 2011, 31, 217–231. [Google Scholar] [CrossRef]
- Moar, W.J.; Berry, C.; Narva, K.E. The structure/function of new insecticidal proteins and regulatory challenges for commercialization. J. Invertebr. Pathol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Sachdev, B.; Sharma, N.; Seth, R.; Bhatnagar, R.K. Interaction of Bacillus thuringiensis vegetative insecticidal protein with ribosomal S2 protein triggers larvicidal activity in Spodoptera frugiperda. Appl. Environ. Microbiol. 2010, 76, 7202–7209. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Williams, L.T. Structural and Functional Diversity in the Fgf Receptor Multigene Family. Adv. Cancer Res. 1993, 60, 1–41. [Google Scholar] [PubMed]
- Tiong, K.H.; Mah, L.Y.; Leong, C.-O. Functional roles of fibroblast growth factor receptors (FGFRs) signaling in human cancers. Apoptosis 2013, 18, 1447–1468. [Google Scholar] [CrossRef] [PubMed]
- Wienken, C.J.; Baaske, P.; Rothbauer, U.; Braun, D.; Duhr, S. Protein-binding assays in biological liquids using microscale thermophoresis. Nat. Commun. 2010. [Google Scholar] [CrossRef] [PubMed]
- Katsuma, S.; Daimon, T.; Mita, K.; Shimada, T. Lepidopteran ortholog of Drosophila breathless is a receptor for the baculovirus fibroblast growth factor. J. Virol. 2006, 80, 5474–5481. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wesche, J.; Haglund, K.; Haugsten, E.M. Fibroblast growth factors and their receptors in cancer. Biochem. J. 2011, 437, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Lambros, M.B.; Horlings, H.M.; Pearson, A.; Sharpe, R.; Natrajan, R.; Geyer, F.C.; van Kouwenhove, M.; Kreike, B.; Mackay, A.; et al. Integrative molecular profiling of triple negative breast cancers identifies amplicon drivers and potential therapeutic targets. Oncogene 2010, 29, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Seckl, M.J. A Therapeutic Target for Smoking-Associated Lung Cancer. Sci. Transl. Med. 2010. [Google Scholar] [CrossRef] [PubMed]
- Pardo, O.E.; Latigo, J.; Jeffery, R.E.; Nye, E.; Poulsom, R.; Spencer-Dene, B.; Lemoine, N.R.; Stamp, G.W.; Aboagye, E.O.; Seckl, M.J. The Fibroblast Growth Factor Receptor Inhibitor PD173074 Blocks Small Cell Lung Cancer Growth in Vitro and in Vivo. Cancer Res. 2009, 69, 8645–8651. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; DeAngelo, D.J.; Kutok, J.L.; Williams, I.R.; Lee, B.H.; Wadleigh, M.; Duclos, N.; Cohen, S.; Adelsperger, J.; Okabe, R. PKC412 inhibits the zinc finger 198-fibroblast growth factor receptor 1 fusion tyrosine kinase and is active in treatment of stem cell myeloproliferative disorder. Proc. Natl. Acad. Sci. USA 2004, 101, 14479–14484. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Mei, S.Q.; Wang, T.T.; Pan, J.H.; Chen, Y.H.; Cai, J. Vip3Aa induces apoptosis in cultured Spodoptera frugiperda (Sf9) cells. Toxicon 2016, 120, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Martínez, P.; Gomis-Cebolla, J.; Ferré, J.; Escriche, B. Changes in gene expression and apoptotic response in Spodoptera exigua larvae exposed to sublethal concentrations of Vip3 insecticidal proteins. Sci. Rep. 2017. [Google Scholar] [CrossRef] [PubMed]
- Jean, S.; Mikryukov, A.; Tremblay, M.G.; Baril, J.; Guillou, F.; Bellenfant, S.; Moss, T. Extended-Synaptotagmin-2 Mediates FGF Receptor Endocytosis and ERK Activation In Vivo. Dev. Cell. 2010, 19, 426–439. [Google Scholar] [CrossRef] [PubMed]

| Primer Name | Sequence (5′→3′) | Function |
|---|---|---|
| pIZT-Fgfr-F pIZT-Fgfr-R Fgi1-Up-F Fgi1-Up-R Fgi1-Do-F Fgi1-Do-R Fgi2-Up-F Fgi2-Up-R Fgi2-Do-F Fgi2-Do-R Fgfr-N-F Fgfr-N-R Sf-actin-RT-F Sf-actin-RT-R Sf-Fg-RT-F Sf-Fg-RT-R | TCGAATTTAAAGCTTGGTACAATGGTAATGAGTCTCGCCGCCATCGC AGGCTTACCTTCGAACCGCGGCTTGATGAAGGGGAAGTCACTA CGAATTTAAAGCTTGGTACGGCAACGGGGTGTCTCGCTCAAAC ATGAGAAACAAGATTACCAAGTTATGTTCGGCGTAGGGTT GAACATAACTTGGTAATCTTGTTTCTCATCTATATGACC AATGGTGATGGTGATGATGAGGCAACGGGGTGTCTCGCTCAAACC CGAATTTAAAGCTTGGTACAAGGTGCTCGGAGAAGGAGAGTTTG AGGATGTCCACAGAGGAGCACGCCGAACGACCAGACAT TTCGGCGTGCTCCTCTGTGGACATCCTTGGCCAGACCGA AATGGTGATGGTGATGATGAAAGGTGCTCGGAGAAGGAGAGTTTG CTGTTCCAGGGGCCCCTGGGACAAACCAGAGAAATTGTCTTGG TCAGTCACGATGCGGCCGCTCCTATGTGTGCTTTCCATGGTCTGGCG TCCTCCGTCTGGACTTGGC CTTCTCCTTGATGTCACGAACG GGCTGTGATAGTGACGCATTG CTTCGCCCGTAGCAGTAGG | Fgfr cloning Fgfr cloning Fgfr RNAi Fgfr RNAi Fgfr RNAi Fgfr RNAi Fgfr RNAi Fgfr RNAi Fgfr RNAi Fgfr RNAi Fgfr-N cloning Fgfr-N cloning Actin qRT-PCR Actin qRT-PCR Fgfr qRT-PCR Fgfr qRT-PCR |
| Plasmids | Relevant Characteristics | Reference |
|---|---|---|
| pET-Vip | Vip3Aa gene cloned into pET-28a (+), His tag binding C-terminal of Vip3Aa | [9] |
| pET-Vip-flag | Vip3Aa gene cloned into pET-28a (+), Flag-His tag binding C-terminal of Vip3Aa | [9] |
| pET-ChiB-flag | ChiB gene cloned into pET-28a (+), Flag-His tag binding C-terminal of ChiB | [9] |
| pET-Vip-RFP | RFP gene cloned into pET-Vip, RFP binding C-terminal of Vip3Aa | [9] |
| pIZT/V5-His | Expression vector, Zeocinr, C-terminal V5-His tag | Invitrogen |
| pIZT-Fgfr | Sf-Fgfr gene cloned into pIZT/V5-His, V5-His tag binding C-terminal of Sf-Fgfr | This study |
| pIZT-fgi1 | Fragment of Sf-Fgfr gene (60-679) and the reverse complemented fragment of Sf-Fgfr (559-60) cloned into pIZT/V5-His | This study |
| pIZT-fgi2 | Fragment of Sf-Fgfr gene(1600-2219) and the reverse complemented Fragment of Sf-Fgfr (2099-1600) cloned into pIZT/V5-His | This study |
| pGEX-6P-1 | Expression vector, Ampr, N-terminal GST tag | Lab collection |
| pGEX-Fgfr-N | Extracellular sequence of Sf-Fgfr gene (Fgfr-N) cloned into pGEX-6P-1, GST tag binding N-terminal of Fgfr-N | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, K.; Hou, X.; Han, L.; Tan, T.; Cao, Z.; Cai, J. Fibroblast Growth Factor Receptor, a Novel Receptor for Vegetative Insecticidal Protein Vip3Aa. Toxins 2018, 10, 546. https://doi.org/10.3390/toxins10120546
Jiang K, Hou X, Han L, Tan T, Cao Z, Cai J. Fibroblast Growth Factor Receptor, a Novel Receptor for Vegetative Insecticidal Protein Vip3Aa. Toxins. 2018; 10(12):546. https://doi.org/10.3390/toxins10120546
Chicago/Turabian StyleJiang, Kun, Xiaoyue Hou, Lu Han, Tongtong Tan, Zhanglei Cao, and Jun Cai. 2018. "Fibroblast Growth Factor Receptor, a Novel Receptor for Vegetative Insecticidal Protein Vip3Aa" Toxins 10, no. 12: 546. https://doi.org/10.3390/toxins10120546
APA StyleJiang, K., Hou, X., Han, L., Tan, T., Cao, Z., & Cai, J. (2018). Fibroblast Growth Factor Receptor, a Novel Receptor for Vegetative Insecticidal Protein Vip3Aa. Toxins, 10(12), 546. https://doi.org/10.3390/toxins10120546





