Biosynthetic Oligoclonal Antivenom (BOA) for Snakebite and Next-Generation Treatments for Snakebite Victims †
Abstract
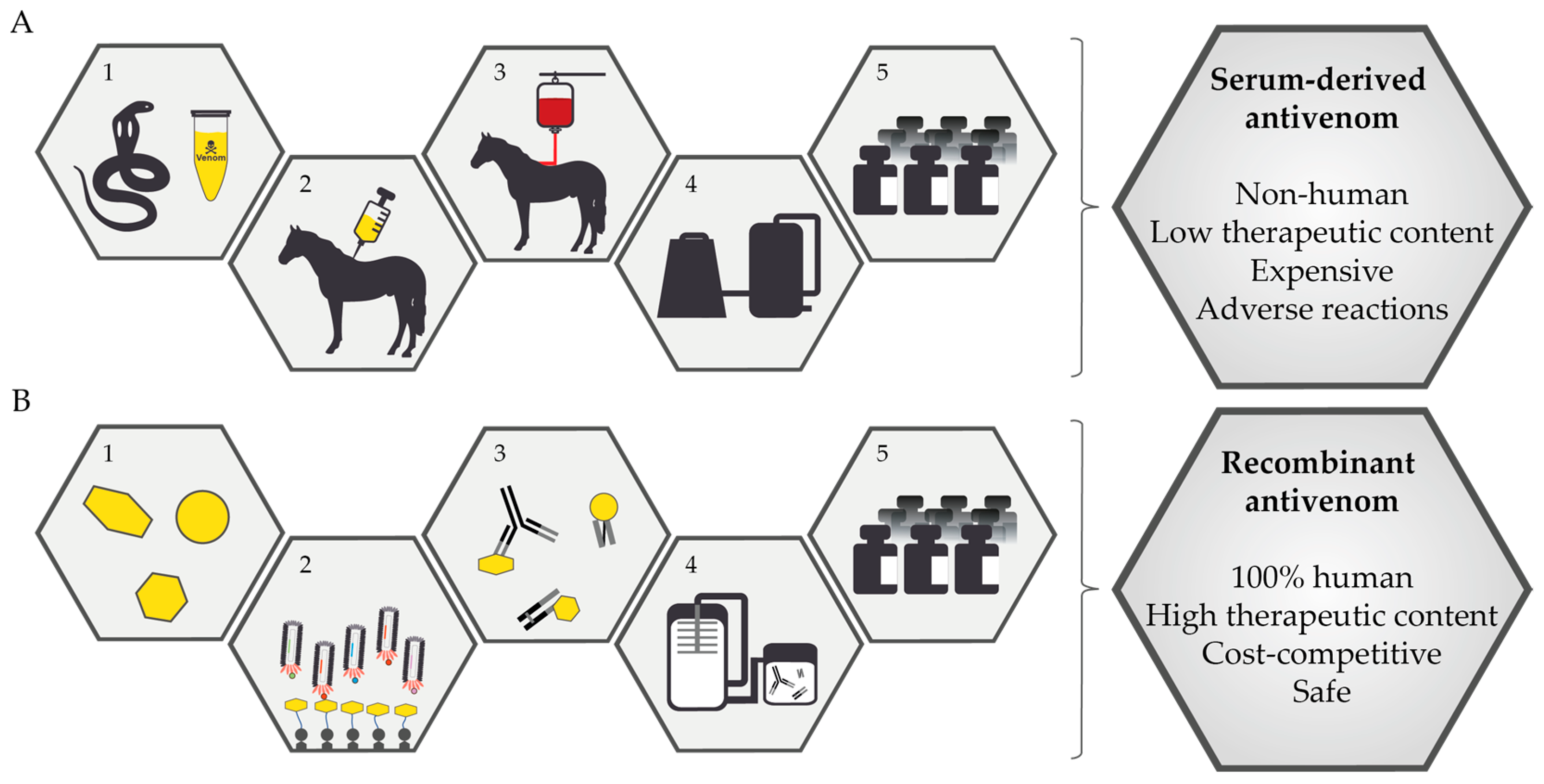
1. Introduction
2. Current Treatment for Snakebite Victims
- 1.
- Inability to abrogate local tissue damage: Snakebites from several snake species cause severe local tissue damage, leading to disfigurement, amputation, and permanent disability. The administration of antivenoms in most cases fails to neutralize this catastrophic pathology, as the heterologous antibodies or antibody fragments in antivenoms have insufficient pharmacokinetics to reach and neutralize toxins in deep tissue before these have started exerting their toxic functions [2].
- 2.
- Allergic reactions and anaphylactic shock: The administration of antivenoms, which are foreign horse-derived antibodies, may lead to acute anaphylactic shock in snakebite victims, which has been demonstrated to be the case for >40% for certain antivenoms [7,8,9,10]. These life-threatening adverse reactions must be managed by attending clinicians.
- 3.
- Serum sickness: Serum sickness is a delayed response to antivenom administration that occurs for 5–56% of treated victims for certain antivenoms [11,12,13]. The incidence of serum sickness is poorly defined, mostly because patients rarely return to health centers or they are not adequately followed after hospital discharge. Despite best efforts, typical antivenoms contain only 5–36% snake venom toxin-binding antibodies [14,15,16]. The ability of these antibodies to neutralize snakebite pathologies depends on the proportion of toxin-neutralizing antibodies and their pharmacokinetics. Hence, a significant number of antivenom vials are administered to each snakebite victim, with extreme cases requiring as much as 15 g of heterologous antibody protein [17]. Such a high dose administration increases the probability of serum sickness.
- 4.
- Inability to neutralize snake venoms from different regions: Snake venoms exhibit significant geographic variations in their toxin composition [18,19,20,21,22,23,24,25,26,27,28,29]. These variations are due to local adaptation, differences in diet, and ontogeny [30]. In a large country, like India, it would be ideal to pool venoms from various regions when designing immunization mixtures to overcome this drawback.
- 5.
- Complex manufacturing processes: Antivenom manufacture is complicated by the dependence of polyclonal antibodies on two biological systems, namely representative snake venoms and individual horse immune systems.
3. Next-Generation Snakebite Therapy
3.1. Biosynthetic Oligoclonal Antibodies (BOA) for Snakebite
- 1.
- Compatibility with human victims: BOA will contain only human antibodies and will thus be compatible with treatment of human patients [50].
- 2.
- Enriched for toxin-neutralizing antibodies: Horse-derived antivenoms contain both toxin-neutralizing and toxin-binding antibodies, but only toxin-neutralizing antibodies are useful for abrogating the pathophysiology of envenomation [33]. Antibody production in animals occurs due to the natural immune response, and there is no control over the antibody clones that expand and produce antibodies [51]. Therefore, horse antibodies show significant differences in their neutralizing capacities. In addition, these antivenoms may also contain antibodies raised against irrelevant infections, to which horses used for antivenom manufacture may have been exposed. Consequently, horse-derived antivenoms contain a small percentage of toxin-neutralizing antibodies [5]. In contrast, recombinant antibodies can be selected precisely for toxin-neutralizing ability. Therefore, the BOA will be enriched for toxin-neutralizing antibodies.
- 3.
- Consistent and reproducible production: The production of polyclonal antibodies in horses is highly variable, and there will always be inherent batch-to-batch variations. The quality of BOAs will provide excellent consistency and reproducibility, and thus, batch-to-batch variation will be obviated [39,50].
- 4.
- Tailor-made antibodies with optimal pharmacokinetics (PK) and pharmacodynamics (PD): Different toxins in snake venoms exhibit distinct biodistribution, PK, and PD, which often contributes to multi-organ failure in snakebite victims. Neutralization of such varied properties of toxins requires antibodies with appropriate biodistribution, PK, and PD. This can be achieved by utilizing full-length immunoglobulin G (IgG) antibodies, antibody fragments, or alternative non-antibody-based binding proteins [35,50]. In the preparation of BOAs, it is possible to include a mixture of full-length antibodies and/or fragments based on the properties of each toxin (toxicokinetics). Tailor-made mixtures of antibodies cannot be produced from horse-derived polyclonal antibodies, but are possible in BOA technology.
- 5.
- Better safety profile: Highly compatible, toxin-neutralizing antibodies with suitable PK and PD are expected to have better safety profiles compared to horse-derived antivenoms [50]. Thus, intravenous administration of a BOA should not cause acute (allergic and anaphylactic) or delayed (serum sickness) reactions.
- 6.
- Rapid administration of antivenoms: Because of inherent acute allergic and anaphylactic reactions, horse-derived antivenoms are administered to snakebite victims only after the victim develops symptoms and has reached a hospital setting. Such delays lead to poor treatment outcomes. The better safety profile of the BOA will allow quicker administration, for example, during transportation to the hospital, thus likely allowing for improved treatment outcomes.
- 7.
- Acceptance among clinicians: Poor efficacy compounded with acute (allergic and anaphylactic) and delayed (serum sickness) reactions has kept many clinicians from venturing to treat snakebite victims with antivenom. With better efficacy and safety profiles, BOA will help in the acceptance of treatment of snakebite victims.
- 8.
- Geographic variation of venoms: Most of the geographic variation in venom composition is due to differences in the abundance of specific toxins in venoms from snake specimens obtained from different regions. Such variations, at times, will make horse-derived antivenoms raised against venoms from one region ineffective against venoms from the same species in another region. Additionally, venoms from the same snake species from different regions may have one or more distinct/unique toxins. In both these cases, the problems can be overcome with BOA by simply including more antibodies or additional antibodies against all offending toxin(s). Such additions will not affect the safety profile of the BOA due to the compatibility of human monoclonal antibodies with the human immune system.
- 9.
- Cross-reactivity with other snake venom toxins: Some toxin-neutralizing antibodies neutralize related toxins not only from the same species, but also from different species [52]. If such cross-reactivity is intelligently engineered into the monoclonal antibodies during development, it may help in preparing polyvalent BOAs from a stock of a limited number of human antibodies [53].
- 10.
- 11.
- Abrogation of local tissue damage: In most cases, horse-derived antivenoms fail to abrogate local tissue damage induced by snake venom toxins [2]. This inability could be either due to a lack of antibodies that neutralize the offending toxin(s) or to the toxin(s) initiating local tissue damaging processes before being neutralized by antibodies [54]. It may be possible to find suitable human antibodies that could neutralize offending toxins. Alternatively, enzymatic processes leading to local tissue damage could be neutralized using small molecule enzyme inhibitors [55,56] (see below). Finally, by having an improved safety profile, it might be possible to administer BOA during transportation en route to the hospital, thereby minimizing the time that the locally-acting snake toxins can exert their toxic actions around the bite wound.
- 12.
- Potential prophylactic use of BOA: The better safety profile of BOA could be of prophylactic use for people who will be exposed to snakebite hazards. The longer PK of full-length antibodies (IgGs), which typically have half-lives of several weeks [50], could provide excellent prophylactic protection, which could reduce mortality, morbidity, and intensity of pathophysiological impact of snakebite.
3.2. Small Molecule Enzyme Inhibitors
- 1.
- Increased treatment window: Treatment for envenomation should ideally start within a short time period following snakebite, as mortality and morbidity increase significantly beyond this window. Small molecule enzyme inhibitors may substantially increase this time window, if they can be administered in the field setting (i.e., if they are stable at elevated temperatures and orally available), and could thus provide more time to reach hospital care.
- 2.
- 3.
- Validated safety in humans: As many of these small molecule inhibitors have been evaluated for their toxicity in human recipients, they have already been proven sufficiently safe for use in the treatment of snakebite envenoming.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chippaux, J.-P. Snakebite envenomation turns again into a neglected tropical disease! J. Venom Anim. Toxins Incl. Trop. Dis. 2017, 23, 38. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, B.; Warrell, D.A.; Suraweera, W.; Bhatia, P.; Dhingra, N.; Jotkar, R.M.; Rodriguez, P.S.; Mishra, K.; Whitaker, R.; Jha, P.; et al. Snakebite mortality in India: A nationally representative mortality survey. PLoS Negl. Trop. Dis. 2011, 5, e1018. [Google Scholar] [CrossRef] [PubMed]
- Warrell, D.A. Clinical toxicology of snakebite in Asia. In Handbook of Clinical Toxicology of Animal Venoms and Poisons; Meier, J., White, J., Eds.; CRC Press: Boca Raton, FL, USA, 1995; pp. 493–594. ISBN 978-0-8493-4489-3. [Google Scholar]
- Laustsen, A.H.; Engmark, M.; Milbo, C.; Johannesen, J.; Lomonte, B.; Gutiérrez, J.M.; Lohse, B. From Fangs to Pharmacology: The Future of Snakebite Envenoming Therapy. Curr. Pharm. Des. 2016, 22, 5270–5293. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; León, G.; Lomonte, B.; Angulo, Y. Antivenoms for snakebite envenomings. Inflamm. Allergy Drug Targets 2011, 10, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Malasit, P.; Warrell, D.A.; Chanthavanich, P.; Viravan, C.; Mongkolsapaya, J.; Singhthong, B.; Supich, C. Prediction, prevention, and mechanism of early (anaphylactic) antivenom reactions in victims of snake bites. Br. Med. J. (Clin. Res. Ed.) 1986, 292, 17–20. [Google Scholar] [CrossRef]
- Theakston, R.D.G.; Warrell, D.A.; Griffiths, E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon 2003, 41, 541–557. [Google Scholar] [CrossRef]
- Lalloo, D.G.; Theakston, R.D.G. Snake antivenoms. J. Toxicol. Clin. Toxicol. 2003, 41, 277–290. [Google Scholar] [CrossRef]
- Ariaratnam, C.A.; Sjöström, L.; Raziek, Z.; Kularatne, S.A.; Arachchi, R.W.; Sheriff, M.H.; Theakston, R.D.; Warrell, D.A. An open, randomized comparative trial of two antivenoms for the treatment of envenoming by Sri Lankan Russell’s viper (Daboia russelii russelii). Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 74–80. [Google Scholar] [CrossRef]
- Lavonas, E.J.; Gerardo, C.J.; O’Malley, G.; Arnold, T.C.; Bush, S.P.; Banner, W.; Steffens, M.; Kerns, W.P. Initial experience with Crotalidae polyvalent immune Fab (ovine) antivenom in the treatment of copperhead snakebite. Ann. Emerg. Med. 2004, 43, 200–206. [Google Scholar] [CrossRef]
- Bush, S.P.; Green, S.M.; Moynihan, J.A.; Hayes, W.K.; Cardwell, M.D. Crotalidae polyvalent immune Fab (ovine) antivenom is efficacious for envenomations by Southern Pacific rattlesnakes (Crotalus helleri). Ann. Emerg. Med. 2002, 40, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Lavonas, E.J.; Kokko, J.; Schaeffer, T.H.; Mlynarchek, S.L.; Bogdan, G.M.; Dart, R.C. Short-term outcomes after Fab antivenom therapy for severe crotaline snakebite. Ann. Emerg. Med. 2011, 57, 128–137. [Google Scholar] [CrossRef]
- Herrera, M.; Paiva, O.K.; Pagotto, A.H.; Segura, A.; Serrano, S.M.T.; Vargas, M.; Villalta, M.; Jensen, S.D.; León, G.; Williams, D.J.; et al. Antivenomic characterization of two antivenoms against the venom of the taipan, Oxyuranus scutellatus, from Papua New Guinea and Australia. Am. J. Trop. Med. Hyg. 2014, 91, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Segura, Á.; Herrera, M.; Villalta, M.; Vargas, M.; Gutiérrez, J.M.; León, G. Assessment of snake antivenom purity by comparing physicochemical and immunochemical methods. Biologicals 2013, 41, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S.; Laing, G.; Smith, D.C.; Theakston, D.; Landon, J. A new antivenom to treat eastern coral snake (Micrurus fulvius fulvius) envenoming. Toxicon 1994, 32, 185–190. [Google Scholar] [CrossRef]
- Harrison, R.A.; Gutiérrez, J.M. Priority actions and progress to substantially and sustainably reduce the mortality, morbidity and socioeconomic burden of tropical snakebite. Toxins 2016, 8, 351. [Google Scholar] [CrossRef] [PubMed]
- Alape-Girón, A.; Sanz, L.; Escolano, J.; Flores-Díaz, M.; Madrigal, M.; Sasa, M.; Calvete, J.J. Snake Venomics of the Lancehead Pitviper Bothrops asper: Geographic, Individual, and Ontogenetic Variations. J. Proteome Res. 2008, 7, 3556–3571. [Google Scholar] [CrossRef] [PubMed]
- Boldrini-Franca, J. Snake venomics and antivenomics of Crotalus durissus subspecies from Brazil: Assessment of geographic variation and its implication on snakebite management. J. Proteomics 2010, 73, 1758–1776. [Google Scholar] [CrossRef]
- Calvete, J.J. Snake population venomics and antivenomics of Bothrops atrox: Paedomorphism along its transamazonian dispersal and implications of geographic venom variability on snakebite management. J. Proteomics 2011, 74, 510–527. [Google Scholar] [CrossRef]
- Núñez, V.; Cid, P.; Sanz, L.; De La Torre, P.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M.; Calvete, J.J. Snake venomics and antivenomics of Bothrops atrox venoms from Colombia and the Amazon regions of Brazil, Perú and Ecuador suggest the occurrence of geographic variation of venom phenotype by a trend towards paedomorphism. J. Proteomics 2009, 73, 57–78. [Google Scholar] [CrossRef]
- Tan, K.Y.; Tan, C.H.; Fung, S.Y.; Tan, N.H. Venomics, lethality and neutralization of Naja kaouthia (monocled cobra) venoms from three different geographical regions of Southeast Asia. J. Proteomics 2015, 120, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Goncalves-Machado, L. Combined venomics, venom gland transcriptomics, bioactivities, and antivenomics of two Bothrops jararaca populations from geographic isolated regions within the Brazilian Atlantic rainforest. J. Proteomics 2016, 135, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H.; Gutiérrez, J.M.; Rasmussen, A.R.; Engmark, M.; Gravlund, P.; Sanders, K.L.; Lohse, B.; Lomonte, B. Danger in the reef: Proteome, toxicity, and neutralization of the venom of the olive sea snake, Aipysurus laevis. Toxicon 2015, 107, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Shashidharamurthy, R.; Jagadeesha, D.K.; Girish, K.S.; Kemparaju, K. Variations in biochemical and pharmacological properties of Indian cobra (Naja naja naja) venom due to geographical distribution. Mol. Cell Biochem. 2002, 229, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Minton, S.A.; Weinstein, S.A. Geographic and ontogenic variation in venom of the western diamondback rattlesnake (Crotalus atrox). Toxicon 1986, 24, 71–80. [Google Scholar] [CrossRef]
- Williams, V.; White, J.; Schwaner, T.D.; Sparrow, A. Variation in venom proteins from isolated populations of tiger snakes (Notechis ater niger, N. scutatus) in South Australia. Toxicon 1988, 26, 1067–1075. [Google Scholar] [CrossRef]
- Daltry, J.C.; Wüster, W.; Thorpe, R.S. Diet and snake venom evolution. Nature 1996, 379, 537–540. [Google Scholar] [CrossRef]
- Jayanthi, G.P.; Gowda, T.V. Geographical variation in India in the composition and lethal potency of Russell’s viper (Vipera russelli) venom. Toxicon 1988, 26, 257–264. [Google Scholar] [CrossRef]
- Wray, K.P.; Margres, M.J.; Seavy, M.; Rokyta, D.R. Early significant ontogenetic changes in snake venoms. Toxicon 2015, 96, 74–81. [Google Scholar] [CrossRef]
- Walsh, G. Biopharmaceutical benchmarks 2014. Nat. Biotech. 2014, 32, 992–1000. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Karatt-Vellatt, A.; Masters, E.W.; Arias, A.S.; Pus, U.; Knudsen, C.; Oscoz, S.; Slavny, P.; Griffiths, D.T.; Luther, A.M.; et al. In vivo neutralization of dendrotoxin-mediated neurotoxicity of black mamba venom by oligoclonal human IgG antibodies. Nature Communications 2018, 9, 3928. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H.; Lohse, B.; Lomonte, B.; Engmark, M.; Gutiérrez, J.M. Selecting key toxins for focused development of elapid snake antivenoms and inhibitors guided by a Toxicity Score. Toxicon 2015, 104, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H. Recombinant Antivenoms, 1st ed.; University of Copenhagen: Copenhagen, Denmark, 2016; ISBN 978-87-93086-61-6. [Google Scholar]
- Laustsen, A.H. Guiding recombinant antivenom development by omics technologies. New Biotechnol. 2018, 45, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H. Toxin synergism in snake venoms. Toxin Rev. 2016, 35, 165–170. [Google Scholar] [CrossRef]
- Dam, S.H.; Friis, R.U.W.; Petersen, S.D.; Martos-Esteban, A.; Laustsen, A.H. Snake Venomics Display: An online toolbox for visualization of snake venomics data. Toxicon 2018, 152, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Méndez, E.; Fuglsang-Madsen, A.; Føns, S.; Lomonte, B.; Gutiérrez, J.M.; Laustsen, A.H. Innovative Immunization Strategies for Antivenom Development. Toxins 2018, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H.; Johansen, K.H.; Engmark, M.; Andersen, M.R. Recombinant snakebite antivenoms: A cost-competitive solution to a neglected tropical disease? PLoS Negl. Trop. Dis. 2017, 11, e0005361. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Dorrestijn, N. Integrating Engineering, Manufacturing, and Regulatory Considerations in the Development of Novel Antivenoms. Toxins 2018, 10, 309. [Google Scholar] [CrossRef]
- Ledsgaard, L.; Kilstrup, M.; Karatt-Vellatt, A.; McCafferty, J.; Laustsen, A.H. Basics of Antibody Phage Display Technology. Toxins 2018, 10, 236. [Google Scholar] [CrossRef]
- Roncolato, E.C.; Campos, L.B.; Pessenda, G.; Costa e Silva, L.; Furtado, G.P.; Barbosa, J.E. Phage display as a novel promising antivenom therapy: A review. Toxicon 2015, 93, 79–84. [Google Scholar] [CrossRef]
- McCafferty, J.; Griffiths, A.D.; Winter, G.; Chiswell, D.J. Phage antibodies: Filamentous phage displaying antibody variable domains. Nature 1990, 348, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.S. Phage display in pharmaceutical biotechnology. Curr. Opin. Biotechnol. 2000, 11, 610–616. [Google Scholar] [CrossRef]
- Rodi, D.J.; Makowski, L. Phage-display technology—Finding a needle in a vast molecular haystack. Curr. Opin. Biotechnol. 1999, 10, 87–93. [Google Scholar] [CrossRef]
- Hoogenboom, H.R.; de Bruïne, A.P.; Hufton, S.E.; Hoet, R.M.; Arends, J.-W.; Roovers, R.C. Antibody phage display technology and its applications. Immunotechnology 1998, 4, 1–20. [Google Scholar] [CrossRef]
- Parmley, S.F.; Smith, G.P. Antibody-selectable filamentous fd phage vectors: Affinity purification of target genes. Gene 1988, 73, 305–318. [Google Scholar] [CrossRef]
- Wright, A.; Morrison, S.L. Effect of glycosylation on antibody function: Implications for genetic engineering. Trends Biotechnol. 1997, 15, 26–32. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Johansen, K.H.; Engmark, M.; Andersen, M.R. Snakebites: Costing recombinant antivenoms. Nature 2016, 538, 41. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Gutiérrez, J.M.; Knudsen, C.; Johansen, K.H.; Bermúdez-Méndez, E.; Cerni, F.A.; Jürgensen, J.A.; Ledsgaard, L.; Martos-Esteban, A.; Øhlenschlæger, M.; et al. Pros and cons of different therapeutic antibody formats for recombinant antivenom development. Toxicon 2018, 146, 151–175. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Engmark, M.; Clouser, C.; Timberlake, S.; Vigneault, F.; Gutiérrez, J.M.; Lomonte, B. Exploration of immunoglobulin transcriptomes from mice immunized with three-finger toxins and phospholipases A2 from the Central American coral snake, Micrurus nigrocinctus. PeerJ 2017, 5, e2924. [Google Scholar] [CrossRef]
- Ledsgaard, L.; Jenkins, T.P.; Davidsen, K.; Krause, K.E.; Martos-Esteban, A.; Engmark, M.; Rørdam Andersen, M.; Lund, O.; Laustsen, A.H. Antibody Cross-Reactivity in Antivenom Research. Toxins 2018, 10, 393. [Google Scholar] [CrossRef]
- Laustsen, A.H. Toxin-centric development approach for next-generation antivenoms. Toxicon 2018, 150, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Prasarnpun, S.; Walsh, J.; Awad, S.S.; Harris, J.B. Envenoming bites by kraits: The biological basis of treatment-resistant neuromuscular paralysis. Brain 2005, 128, 2987–2996. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, C.; Laustsen, A.H. Recent Advances in Next Generation Snakebite Antivenoms. Trop. Med. Infect. Dis. 2018, 3, 42. [Google Scholar] [CrossRef]
- Arias, A.S.; Rucavado, A.; Gutiérrez, J.M. Peptidomimetic hydroxamate metalloproteinase inhibitors abrogate local and systemic toxicity induced by Echis ocellatus (saw-scaled) snake venom. Toxicon 2017, 132, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M.; Doley, R. Structure, function and evolution of three-finger toxins: Mini proteins with multiple targets. Toxicon 2010, 56, 855–867. [Google Scholar] [CrossRef]
- Kini, R.M. Excitement ahead: Structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon 2003, 42, 827–840. [Google Scholar] [CrossRef]
- Kini, R.M. Anticoagulant proteins from snake venoms: Structure, function and mechanism. Biochem. J. 2006, 397, 377–387. [Google Scholar] [CrossRef]
- Lewin, M.; Samuel, S.; Merkel, J.; Bickler, P. Varespladib (LY315920) Appears to Be a Potent, Broad-Spectrum, Inhibitor of Snake Venom Phospholipase A2 and a Possible Pre-Referral Treatment for Envenomation. Toxins 2016, 8, 248. [Google Scholar] [CrossRef]
- Lewin, M.R.; Gilliam, L.L.; Gilliam, J.; Samuel, S.P.; Bulfone, T.C.; Bickler, P.E.; Gutiérrez, J.M. Delayed LY333013 (Oral) and LY315920 (Intravenous) Reverse Severe Neurotoxicity and Rescue Juvenile Pigs from Lethal Doses of Micrurus fulvius (Eastern Coral Snake) Venom. Toxins 2018, 10, 479. [Google Scholar] [CrossRef]
- Lewin, M.R.; Gutiérrez, J.M.; Samuel, S.P.; Herrera, M.; Bryan-Quirós, W.; Lomonte, B.; Bickler, P.E.; Bulfone, T.C.; Williams, D.J. Delayed Oral LY333013 Rescues Mice from Highly Neurotoxic, Lethal Doses of Papuan Taipan (Oxyuranus scutellatus) Venom. Toxins 2018, 10, 380. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kini, R.M.; Sidhu, S.S.; Laustsen, A.H. Biosynthetic Oligoclonal Antivenom (BOA) for Snakebite and Next-Generation Treatments for Snakebite Victims. Toxins 2018, 10, 534. https://doi.org/10.3390/toxins10120534
Kini RM, Sidhu SS, Laustsen AH. Biosynthetic Oligoclonal Antivenom (BOA) for Snakebite and Next-Generation Treatments for Snakebite Victims. Toxins. 2018; 10(12):534. https://doi.org/10.3390/toxins10120534
Chicago/Turabian StyleKini, R. Manjunatha, Sachdev S. Sidhu, and Andreas Hougaard Laustsen. 2018. "Biosynthetic Oligoclonal Antivenom (BOA) for Snakebite and Next-Generation Treatments for Snakebite Victims" Toxins 10, no. 12: 534. https://doi.org/10.3390/toxins10120534
APA StyleKini, R. M., Sidhu, S. S., & Laustsen, A. H. (2018). Biosynthetic Oligoclonal Antivenom (BOA) for Snakebite and Next-Generation Treatments for Snakebite Victims. Toxins, 10(12), 534. https://doi.org/10.3390/toxins10120534





