Diarrhetic Shellfish Toxin Monitoring in Commercial Wild Harvest Bivalve Shellfish in New South Wales, Australia
Abstract
1. Introduction
2. Results
2.1. Wild Harvest Shellfish End-Product Market Survey
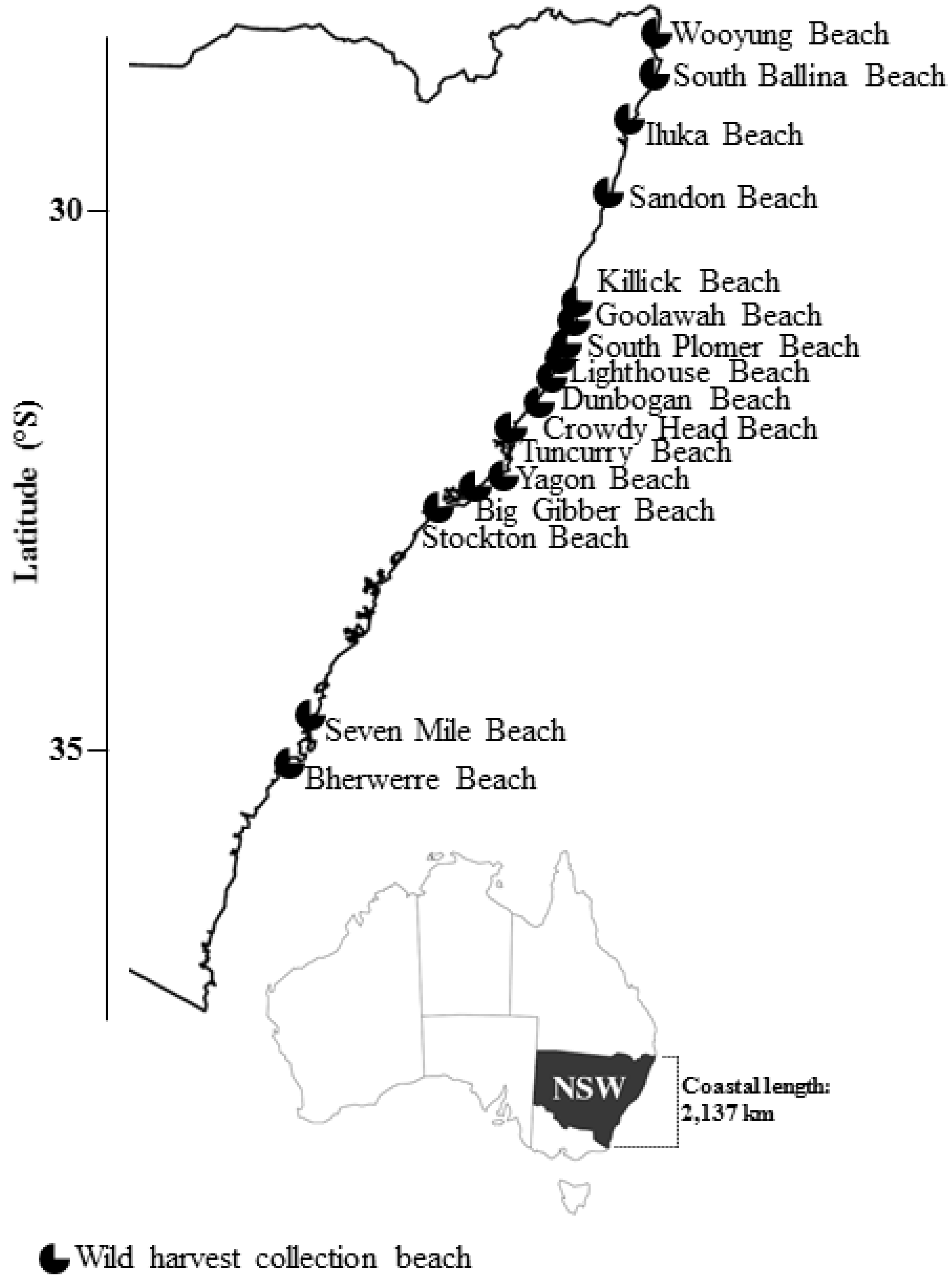
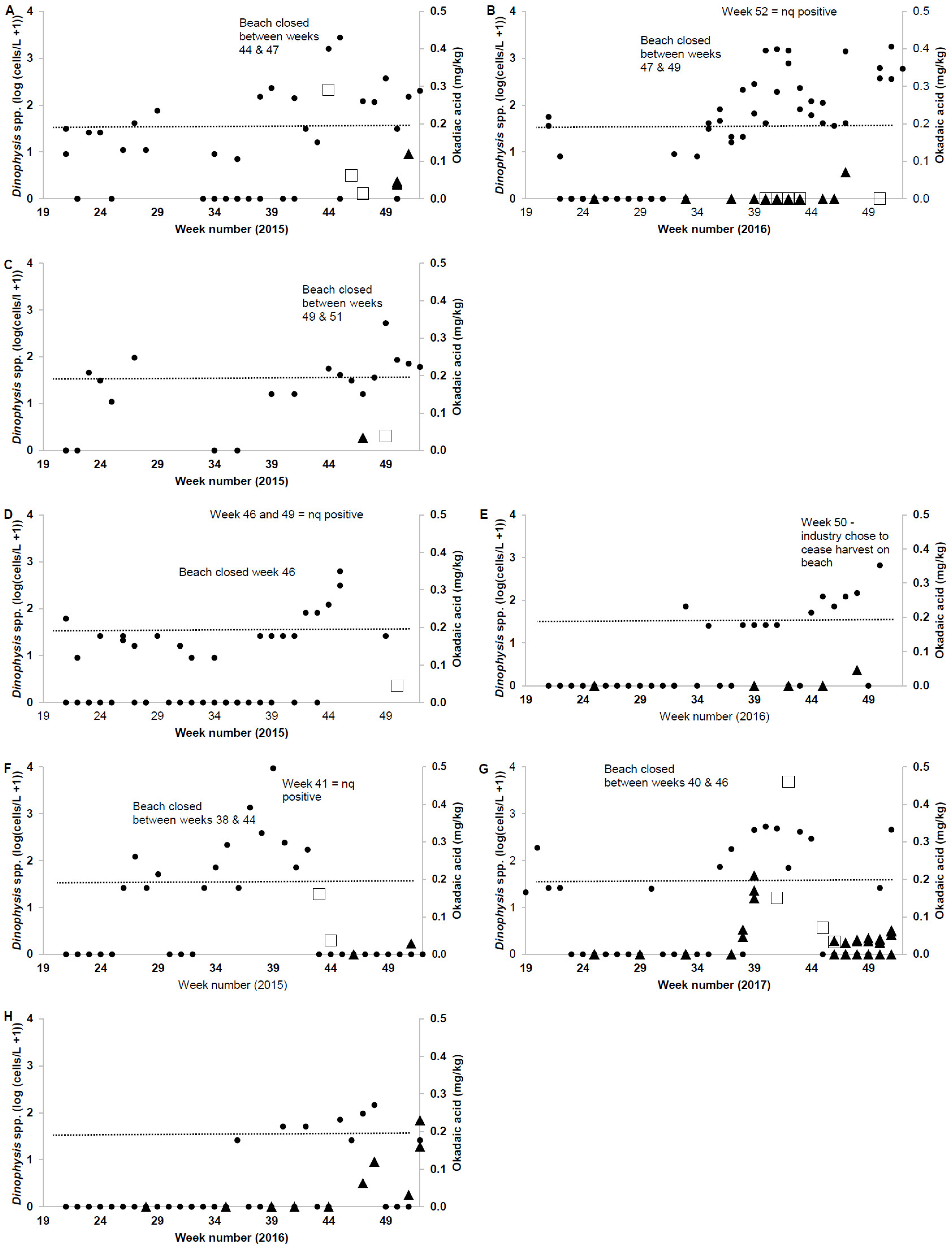
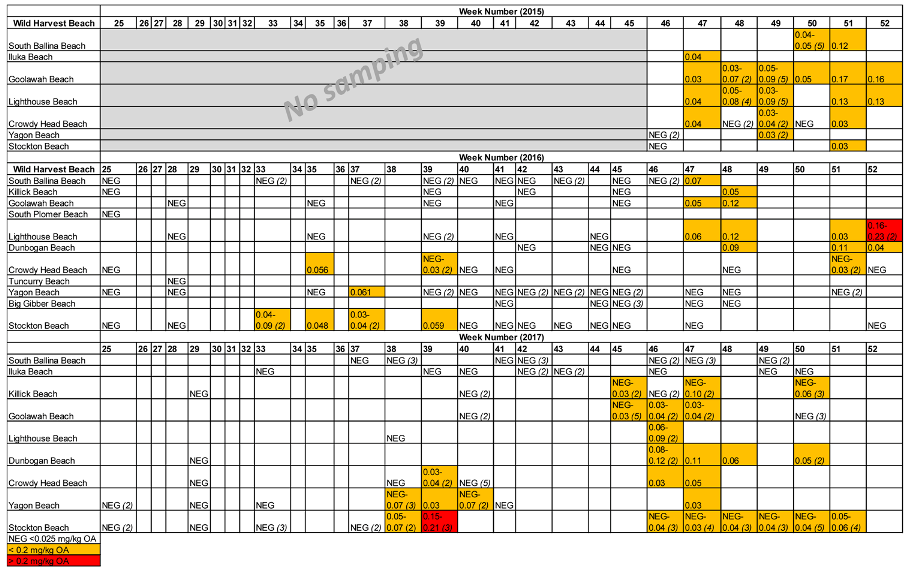
2.2. Wild Shellfish Harvest Beaches Phytoplankton and Biotoxin Samples
2.3. Comparison of Market Survey and Wild Shellfish Harvest Data
2.4. Phytoplankton and Biotoxin Samples from Oyster and Mussel Harvest Areas
3. Discussion
4. Materials and Methods
4.1. End-Product Market Survey
4.1.1. End-Product Sample Collection (Shellfish)
4.1.2. Biotoxin Testing of Shellfish Samples
4.2. Routine Monitoring at Shellfish Harvest or Collection Areas
4.2.1. Sample Collection for Phytoplankton Analyses (Water)
4.2.2. Sample Collection for Biotoxin Testing (Shellfish)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Globefish. Globefish-Analysis and Information on World Fish Trade. Available online: http://www.fao.org/in-action/globefish/market-reports/resource-detail/en/c/1136590/ (accessed on 20 July 2018).
- Campbell, A.; Hudson, D.; McLeod, C.; Nicholls, C.; Pointon, A. Tactical Research Fund: Review of the 2012 Paralytic Shellfish Toxin Event in Tasmania Associated with the Dinoflagellate Alga, Alexandrium tamarense. Fisheries Research and Development Corporation; FRDC Project 2012/060 2013; p. 94. Available online: http://www.safefish.com.au/Reports/Technical-Reports/Paralytic-Shellfish-Toxin-Event-2012-2013 (accessed on 20 July 2018).
- Ajani, P.; Harwood, D.T.; Murray, S.A. Recent trends in marine phycotoxins from Australian coastal waters. Mar. Drugs 2017, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Kiermeier, A.; McLeod, C.; Sumner, J. Marinebiotoxins in Shellfish-Saxitoxin Group: Scientific Opinion of the Panel on Contamination in the Food Chain’ by the European Food Safety Authority (EFSA); South Australian Research and Development Institute (SARDI): Adelaide, SA, Australia, 2009; p. 11.
- Lawrence, J.; Loreal, H.; Toyofuku, H.; Hess, P.; Iddya, K.; Ababouch, L. Assessment and management of biotoxin risks in bivalve molluscs. FAO Tech. Pap. 2011, 551, 358. Available online: http://www.fao.org/docrep/015/i2356e/i2356e.pdf (accessed on 20 July 2018).
- Taylor, F.J.R.; Fukuyo, Y.; Larsen, J.; Hallegraeff, G.M. Taxonomy of harmful dinoflagellates. In Manual on Harmful Marine Microalgae, 2nd ed.; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Eds.; UNESCO: Paris, France, 2004; pp. 389–431. [Google Scholar]
- Reguera, B.; Riobó, P.; Rodríguez, F.; Díaz, P.; Pizarro, G.; Paz, B.; Franco, J.; Blanco, J. Dinophysis toxins: Causative organisms, distribution and fate in shellfish. Mar. Drugs 2014, 12, 394–461. [Google Scholar] [CrossRef] [PubMed]
- Reguera, B.; Velo-Suárez, L.; Raine, R.; Park, M.G. Harmful Dinophysis species: A review. Harmful Algae 2012, 14, 87–106. [Google Scholar] [CrossRef]
- Suganuma, M.; Fujiki, H.; Suguri, H.; Yoshizawa, S.; Hirota, M.; Nakayasu, M.; Ojika, M.; Wakamatsu, K.; Yamada, K.; Sugimura, T. Okadaic acid: An additional non-phorbol-12-tetradecanoate-13-acetate-type tumor promoter. Proc. Natl. Acad. Sci. USA 1988, 85, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, M.; Tatematsu, M.; Yatsunami, J.; Yoshizawa, S.; Okabe, S.; Uemura, D.; Fujiki, H. An alternative theory of tissue specificity by tumor promotion of okadaic acid in glandular stomach of sd rats. Carcinogenesis 1992, 13, 1841–1845. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Suganuma, M. Unique features of the okadaic acid activity class of tumor promoters. J. Cancer Res. Clin. Oncol. 1999, 125, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Shimatani, M.; Sugitani, H.; Oshima, Y.; Yasumoto, T. Isolation and structural elucidation of the causative toxin of the diarrhetic shellfish poisoning. Nippon Suisan Gakkaishi 1982, 48, 549–552. [Google Scholar] [CrossRef]
- Xie, H.; Lazure, P.; Gentien, P. Small scale retentive structures and Dinophysis. J. Mar. Syst. 2007, 64, 173–188. [Google Scholar] [CrossRef]
- Farrell, H.; Gentien, P.; Fernand, L.; Lunven, M.; Reguera, B.; González-Gil, S.; Raine, R. Scales characterising a high density thin layer of Dinophysis acuta Ehrenberg and its transport within a coastal jet. Harmful Algae 2012, 15, 36–46. [Google Scholar] [CrossRef]
- Velo-Suárez, L.; Reguera, B.; González-Gil, S.; Lunven, M.; Lazure, P.; Nézan, E.; Gentien, P. Application of a 3d lagrangian model to explain the decline of a Dinophysis acuminata bloom in the Bay of Biscay. J. Mar. Syst. 2010, 83, 242–252. [Google Scholar] [CrossRef]
- Park, M.G.; Kim, S.; Kim, H.S.; Myung, G.; Kang, Y.; Yih, W. First successful culture of the marine dinoflagellate Dinophysis acuminata. Aquat. Microb. Ecol. 2006, 45, 101–106. [Google Scholar] [CrossRef]
- Nishitani, G.; Nagai, S.; Sakiyama, S.; Kamiyama, T. Successful cultivation of the toxic dinoflagellate Dinophysis caudata (Dinophyceae). Plankton Benthos Res. 2008, 3, 78–85. [Google Scholar] [CrossRef]
- Nagai, S.; Nitshitani, G.; Tomaru, Y.; Sakiyama, S.; Kamiyama, T. Predation by the toxic dinoflagellate Dinophysis fortii on the ciliate Myrionecta rubra and observation of sequestration of ciliate chloroplasts1. J. Phycol. 2008, 44, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, G.; Nagai, S.; Takano, Y.; Sakiyama, S.; Baba, K.; Kamiyama, T. Growth characteristics and phylogenetic analysis of the marine dinoflagellate Dinophysis infundibulus (Dinophyceae). Aquat. Microb. Ecol. 2008, 52, 209–221. [Google Scholar] [CrossRef]
- Rodríguez, F.; Escalera, L.; Reguera, B.; Rial, P.; Riobó, P.; de Jesús da Silva, T. Morphological variability, toxinology and genetics of the dinoflagellate Dinophysis tripos (Dinophysiaceae, Dinophysiales). Harmful Algae 2012, 13, 26–33. [Google Scholar] [CrossRef]
- Nagai, S.; Suzuki, T.; Kamiyama, T. Successful cultivation of the toxic dinoflagellate Dinophysis tripos (Dinophyceae). Plankton Benthos Res. 2013, 8, 171–177. [Google Scholar] [CrossRef]
- Basti, L.; Uchida, H.; Matsushima, R.; Watanabe, R.; Suzuki, T.; Yamatogi, T.; Nagai, S. Influence of temperature on growth and production of pectenotoxin-2 by a monoclonal culture of Dinophysis caudata. Mar. Drugs 2015, 13, 7124–7137. [Google Scholar] [CrossRef] [PubMed]
- Jaén, D.; Mamán, L.; Domínguez, R.; Martín, E. First report of Dinophysis acuta in culture. Harmful Algal News 2009, 39, 1–2. [Google Scholar]
- Jefferson, R. Aquaculture Production Report 2016–2017; NSW Department of Primary Industries: Taylors Beach, NSW, Australia, 2018; p. 12. Available online: https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0009/750726/Aquaculture-Production-Report-2016-2017.pdf (accessed on 20 July 2018).
- NSW FA. Marine Biotoxin Management Plan; NSW Food Authority: Newington, NSW, Australia, 2014; p. 36. Available online: http://www.foodauthority.nsw.gov.au/_Documents/industry/marine_biotoxin_management_plan.pdf (accessed on 20 July 2018).
- Ajani, P.; Brett, S.; Krogh, M.; Scanes, P.; Webster, G.; Armand, L. The risk of harmful algal blooms (HABS) in the oyster-growing estuaries of New South Wales, Australia. Environ. Monit. Assess. 2013, 185, 5295–5316. [Google Scholar] [CrossRef] [PubMed]
- Ajani, P.; Hallegraeff, G.M.; Pritchard, T. Historic overview of algal blooms in marine and estuarine waters of New South Wales, Australia. Proc. Linn. Soc. NSW 2001, 123, 1–22. [Google Scholar]
- Ajani, P.; Ingleton, T.; Pritchard, T.; Armand, L. Microalgal blooms in the coastal waters of New South Wales, Australia. Proc. Linn. Soc. NSW 2011, 133, 15–31. [Google Scholar]
- NSW FA. Phytoplankton and Biotoxins in Nsw Shellfish Aquaculture Areas; NSW Food Authority: Newington, NSW, Australia, 2017; p. 48. Available online: http://www.foodauthority.nsw.gov.au/_Documents/scienceandtechnical/phytoplankton_and_biotoxin_risk_assessment.pdf (accessed on 20 July 2018).
- FSANZ. Australia New Zealand Food Standards Code. Food Standards Australia New Zealand, 2016. Available online: https://www.legislation.gov.au/Details/F2017C00305 (accessed on 20 July 2018).
- EC-853. No 853/2004 of the European Parliament and of the Council of April 29, 2004. Laying down specific hygiene rules for food of animal origin. Off. J. Eur. Union 2004, 139, 55. [Google Scholar]
- NSSP/US FDA. National shellfish sanitation program. In Guide for the Control of Moluscan Shellfish: 2018 Revision; 2018. Available online: https://www.fda.gov/food/guidanceregulation/federalstatefoodprograms/ucm2006754.htm (accessed on 20 July 2018).
- Dyson, K.; Huppert, D.D. Regional economic impacts of razor clam beach closures due to harmful algal blooms (HABs) on the Pacific coast of Washington. Harmful Algae 2010, 9, 264–271. [Google Scholar] [CrossRef]
- Trainer, V.L.; Moore, L.; Bill, B.D.; Adams, N.G.; Harrington, N.; Borchert, J.; Da Silva, D.A.M.; Eberhart, B.-T.L. Diarrhetic shellfish toxins and other lipophilic toxins of human health concern in Washington State. Mar. Drugs 2013, 11, 1815–1835. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Hoagland, P.; Kaoru, Y.; White, A.W. Estimated Annual Economic Impacts from Harmful Algal Blooms (HABs) in the United States; DTIC Document: 2000. Available online: http://www.whoi.edu/cms/files/Economics_report_18564_23050.pdf (accessed on 20 July 2018).
- MacKenzie, L.; White, D.; Adamson, J. Temporal variation and tissue localization of paralytic shellfish toxins in the New Zealand tuatua (surfclam), Paphies subtriangulata. J. Shellfish Res. 1996, 15, 735–740. [Google Scholar]
- Farrell, H.; Murray, S.A.; Zammit, A.; Edwards, A.W. Management of ciguatoxin risk in eastern Australia. Toxins 2017, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Farrell, H.; Zammit, A.; Harwood, D.T.; McNabb, P.; Shadboldt, C.; Manning, J.; Turahui, J.; van den Berg, D.; Szabo, L. Clinical diagnosis and chemical confirmation of ciguatera fish poisoning in New South Wales, Australia. CDI 2016, 40, E1–E6. [Google Scholar] [PubMed]
- Farrell, H.; Zammit, A.; Harwood, D.T.; Murray, S. Is ciguatera moving south in Australia? Harmful Algal News 2016, 54, 5–6. [Google Scholar]
- Quaine, J.; Kraa, E.; Holloway, J.; White, K.; McCarthy, R.; Delpech, V.; Trent, M.; McAnulty, J. Outbreak of gastroenteritis linked to eating pipis. NSW Public Health Bull. 1997, 8, 103–104. [Google Scholar]
- MacKenzie, L.; Holland, P.; McNabb, P.; Beuzenberg, V.; Selwood, A.; Suzuki, T. Complex toxin profiles in phytoplankton and greenshell mussels (Perna canaliculus), revealed by LC-MS/MS analysis. Toxicon 2002, 40, 1321–1330. [Google Scholar] [CrossRef]
- Hallegraeff, G.M.; Lucas, I.A.N. The marine dinoflagellate genus Dinophysis (Dinophyceae): Photosynthetic, neritic and non-photosynthetic, oceanic species. Phycologia 1988, 27, 25–42. [Google Scholar] [CrossRef]
- McCarthy, P. Census of Australian Marine Dinoflagellates. 2018. Available online: http://www.anbg.gov.au/abrs/Dinoflagellates/index_Dino.html (accessed on 20 July 2018).
- Burgess, V.; Shaw, G. Pectenotoxins—An issue for public health: A review of their comparative toxicology and metabolism. Environ. Int. 2001, 27, 275–283. [Google Scholar] [CrossRef]
- Farrell, H.; Brett, S.; Webster, G.; Baker, P.; Zammit, A. A summary of harmful algal bloom monitoring and risk assessment in New South Wales, australia. In Proceedings of the 16th International Conference on Harmful Algae, Wellington, New Zealand, 27–31 October 2014; pp. 254–257. [Google Scholar]
- Madigan, T.L.; Lee, K.G.; Padula, D.J.; McNabb, P.; Pointon, A.M. Diarrhetic shellfish poisoning (DSP) toxins in South Australian shellfish. Harmful Algae 2006, 5, 119–123. [Google Scholar] [CrossRef]
- Wallace, G.M. Diarrhetic Shellfish Toxins in Tasmanian Coastal Waters: Causative Dinoflagellate Organisms, Dissolved Toxins and Shellfish Depuration. Ph.D. Thesis, University of Tasmania, Tasmania, Australia, 2011. [Google Scholar]
- Sydney Fish Market. Annual Report 2017. 2017. Available online: https://www.sydneyfishmarket.com.au/our-company/annual-report (accessed on 20 July 2018).
- Pitcher, G.C.; Krock, B.; Cembella, A.D. Accumulation of diarrhetic shellfish poisoning toxins in the oyster Crassostrea gigas and the mussel Choromytilus meridionalis in the southern benguela ecosystem. Afr. J. Mar. Sci. 2011, 33, 273–281. [Google Scholar] [CrossRef]
- FAO Food and Nutrition Paper 80. In UN Food and Agriculture Organization (FAO); FAO: Rome, Italy, 2004; Available online: http://www.fao.org/docrep/007/y5486e/y5486e00.htm (accessed on 20 July 2018).
- Hégaret, H.; Wikfors, G.H.; Shumway, S.E. Biotoxin contamination and shellfish safety. In Shellfish Safety and Quality; Shumway, S.E., Rodrick, G.E., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2009; pp. 43–82. [Google Scholar]
- Mons, M.P.; Speijers, G.J.A. Paralytic Shellfish Poisoning: A Review. 1998. Available online: https://www.rivm.nl/bibliotheek/rapporten/388802005.html (accessed on 20 July 2018).
- Fernández, M.L.; Shumway, S.E. Managment of shellfish resources. In Manual on Harmful Marine Microalgae; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Eds.; UNESCO: Paris, France, 2004. [Google Scholar]
- Sidari, L.; Nichetto, P.; Cok, S.; Sosa, S.; Tubaro, A.; Honsell, G.; Della Loggia, R. Phytoplankton selection by mussels, and diarrhetic shellfish poisoning. Mar. Biol. 1998, 131, 103–111. [Google Scholar] [CrossRef]
- Ajani, P.; Lee, R.; Pritchard, T.; Krogh, M. Phytoplankton dynamics at a long-term coastal station off Sydney, Australia. J. Coastal Res. 2001, 60–73. [Google Scholar]
- Takahashi, E.; Yu, Q.; Eaglesham, G.; Connell, D.W.; McBroom, J.; Costanzo, S.; Shaw, G.R. Occurrence and seasonal variations of algal toxins in water, phytoplankton and shellfish from North Stradbroke Island, Queensland, Australia. Mar. Environ. Res. 2007, 64, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Ajani, P.; Larsson, M.E.; Rubio, A.; Bush, S.; Brett, S.; Farrell, H. Modelling bloom formation of the toxic dinoflagellates Dinophysis acuminata and Dinophysis caudata in a highly modified estuary, south eastern Australia. Estuar. Coastal Shelf Sci. 2016, 183, 95–106. [Google Scholar] [CrossRef]
- Burgess, V.; Shaw, G. Investigations into the Toxicology of Pectenotoxin-2-Seco Acid and 7-Epi Pectenotoxin 2-Seco Acid to Aid in a Health Risk Assessment for the Consumption of Shellfish Contaminated with These Shellfish Toxins in Australia. Available online: http://www.frdc.com.au/Archived-Reports/FRDC%20Projects/2001-258-DLD.pdf (accessed on 20 July 2018).
- Escalera, L.; Reguera, B.; Moita, T.; Pazos, Y.; Cerejo, M.; Cabanas, J.M.; Ruiz-Villarreal, M. Bloom dynamics of Dinophysis acuta in an upwelling system: In situ growth versus transport. Harmful Algae 2010, 9, 312–322. [Google Scholar] [CrossRef]
- Taylor, M.; McIntyre, L.; Ritson, M.; Stone, J.; Bronson, R.; Bitzikos, O.; Rourke, W.; Galanis, E.; Outbreak Investigation, T. Outbreak of diarrhetic shellfish poisoning associated with mussels, British Columbia, Canada. Mar. Drugs 2013, 11, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xu, X.; Wei, J.; Chen, J.; Miu, R.; Huang, L.; Zhou, X.; Fu, Y.; Yan, R.; Wang, Z.; et al. Food-borne disease outbreak of diarrhetic shellfish poisoning due to toxic mussel consumption: The first recorded outbreak in China. PLoS ONE 2013, 8, e65049. [Google Scholar] [CrossRef] [PubMed]
- Alves, T.; Mafra, L. Diel variations in cell abundance and trophic transfer of diarrheic toxins during a massive Dinophysis bloom in southern Brazil. Toxins 2018, 10, 232. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T. Spring Bay mussels recalled in tasmania over algal toxin find. ABC News. 20 March 2016. Available online: https://www.abc.net.au/news/2016-03-19/spring-bay-mussels-recalled-in-tasmania-over-algal-toxin-find/7260250 (accessed on 20 July 2018).
- Hallegraeff, G.M. Harmful algal blooms: A global overview. In Manual on Harmful Marine Microalgae; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Eds.; UNESCO: Paris, France, 2003; pp. 24–49. [Google Scholar]
- Hallegraeff, G.M. Ocean climate change, phytoplankton community responses, and harmful algal blooms: A formidable predictive challenge. J. Phycol. 2010, 46, 220–235. [Google Scholar] [CrossRef]
- Pizarro, G.; Moroño, Á.; Paz, B.; Franco, J.; Pazos, Y.; Reguera, B. Evaluation of passive samplers as a monitoring tool for early warning of Dinophysis toxins in shellfish. Mar. Drugs 2013, 11, 3823. [Google Scholar] [CrossRef] [PubMed]
- Hattenrath-Lehmann, T.K.; Lusty, M.W.; Wallace, R.B.; Haynes, B.; Wang, Z.; Broadwater, M.; Deeds, J.R.; Morton, S.L.; Hastback, W.; Porter, L.; et al. Evaluation of rapid, early warning approaches to track shellfish toxins associated with Dinophysis and Alexandrium blooms. Mar. Drugs 2018, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Economou, V.; Papadopoulou, C.; Brett, M.; Kansouzidou, A.; Charalabopoulos, K.; Filioussis, G.; Seferiadis, K. Diarrheic shellfish poisoning due to toxic mussel consumption: The first recorded outbreak in Greece. Food Addit. Contam. 2007, 24, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.F.; Niedzwiadek, B.; Menard, C. Quantitative determination of paralytic shellfish poisoning toxins in shellfish using prechromatographic oxidation and liquid chromatography with fluorescence detection: Collaborative study. J. AOAC Int. 2005, 88, 1714–1732. [Google Scholar] [PubMed]
- van den Top, H.J.; Gerssen, A.; McCarron, P.; van Egmond, H.P. Quantitative determination of marine lipophilic toxins in mussels, oysters and cockles using liquid chromatography-mass spectrometry: Inter-laboratory validation study. Food Addit. Contam. 2011, 28, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Villar-Gonzalez, A.; Rodriguez-Velasco, M.L.; Gago-Martinez, A. Determination of lipophilic toxins by LC/MS/MS: Single-laboratory validation. J. AOAC Int. 2011, 94, 909–922. [Google Scholar] [PubMed]
- AOAC. Domoic acid in mussels, liquid chromatographic method. J. AOAC Int. 2000. [Google Scholar]
- ASQAAC. The Australian Shellfish Quality Assurance Program Operations Manual; Australian Shellfish Quality Assurance Advisory Committee: Canberra, Australia, 2016; p. 46. [Google Scholar]

| Toxin Group | EU [31] | USA [32] | Australia [30] |
|---|---|---|---|
| Amnesic shellfish toxin (AST, domoic acid equivalent) | 20 mg/kg | 20 mg/kg | 20 mg/kg |
| Diarrhetic shellfish toxins (DSTs, okadaic acid equivalent) | 0.16 mg/kg | 0.16 mg/kg | 0.20 mg/kg |
| Paralytic shellfish toxins (PSTs, saxitoxin equivalent) | 0.80 mg/kg | 0.80 mg/kg | 0.80 mg/kg |
| Neurotoxic shellfish toxins (NSTs, brevetoxin-2 equivalent) | n/a 1 | 0.8 ppm or 200 MU/kg | 200 MU/kg |
| Azaspiracid shellfish toxins (AZTs) | 0.16 mg/kg | 0.16 mg/kg | n/a 2 |
| Shellfish Type | n = 321 | AST (No. Positive/Above Regulatory Limit) | DST (No. Positive/Above Regulatory Limit) | PST (No. Positive/Above Regulatory Limit) |
|---|---|---|---|---|
| Pipis (Plebidonax deltoides) | 271 | 3/0 | 110/2 | 0/0 |
| Cockles (Katelysia spp. Anadara granosa) 1 | 47 | 0/0 | 0/0 | 0/0 |
| Strawberry clam (cockle) (Notocallista kingii) | 3 | 2/0 | 0/0 | 0/0 |
| Wild Harvest Beach (North–South) | 2015 (Positive/Total) | 2016 (Positive/Total) | 2017 (Positive/Total) |
|---|---|---|---|
| South Ballina Beach | 7/7 | 1/16 | 0/15 |
| Iluka Beach | 1/1 | - | 0/10 |
| Killick Beach | - | 1/5 | 3/12 |
| Goolawah Beach | 12/12 | 2/7 | 6/14 |
| South Plomer Beach | - | 0/1 | - |
| Lighthouse Beach | 13/13 | 5/11 | 2/3 |
| Dunbogan Beach | - | 3/6 | 6/7 |
| Crowdy Head Beach | 6/9 | 3/11 | 4/12 |
| Tuncurry Beach | - | 0/1 | - |
| Yagon Beach | 2/5 | 1/19 | 5/12 |
| Big Gibber Beach | - | 0/7 | - |
| Stockton Beach | 1/2 | 6/16 | 20/35 |
| Unconfirmed 1 | 0/2 | - | - |
| Total | 42/51 | 22/100 | 46/120 |
| Wild Harvest Monitoring Data | 2015 | 2016 | 2017 | All |
|---|---|---|---|---|
| No. of phytoplankton samples collected | 310 | 411 | 376 | 1097 |
| No. of phytoplankton samples >500 cells/L Dinophysis spp. | 7 | 8 | 2 | 17 |
| South Ballina Beach/Max cells/L Dinophysis spp. | 2760 | 1760 | - | - |
| Iluka Beach/Max cells/L Dinophysis spp. | 520 | - | - | - |
| Killick Beach/Max cells/L Dinophysis spp. | 625 | 650 | - | - |
| South Plomer/Max cells/L Dinophysis spp. | 825 | - | - | - |
| Stockton Beach/Max cells/L Dinophysis spp. | 9330 | - | 530 | - |
| Bherwerre Beach/Max cells/L Dinophysis spp. | - | - | 500 | - |
| No. of DST tests | 10 | 10 | 6 | 26 |
| No. of DST positive results | 8 1 | 1 2 | 4 | 4 |
| No. of DST results > 0.2 mg/kg OA | 1 | 0 | 1 | 2 |
| Estuary (North–South) | Latitude (S) | No. of Sample Sites | Total Samples Per Site (November 2015–December 2017) | D. acuminate (No. of Observations/Max. Cells/L /Month of Max. Concentration) | D. caudate (No. of Observations/Max. Cells/L /Month of Max. Concentration) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Tweed River | 28°10′ | 1 | 45 | - | - | - | 3 | 700 | December |
| Richmond River | 28°53′ | 1 | 20 | 6 | 650 | December | 6 | 400 | |
| Clarence River | 29°25′ | 1 | 29 | 8 | 850 | November | 7 | 200 | |
| Wooli River | 29°53′ | 1 | 20 | 3 | 200 | 3 | 200 | ||
| Bellinger and Kalang Rivers | 30°30′ | 2 | 29, 14 | 6 | 2400 | November | 8 | 200 | |
| Nambucca River | 30°39′ | 2 | 48, 43 | 10 | 3200 | November | 7 | 250 | |
| Macleay River | 30°52′ | 2 | 44, 20 | 3 | 50 | 2 | 50 | ||
| Hastings River | 31°25′ | 3 | 45, 38, 23 | 27 | 1100 | November | 13 | 1000 | December |
| Camden Haven River | 31°38′ | 3 | 56, 34, 34 | 29 | 650 | November | 7 | 900 | |
| Manning River | 31°53′ | 3 | 53, 52, 41 | 39 | 1700 | October | 19 | 300 | May |
| Wallis Lake | 32°13′ | 3 | 58, 58, 59 | 54 | 3700 | October | 27 | 550 | December |
| Port Stephens | 32°42′ | 10 | 29, 34, 56, 56, 56, 57, 57, 56, 56, 56 | 44 | 1000 | August | 28 | 850 | February |
| Brisbane Water | 33°31′ | 4 | 55, 52, 37, 56 | 18 | 300 | 24 | 1300 | April | |
| Patonga River | 33°32′ | 1 | 52 | 4 | 100 | 1 | 50 | ||
| Hawkesbury River | 33°34′ | 3 | 61, 59, 20 | 14 | 300 | 25 | 350 | ||
| Georges River | 34°01′ | 1 | 57 | 6 | 300 | 4 | 500 | March | |
| Shoalhaven and Crookhaven Rivers | 34°53′ | 3 | 48, 48, 48 | 30 | 250 | 6 | 100 | ||
| Clyde River | 35°42′ | 3 | 42, 56, 56 | 9 | 150 | 21 | 250 | ||
| Tuross Lake | 36°04′ | 1 | 53 | 1 | 100 | - | - | ||
| Wagonga Inlet | 36°13′ | 2 | 62, 62 | 27 | 350 | 36 | 1500 | November | |
| Bermagui River | 36°26′ | 1 | 23 | 3 | 150 | - | - | ||
| Wapengo Lake | 36°38′ | 2 | 57, 56 | 16 | 150 | 1 | 50 | ||
| Nelson Lagoon | 36°41′ | 1 | 31 | 1 | 50 | - | - | ||
| Merimbula | 36°54′ | 2 | 59, 59 | 15 | 400 | 25 | 1300 | November | |
| Pambula Lake | 36°57′ | 1 | 60 | 11 | 250 | 2 | 100 | ||
| Twofold Bay | 37°05′ | 3 | 48, 48, 48 | 42 | 1400 | March | 19 | 300 | |
| Wonboyn River | 37°17′ | 2 | 57, 57 | 18 | 250 | 41 | 1000 | June | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farrell, H.; Ajani, P.; Murray, S.; Baker, P.; Webster, G.; Brett, S.; Zammit, A. Diarrhetic Shellfish Toxin Monitoring in Commercial Wild Harvest Bivalve Shellfish in New South Wales, Australia. Toxins 2018, 10, 446. https://doi.org/10.3390/toxins10110446
Farrell H, Ajani P, Murray S, Baker P, Webster G, Brett S, Zammit A. Diarrhetic Shellfish Toxin Monitoring in Commercial Wild Harvest Bivalve Shellfish in New South Wales, Australia. Toxins. 2018; 10(11):446. https://doi.org/10.3390/toxins10110446
Chicago/Turabian StyleFarrell, Hazel, Penelope Ajani, Shauna Murray, Phil Baker, Grant Webster, Steve Brett, and Anthony Zammit. 2018. "Diarrhetic Shellfish Toxin Monitoring in Commercial Wild Harvest Bivalve Shellfish in New South Wales, Australia" Toxins 10, no. 11: 446. https://doi.org/10.3390/toxins10110446
APA StyleFarrell, H., Ajani, P., Murray, S., Baker, P., Webster, G., Brett, S., & Zammit, A. (2018). Diarrhetic Shellfish Toxin Monitoring in Commercial Wild Harvest Bivalve Shellfish in New South Wales, Australia. Toxins, 10(11), 446. https://doi.org/10.3390/toxins10110446





