First Report of Okadaic Acid and Pectenotoxins in Individual Cells of Dinophysis and in Scallops Argopecten purpuratus from Perú
Abstract
1. Introduction
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
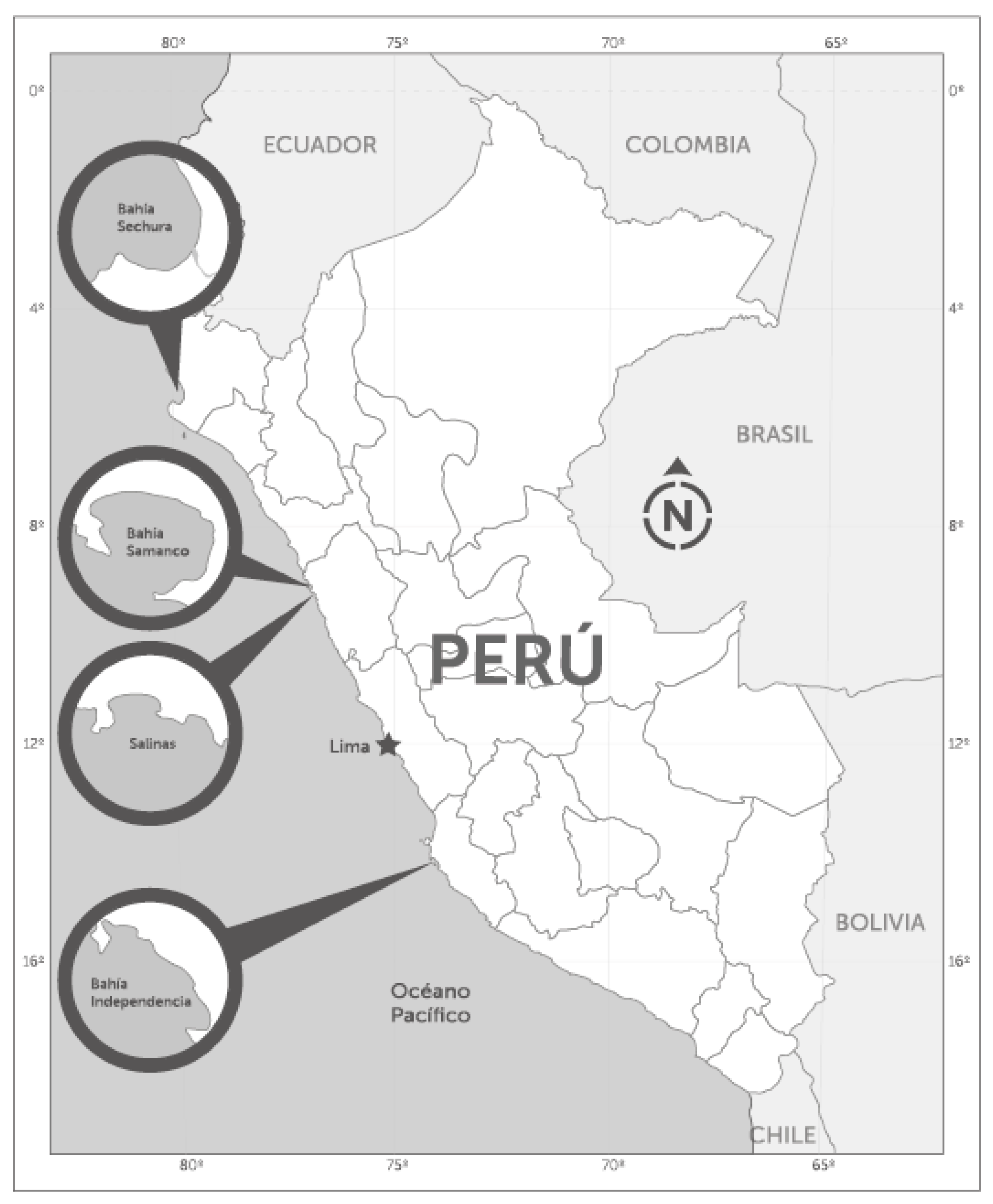
5.1. Field Sampling
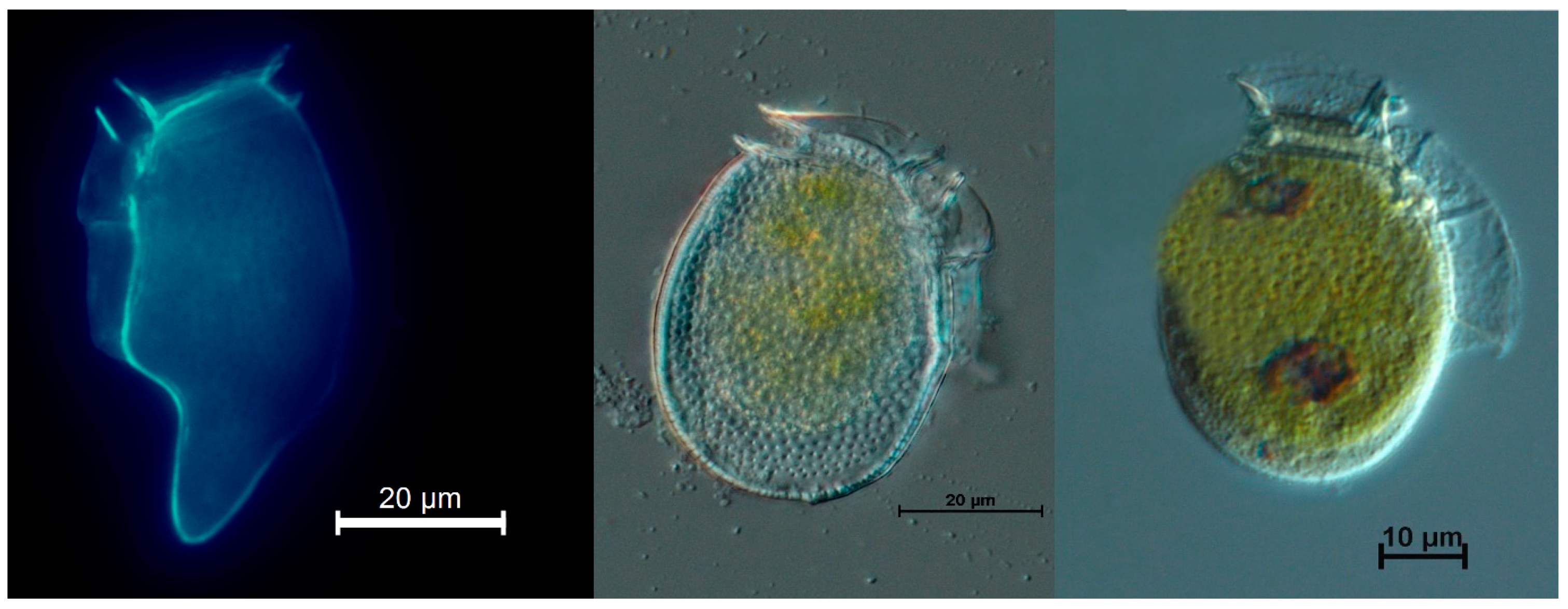
5.2. Single Cell Isolations
5.3. Standards and Reagents
5.4. Toxins Extraction
5.4.1. From Isolated Cells of Dinophysis
5.4.2. From Shellfish Meat
5.5. LC-MS/MS Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yasumoto, T.; Murata, M.; Oshima, Y.; Sano, M.; Matsumoto, G.K.; Clardy, J. Diarrhetic shellfish toxins. Tetrahedron 1985, 41, 1019–1025. [Google Scholar] [CrossRef]
- Yasumoto, T.; Oshima, Y.; Yamaguchi, M. Occurrence of a new type of shellfish poisoning in the Tohoku district. NIPPON SUISAN GAKKAISHI 1978, 44, 1249–1255. [Google Scholar] [CrossRef]
- Yasumoto, T.; Murata, M. Polyether Toxins Involved in Seafood Poisoning. In Marine Toxins; Hall, S., Strichartz, G., Eds.; American Chemical Society: Washington, DC, USA, 1990; Volume 418, pp. 120–132. [Google Scholar]
- Fux, E.; Smith, J.L.; Tong, M.; Guzmán, L.; Anderson, D.M. Toxin profiles of five geographical isolates of Dinophysis spp. from North and South America. Toxicon 2011, 57, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Reguera, B.; Riobó, P.; Rodríguez, F.; Díaz, P.; Pizarro, G.; Paz, B.; Franco, J.; Blanco, J. Dinophysis Toxins: Causative Organisms, Distribution and Fate in Shellfish. Mar. Drugs 2014, 12, 394–461. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Moroño, Á.; Fernández, M.L. Toxic episodes in shellfish, produced by lipophilic phycotoxins: An overview. Galician J. Mar. Resour. 2005, 1, 1–70. [Google Scholar]
- Van Dolah, F.M. Marine Algal Toxins: Origins, Health Effects, and Their Increased Occurrence. Environ. Health Perspect. 2000, 108, 9. [Google Scholar] [CrossRef] [PubMed]
- Reguera, B. Biología, Autoecología y Toxinología de las Principales Especies del Género Dinophysis Asociadas a Episodios de Intoxicación Diarreogénica por Bivalvos (DSP); Universidad de Barcelona: Barcelona, España, 2003. [Google Scholar]
- Jørgensen, K.; Andersen, P. Relation between the concentration of Dinophysis acuminata and diarrheic shellfish poisoning toxins in blue mussels (Mytilus edulis) during a toxic episode in the Limfjord (DENMARK), 2006. J. Shellfish Res. 2007, 26, 1081–1087. [Google Scholar] [CrossRef]
- Nincevic-Gladan, Z.; Skejic, S.; Arapov, J.; Buzancic, M.; Bojanic, N.; Ujevic, I.; Kuspilic, G.; Grbec, B.; Vidjack, O. Seasonal variability in Dinophysis spp. abundances and diarrhetic shellfish poisoning outbreaks along the eastern Adriatic coast. Botanica Mar. 2008, 51, 449–463. [Google Scholar] [CrossRef]
- Hossen, V.; Jourdan-da Silva, N.; Guillois-Bécel, Y.; Marchal, J.; Krys, S. Food poisoning outbreaks linked to mussels contaminated with okadaic acid and ester dinophysistoxin-3 in France, June 2009. Eurosurveillance 2011, 16. [Google Scholar] [CrossRef]
- Reguera, B.; Velo-Suárez, L.; Raine, R.; Park, M.G. Harmful Dinophysis species: A review. Harmful Algae 2012, 14, 87–106. [Google Scholar] [CrossRef]
- Sanchez, S.; Bernales, A.; Delgado, E.; Carmen Chang, F.D.; Jacobo, N.; Quispe, J. Variability and Biogeographical Distribution of Harmful Algal Blooms in Bays of High Productivity Off Peruvian Coast (2012–2015). J. Environ. Anal.Toxicol. 2017, 7. [Google Scholar] [CrossRef]
- Ochoa, N.; Gómez, O.; Sánchez, S.; Delgado, E. Diversidad de Diatomeas y Dinoflagelados Marinos del Perú. Bol. Inst. Mar. Perú 1999, 18, 1–14. [Google Scholar]
- Blanco, J.; Álvarez, G.; Uribe, E. Identification of pectenotoxins in plankton, filter feeders, and isolated cells of a Dinophysis acuminata with an atypical toxin profile, from Chile. Toxicon 2007, 49, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.L.; Reguera, B.; González-Gil, S.; Míguez, A. Pectenotoxin-2 in single-cell isolates of Dinophysis caudata and Dinophysis acuta from the Galician Rías (NW Spain). Toxicon 2006, 48, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.T.; Krock, B.; Hansen, P.J. Production and excretion of okadaic acid, pectenotoxin-2 and a novel dinophysistoxin from the DSP-causing marine dinoflagellate Dinophysis acuta—Effects of light, food availability and growth phase. Harmful Algae 2013, 23, 34–45. [Google Scholar] [CrossRef]
- Pizarro, G.; Paz, B.; Gonzalez-Gil, S.; Franco, J.M.; Reguera, B. Seasonal variability of lipophilic toxins during a Dinophysis acuta bloom in Western Iberia: Differences between picked cells and plankton concentrates. Harmful Algae 2009, 8, 926–937. [Google Scholar] [CrossRef]
- Pigozzi, S.; Ceredi, A.; Milandri, A.; Pompei, M.; Buzzichelli, S.; Macori, G.; Susini, F.; Forletta, R. Pectenotoxin and okadaic acid-based toxin profiles in phytoplankton and shellfish from Orbetello Lagoon, Italy. In Proceedings of the 7th International Conference on Molluscan Shellfish Safety, Nantes, France, 14–19 June 2009; Available online: https://www.researchgate.net/profile/Guerrino_Macori2/publication/304776353_Pectenotoxin_and_okadaic_acid-based_toxin_profiles_in_phytoplankton_and_shellfish_from_Orbetello_Lagoon_Italy/links/577a35e608ae355e74f05c29/Pectenotoxin-and-okadaic-acid-based-toxin-profiles-in-phytoplankton-and-shellfish-from-Orbetello-Lagoon-Italy.pdf (accessed on 17 November 2018). [CrossRef]
- MacKenzie, L.; Beuzenberg, V.; Holland, P.; McNabb, P.; Suzuki, T.; Selwood, A. Pectenotoxin and okadaic acid-based toxin profiles in Dinophysis acuta and Dinophysis acuminata from New Zealand. Harmful Algae 2005, 4, 75–85. [Google Scholar] [CrossRef]
- Nagai, S.; Suzuki, T.; Nishikawa, T.; Kamiyama, T. Differences in the production and excretion kinetics of okadaic acid, dinophysistoxin-1, and pectenotoxin-2 between cultures of Dinophysis acuminata and Dinophysis fortii isolated from western Japan. J.Phycol. 2011, 47, 1326–1337. [Google Scholar] [CrossRef] [PubMed]
- Utermöhl, H. Zur Vervollkommung der quantitativen phytoplankton-methodik. Mitt Int. Ver Limnol. 1958, 9, 38. [Google Scholar]
- Raho, N.; Pizarro, G.; Escalera, L.; Reguera, B.; Marín, I. Morphology, toxin composition and molecular analysis of Dinophysis ovum Schütt, a dinoflagellate of the “Dinophysis acuminata complex”. Harmful Algae 2008, 7, 839–848. [Google Scholar] [CrossRef]
- European Union Reference Laboratory for Marine Biotoxins. EU-Harmonised Standard Operating Procedure for Determination of Lipophilic Marine Biotoxins in Molluscs by LC-MS/MS, V.5. Available online: http://aesan.msssi.gob.es/en/CRLMB/web/home.shtml (accessed on 15 March 2017).
- Gerssen, A.; Mulder, P.P.J.; McElhinney, M.A.; de Boer, J. Liquid chromatography–tandem mass spectrometry method for the detection of marine lipophilic toxins under alkaline conditions. J. Chromatogr. A 2009, 1216, 1421–1430. [Google Scholar] [CrossRef] [PubMed]

| Date (d/m/y) | Location | Sampling Depth (m) | Species | Picked Cells (No.) | OA (pg Cell−1) | PTX2 (pg Cell−1) |
|---|---|---|---|---|---|---|
| 27/01/2017 | Independencia Bay | 0–8 | D. acuminata-complex | 150 | <LOD * | 9.6 |
| 09/02/2017 | Samanco Bay | 0–15 | D. caudata | 92 | <LOD * | 9.2 |
| 10/04/2017 | Salinas | 0–15 | D. acuminata-complex | 204 | <LOD * | 9.3 |
| 24/03/2018 | Sechura Bay (Vichayo) | 0–15 | D. acuminata-complex | 440 | 0.8 | 11.1 |
| 24/03/2018 | Sechura Bay (Puerto Rico) | 0–15 | D. acuminata-complex | 400 | 0.3 | 1.5 |
| 14/04/2018 | Sechura Bay (Parachique) | 0–15 | D. caudata | 400 | <LOD * | 5.8 |
| Date d/m/y | Place | OA µg kg−1 Post-Hydrolysis | PTX2 µg kg−1 |
|---|---|---|---|
| 27/01/2017 | Independencia Bay-El Queso | <LOD * | 22.2 |
| 09/02/2017 | Samanco Bay | <LOD * | 20.3 |
| 10/04/2017 | Salinas | <LOD * | 10.3 |
| 14/04/2018 | Sechura Bay-Puerto Rico | 10.4 | 34.8 |
| 14/04/2018 | Sechura Bay-Barrancos | 8.6 | 20.8 |
| 14/04/2018 | Sechura Bay-San Pedro | 15.2 | 27.6 |
| 05/05/2018 | Sechura Bay-Las Delicias | 7.7 | 10.7 |
| Toxins | ESI Mode | Ion | Cone Voltage (V) | Collision Energy (CE) (eV) | Dwell (s) | |
|---|---|---|---|---|---|---|
| Precursor (m/z) | Product (m/z) | |||||
| OA | ESI− | 803.5 | 255.1 * | 30 | 50 | 0.05 |
| 113.0 | 60 | |||||
| DTX1 | ESI− | 817.5 | 255.1 * | 30 | 50 | 0.05 |
| 113.0 | 60 | |||||
| DTX2 | ESI− | 803.5 | 255.1 * | 30 | 50 | 0.05 |
| 113.0 | 60 | |||||
| PTX1 | ESI+ | 892.5 | 821.5 * | 30 | 30 | 0.02 |
| 213.3 | 40 | |||||
| PTX2 | ESI+ | 876.6 | 823.5 * | 30 | 20 | 0.20 |
| 213.1 | 40 | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcántara-Rubira, A.; Bárcena-Martínez, V.; Reyes-Paulino, M.; Medina-Acaro, K.; Valiente-Terrones, L.; Rodríguez-Velásquez, A.; Estrada-Jiménez, R.; Flores-Salmón, O. First Report of Okadaic Acid and Pectenotoxins in Individual Cells of Dinophysis and in Scallops Argopecten purpuratus from Perú. Toxins 2018, 10, 490. https://doi.org/10.3390/toxins10120490
Alcántara-Rubira A, Bárcena-Martínez V, Reyes-Paulino M, Medina-Acaro K, Valiente-Terrones L, Rodríguez-Velásquez A, Estrada-Jiménez R, Flores-Salmón O. First Report of Okadaic Acid and Pectenotoxins in Individual Cells of Dinophysis and in Scallops Argopecten purpuratus from Perú. Toxins. 2018; 10(12):490. https://doi.org/10.3390/toxins10120490
Chicago/Turabian StyleAlcántara-Rubira, Alex, Víctor Bárcena-Martínez, Maribel Reyes-Paulino, Katherine Medina-Acaro, Lilibeth Valiente-Terrones, Angélica Rodríguez-Velásquez, Rolando Estrada-Jiménez, and Omar Flores-Salmón. 2018. "First Report of Okadaic Acid and Pectenotoxins in Individual Cells of Dinophysis and in Scallops Argopecten purpuratus from Perú" Toxins 10, no. 12: 490. https://doi.org/10.3390/toxins10120490
APA StyleAlcántara-Rubira, A., Bárcena-Martínez, V., Reyes-Paulino, M., Medina-Acaro, K., Valiente-Terrones, L., Rodríguez-Velásquez, A., Estrada-Jiménez, R., & Flores-Salmón, O. (2018). First Report of Okadaic Acid and Pectenotoxins in Individual Cells of Dinophysis and in Scallops Argopecten purpuratus from Perú. Toxins, 10(12), 490. https://doi.org/10.3390/toxins10120490




