Effects of a Fusarium Toxin-Contaminated Maize Treated with Sodium Sulfite on Male Piglets in the Presence of an LPS-Induced Acute Inflammation
Abstract
1. Introduction
2. Results
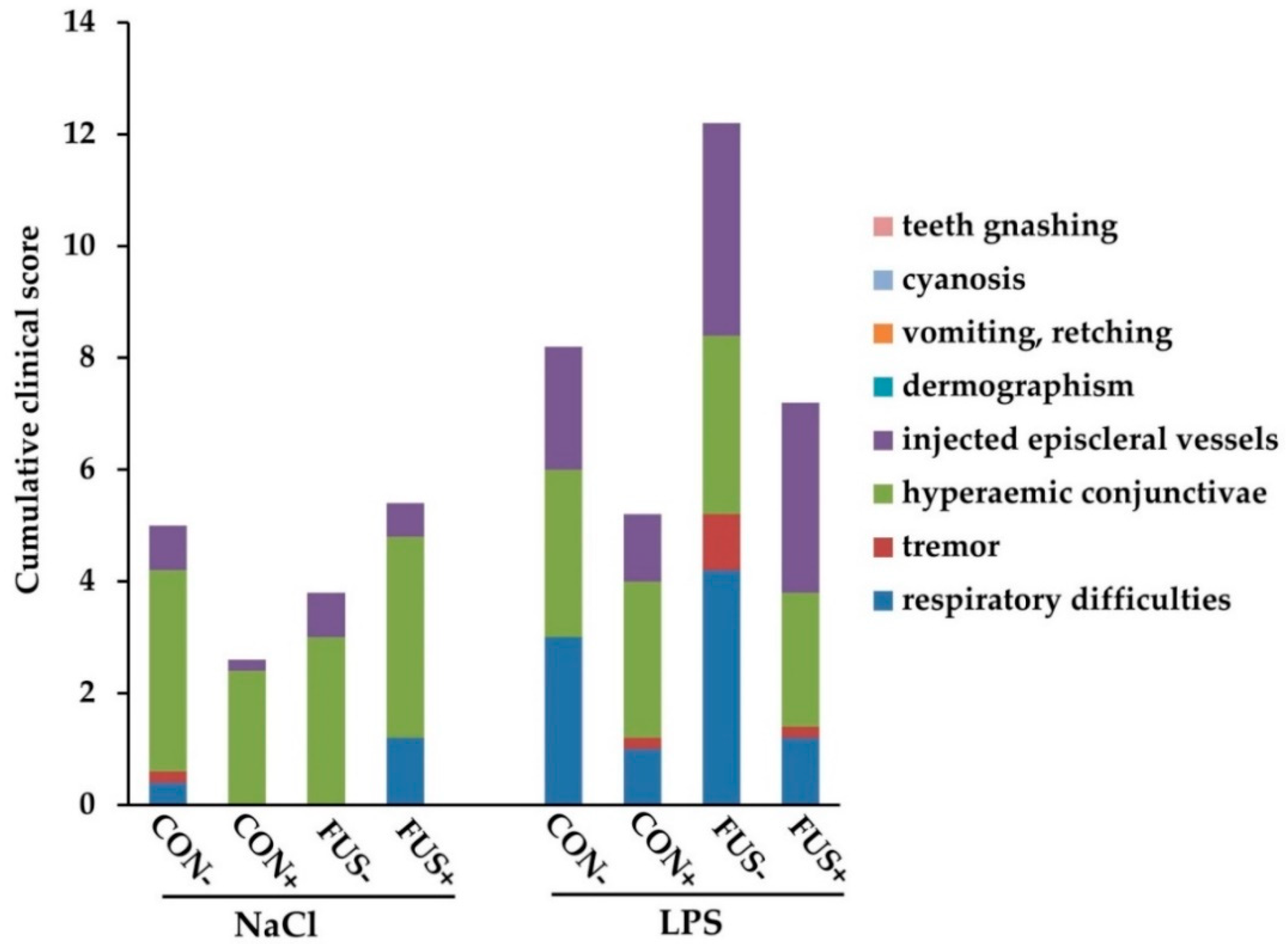
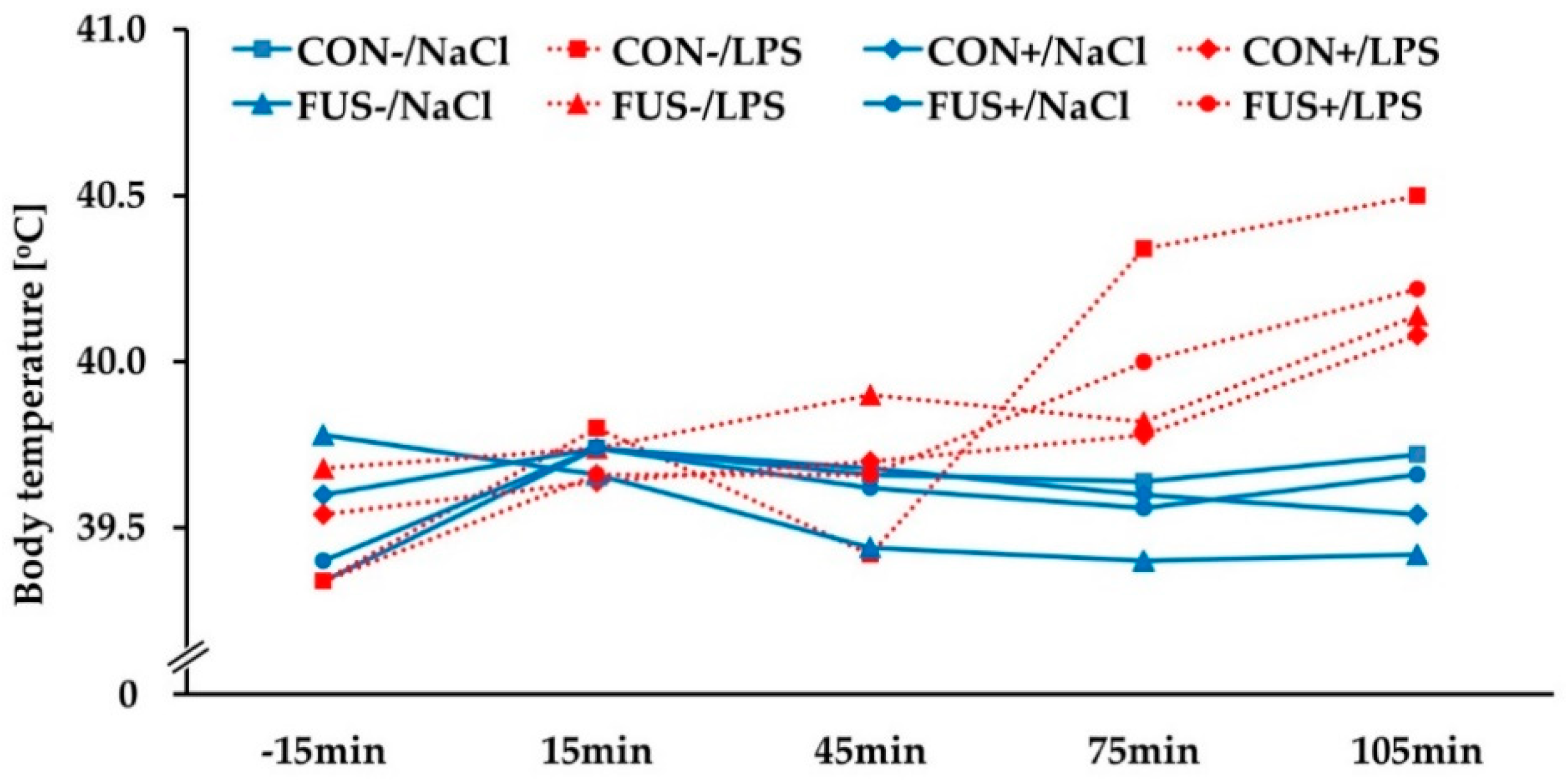
2.1. Clinical Signs
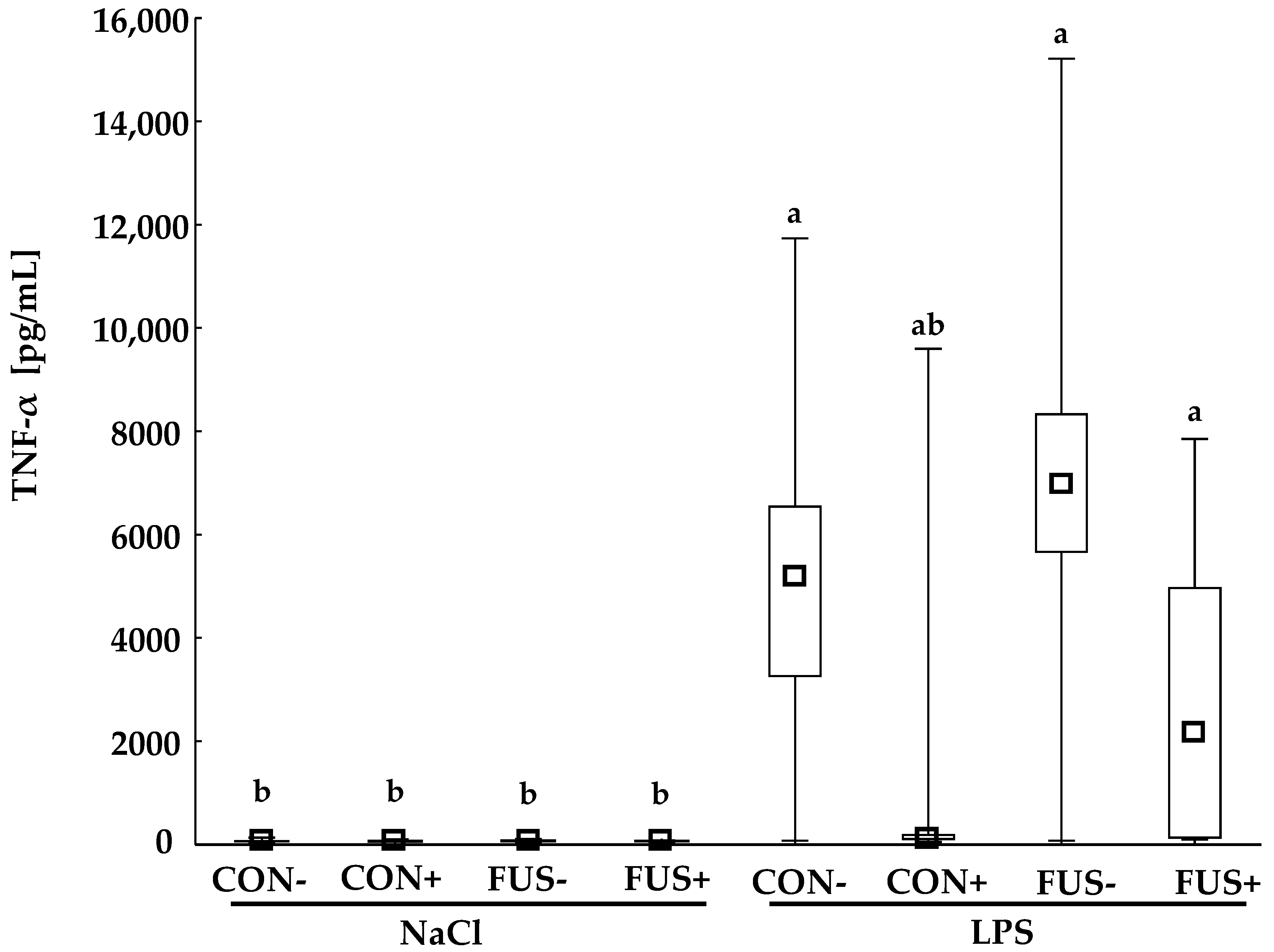
2.2. TNF-α
2.3. Organ Weights
2.4. Haematology
2.5. Clinical Chemistry
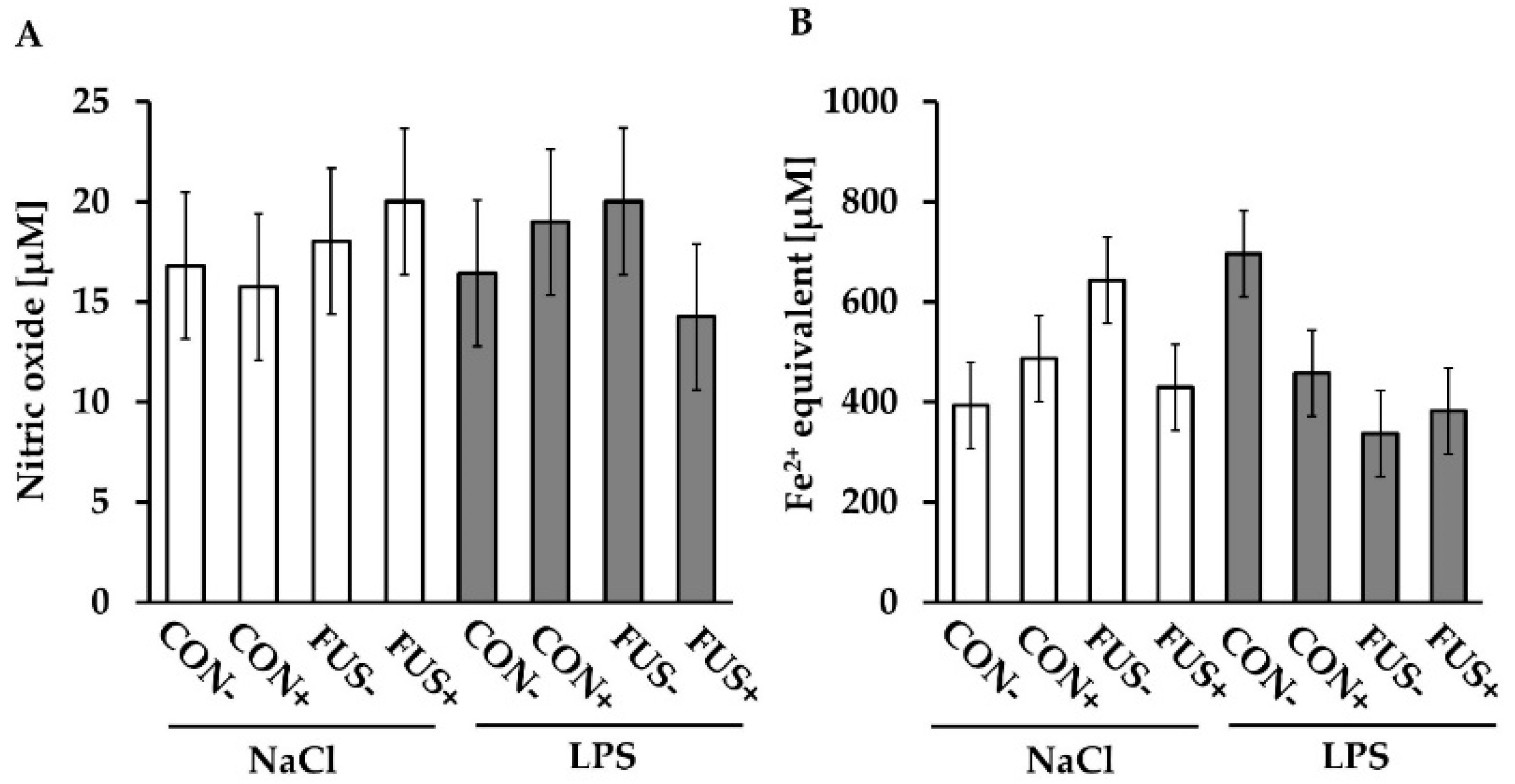
2.6. Redox Status
3. Discussion
4. Materials and Methods
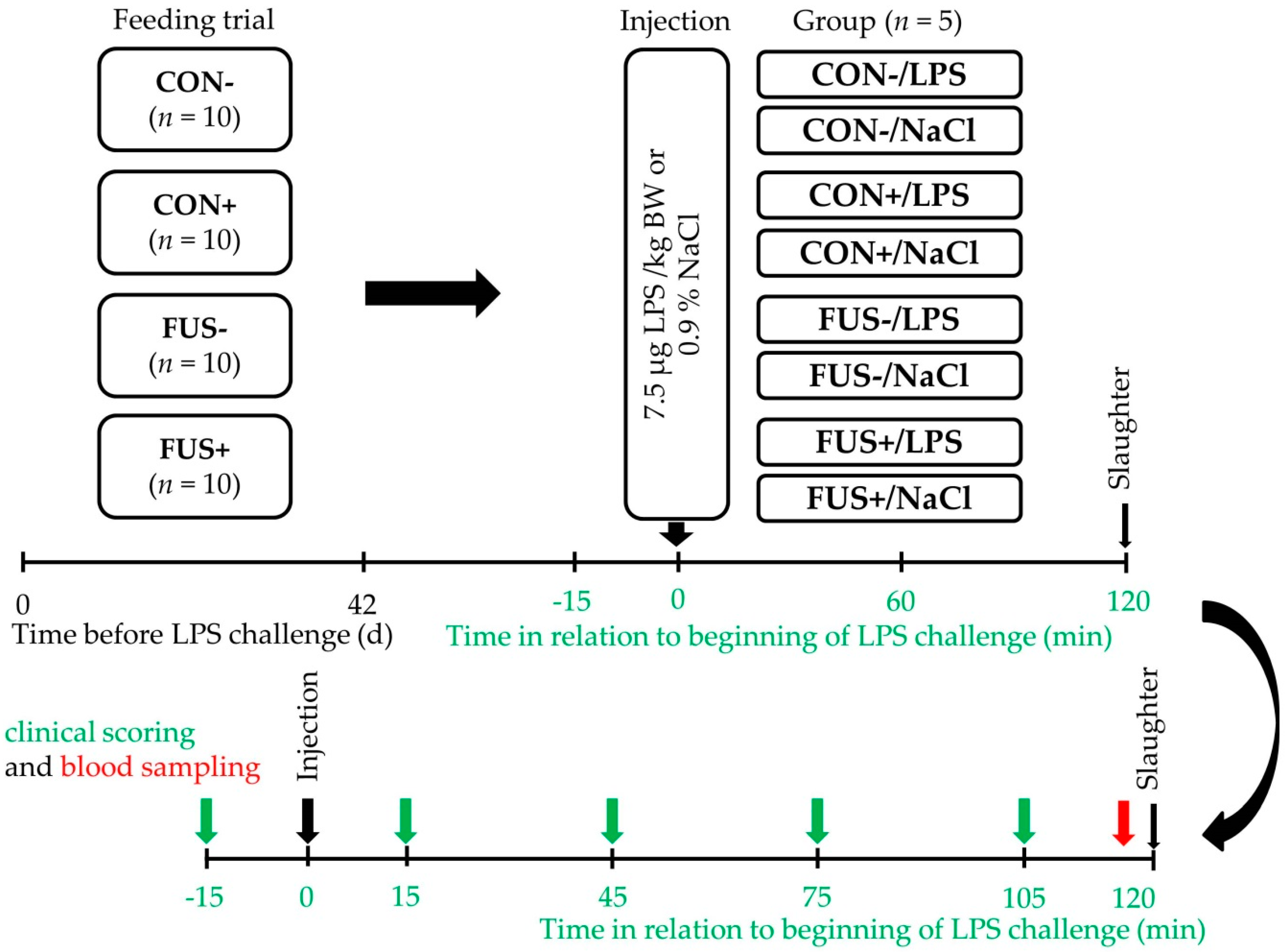
4.1. Experimental Design and Procedures
4.2. Analyses
4.2.1. Haematology and Biochemichal Analysis
4.2.2. Oxidative Status
Griess Assay
Ferric Reducing Ability
4.3. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EFSA. Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to Deoxynivalenol (DON) as undesirable substance in animal feed. EFSA J. 2004, 73, 1–41. [Google Scholar]
- Rotter, B.A.; Prelusky, D.B.; Pestka, J.J. Toxicology of deoxynivalenol (vomitoxin). J. Toxicol. Environ. Health 1996, 48, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Ghareeb, K.; Bohm, J.; Zentek, J. Decontamination and detoxification strategies for the Fusarium mycotoxin deoxynivalenol in animal feed and the effectiveness of microbial biodegradation. Food Addit. Contam. Part A Chem. 2010, 27, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Paulick, M.; Rempe, I.; Kersten, S.; Schatzmayr, D.; Schwartz-Zimmermann, H.E.; Dänicke, S. Effects of increasing concentrations of sodium sulfite on deoxynivalenol and deoxynivalenol sulfonate concentrations of maize kernels and maize meal preserved at various moisture content. Toxins 2015, 7, 791–811. [Google Scholar] [CrossRef] [PubMed]
- Paulick, M.; Winkler, J.; Kersten, S.; Schatzmayr, D.; Schwartz-Zimmermann, H.E.; Dänicke, S. Studies on the bioavailability of deoxynivalenol (DON) and DON sulfonate (DONS) 1, 2, and 3 in pigs fed with sodium sulfite-treated DON-contaminated maize. Toxins 2015, 7, 4622–4644. [Google Scholar] [CrossRef] [PubMed]
- Young, J.C.; Trenholm, H.L.; Friend, D.W.; Prelusky, D.B. Detoxification of deoxynivalenol with sodium bisulfite and evaluation of the effects when pure mycotoxin or contaminated corn was treated and given to pigs. J. Agric. Food Chem. 1987, 35, 259–261. [Google Scholar] [CrossRef]
- Dänicke, S.; Beyer, M.; Breves, G.; Valenta, H.; Humpf, H.U. Effects of oral exposure of pigs to deoxynivalenol (DON) sulfonate (DONS) as the non-toxic derivative of DON on tissue residues of DON and de-epoxy-DON and on DONS blood levels. Food Addit. Contam. Part A Chem. 2010, 27, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Paulick, M.; Winkler, J.; Kersten, S.; Schatzmayr, D.; Frahm, J.; Kluess, J.; Schwartz-Zimmermann, H.E.; Dänicke, S. Effects of oral exposure to sodium sulphite-treated deoxynivalenol (DON)-contaminated maize on performance and plasma concentrations of toxins and metabolites in piglets. Arch. Anim. Nutr. 2018, 72, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.T.; Kluess, J.; Berk, A.; Paulick, M.; Frahm, J.; Schatzmayr, D.; Winkler, J.; Kersten, S.; Dänicke, S. Detoxification of Fusarium-contaminated maize with sodium sulphite–in vivo efficacy with special emphasis on mycotoxin residues and piglet health. Arch. Anim. Nutr. 2018, 72, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Brezina, U. Kinetics and metabolism of the Fusarium toxin deoxynivalenol in farm animals: Consequences for diagnosis of exposure and intoxication and carry over. Food Chem. Toxicol. 2013, 60, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Brüssow, K.P.; Valenta, H.; Ueberschar, K.H.; Tiemann, U.; Schollenberger, M. On the effects of graded levels of Fusarium toxin contaminated wheat in diets for gilts on feed intake, growth performance and metabolism of deoxynivalenol and zearalenone. Mol. Nutr. Food Res. 2005, 49, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Beineke, A.; Goyarts, T.; Valenta, H.; Beyer, M.; Humpf, H.U. Effects of a Fusarium toxin-contaminated triticale, either untreated or treated with sodium metabisulphite (Na2S2O5, SBS), on weaned piglets with a special focus on liver function as determined by the (13)C-methacetin breath test. Arch. Anim. Nutr. 2008, 62, 263–286. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Kersten, S.; Valenta, H.; Breves, G. Inactivation of deoxynivalenol-contaminated cereal grains with sodium metabisulfite: A review of procedures and toxicological aspects. Mycotoxin Res. 2012, 28, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Palsson-McDermott, E.M.; O’Neill, L.A. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 2004, 113, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Kuzmich, N.N.; Sivak, K.V.; Chubarev, V.N.; Porozov, Y.B.; Savateeva-Lyubimova, T.N.; Peri, F. TLR4 Signaling Pathway Modulators as Potential Therapeutics in Inflammation and Sepsis. Vaccines 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Islam, Z.; Pestka, J.J. LPS priming potentiates and prolongs proinflammatory cytokine response to the trichothecene deoxynivalenol in the mouse. Toxicol. Appl. Pharmacol. 2006, 211, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.R.; Harkema, J.R.; Yan, D.; Pestka, J.J. Amplified proinflammatory cytokine expression and toxicity in mice coexposed to lipopolysaccharide and the trichothecene vomitoxin (deoxynivalenol). J. Toxicol. Environ. Health A 1999, 57, 115–136. [Google Scholar] [PubMed]
- Döll, S.; Schrickx, J.A.; Dänicke, S.; Fink-Gremmels, J. Deoxynivalenol-induced cytotoxicity, cytokines and related genes in unstimulated or lipopolysaccharide stimulated primary porcine macrophages. Toxicol. Lett. 2009, 184, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.R.; Harkema, J.R.; Hotchkiss, J.A.; Yan, D.; Roth, R.A.; Pestka, J.J. Lipopolysaccharide and the Trichothecene Vomitoxin (Deoxynivalenol)Synergistically Induce Apoptosis in Murine Lymphoid Organs. Toxicol. Sci. Off. J. Soc. Toxicol. 2000, 53, 253–263. [Google Scholar] [CrossRef]
- Dänicke, S.; Valenta, H.; Ganter, M.; Brosig, B.; Kersten, S.; Diesing, A.K.; Kahlert, S.; Panther, P.; Kluess, J.; Rothkötter, H.J. Lipopolysaccharides (LPS) modulate the metabolism of deoxynivalenol (DON) in the pig. Mycotoxin Res. 2014, 30, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Bannert, E.; Tesch, T.; Kluess, J.; Frahm, J.; Kersten, S.; Kahlert, S.; Renner, L.; Rothkötter, H.J.; Dänicke, S. Metabolic and hematological consequences of dietary deoxynivalenol interacting with systemic Escherichia coli lipopolysaccharide. Toxins 2015, 7, 4773–4796. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, W. Klinische Propädeutik der Haus-und Heimtiere [Clinical Propaedeutic of Domestic and Pet Animals], 7th ed.; MVS Medizinverlage Stuttgart GmbH & Co., KG: Stuttgart, Germany, 2009. [Google Scholar]
- Kraft, W.; Dürr, U.M. Klinische Labordiagnostik in der Tiermedizin. [Clinical Laboratory Diagnostician in Veterinary Medicine], 7th ed.; Schattauer GmbH: Stuttgart, Germany, 2014. [Google Scholar]
- Stanek, C.; Reinhardt, N.; Diesing, A.K.; Nossol, C.; Kahlert, S.; Panther, P.; Kluess, J.; Rothkotter, H.J.; Kuester, D.; Brosig, B.; et al. A chronic oral exposure of pigs with deoxynivalenol partially prevents the acute effects of lipopolysaccharides on hepatic histopathology and blood clinical chemistry. Toxicol. Lett. 2012, 215, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Stelter, K.; Frahm, J.; Paulsen, J.; Berk, A.; Kleinwachter, M.; Selmar, D.; Dänicke, S. Effects of oregano on performance and immunmodulating factors in weaned piglets. Arch. Anim. Nutr. 2013, 67, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Saetre, T.; Hovig, T.; Roger, M.; Gundersen, Y.; Aasen, A.O. Hepatocellular damage in porcine endotoxemia: Beneficial effects of selective versus non-selective nitric oxide synthase inhibition? Scand. J. Clin. Lab. Investig. 2001, 61, 503–512. [Google Scholar] [CrossRef]
- Hinshaw, L.B.; Emerson, T.E., Jr.; Chang, A.C.; Duerr, M.; Peer, G.; Fournel, M. Study of septic shock in the non-human primate: Relationship of pathophysiological response to therapy with anti-TNF antibody. Circ. Shock. 1994, 44, 221–229. [Google Scholar] [PubMed]
- Dänicke, S.; Hegewald, A.-K.; Kahlert, S.; Kluess, J.; Rothkotter, H.J.; Breves, G.; Döll, S. Studies on the toxicity of deoxynivalenol (DON), sodium metabisulfite, DON-sulfonate (DONS) and de-epoxy-DON for porcine peripheral blood mononuclear cells and the Intestinal Porcine Epithelial Cell lines IPEC-1 and IPEC-J2, and on effects of DON and DONS on piglets. Food Chem. Toxicol. 2010, 48, 2154–2162. [Google Scholar] [PubMed]
- Rempe, I.; Kersten, S.; Valenta, H.; Dänicke, S. Hydrothermal treatment of naturally contaminated maize in the presence of sodium metabisulfite, methylamine and calcium hydroxide; effects on the concentration of zearalenone and deoxynivalenol. Mycotoxin Res. 2013, 29, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Elmas, O.; Aslan, M.; Caglar, S.; Derin, N.; Agar, A.; Aliciguzel, Y.; Yargicoglu, P. The prooxidant effect of sodium metabisulfite in rat liver and kidney. Regul. Toxicol. Pharmacol. 2005, 42, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Tejnorova, I. Sulfite oxidase activity in liver and kidney tissue in five laboratory animal species. Toxicol. Appl. Pharmacol. 1978, 44, 251–256. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘antioxidant power’’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
| Maize | Treatment | Injection | BW [kg] | Heart | Liver | Spleen | Kidney | Lung | Stomach |
|---|---|---|---|---|---|---|---|---|---|
| CON | − | NaCl | 30.6 | 4.8 | 25.5 | 1.5 | 4.8 | 13.0 | 7.2 |
| CON | − | LPS | 29.6 | 4.7 | 28.9 | 2.0 | 4.6 | 11.1 | 8.0 |
| CON | + | NaCl | 29.8 | 4.7 | 26.3 | 1.6 | 4.6 | 13.8 | 7.4 |
| CON | + | LPS | 30.8 | 4.7 | 25.4 | 1.6 | 4.3 | 13.8 | 8.3 |
| FUS | − | NaCl | 25.8 | 4.8 | 25.9 | 1.6 | 4.8 | 14.6 | 9.4 |
| FUS | − | LPS | 26.3 | 4.4 | 27.4 | 1.8 | 4.8 | 13.8 | 10.1 |
| FUS | + | NaCl | 31.4 | 4.7 | 26.1 | 1.6 | 4.5 | 13.1 | 7.9 |
| FUS | + | LPS | 30.4 | 4.7 | 26.4 | 1.7 | 4.6 | 10.3 | 7.9 |
| ANOVA (p-value) | |||||||||
| Maize | 0.087 | 0.712 | 0.897 | 0.888 | 0.482 | 0.995 | <0.001 | ||
| Treatment | 0.015 | 0.846 | 0.261 | 0.141 | 0.126 | 0.645 | 0.010 | ||
| Injection | 0.495 | 0.503 | 0.181 | 0.015 | 0.642 | 0.082 | 0.048 | ||
| Maize × treatment | 0.023 | 0.697 | 0.524 | 0.881 | 0.831 | 0.009 | 0.001 | ||
| Maize × injection | 0.495 | 0.606 | 0.834 | 0.590 | 0.382 | 0.580 | 0.392 | ||
| Treatment × injection | 0.495 | 0.388 | 0.081 | 0.082 | 0.890 | 0.973 | 0.636 | ||
| Maize × treatment × injection | 0.742 | 0.668 | 0.319 | 0.242 | 0.793 | 0.211 | 0.545 | ||
| PSEM # | 1.4 | 0.2 | 1.1 | 0.1 | 0.2 | 1.1 | 0.4 | ||
| Maize | Treatment | Injection | Leukocytes (10–22) Φ | Lymphocytes (6–16) Φ | Segmented Neutrophils (1–8.2) Φ | Banded Neutrophils (0–1.5) Φ | Monocytes (0–1) Φ | Eosinophils (0–1.3) Φ |
|---|---|---|---|---|---|---|---|---|
| CON | − | NaCl | 18.7 | 11.9 | 4.7 | 0.5 | 0.7 | 0.3 |
| CON | − | LPS | 9.8 | 6.6 | 2.3 | 0.4 | 0.2 | 0.2 |
| CON | + | NaCl | 13.4 | 8.7 | 3.7 | 0.4 | 0.2 | 0.3 |
| CON | + | LPS | 14.0 | 10.2 | 2.6 | 0.5 | 0.4 | 0.3 |
| FUS | − | NaCl | 14.4 | 8.4 | 4.8 | 0.6 | 0.4 | 0.2 |
| FUS | − | LPS | 7.8 | 6.0 | 1.3 | 0.2 | 0.2 | 0.1 |
| FUS | + | NaCl | 20.8 | 12.2 | 6.8 | 0.9 | 0.7 | 0.3 |
| FUS | + | LPS | 13.0 | 8.0 | 3.7 | 0.7 | 0.3 | 0.2 |
| ANOVA (p-value) | ||||||||
| Maize | 0.974 | 0.388 | 0.091 | 0.126 | 0.836 | 0.218 | ||
| Treatment | 0.034 | 0.062 | 0.067 | 0.028 | 0.960 | 0.258 | ||
| Injection | <0.001 | 0.003 | <0.001 | 0.049 | 0.002 | 0.380 | ||
| Maize × treatment | 0.012 | 0.112 | 0.011 | 0.019 | 0.022 | 0.691 | ||
| Maize × injection | 0.206 | 0.425 | 0.095 | 0.112 | 0.491 | 0.843 | ||
| Treatment × injection | 0.092 | 0.132 | 0.366 | 0.299 | 0.064 | 0.948 | ||
| Maize × treatment × injection | 0.033 | 0.012 | 0.664 | 0.809 | 0.011 | 0.858 | ||
| PSEM # | 1.7 | 1.1 | 0.7 | 0.1 | 0.1 | 0.1 | ||
| Maize | Treatment | Injection | Protein [g/L] < 86 Φ | Albumin [g/L] (19–39) Φ | AST † [U/L] < 35 Φ | γ-GT ‡ [U/L] < 45 Φ | Total Bilirubin [µmol/L] < 4.3 Φ |
|---|---|---|---|---|---|---|---|
| CON | − | NaCl | 48.0 | 32.7 | 36.8 | 24.0 | 3.1 |
| CON | − | LPS | 45.5 | 32.4 | 46.3 | 29.1 | 3.5 |
| CON | + | NaCl | 45.0 | 32.4 | 39.7 | 25.4 | 2.4 |
| CON | + | LPS | 45.2 | 33.0 | 49.4 | 24.4 | 3.0 |
| FUS | − | NaCl | 46.0 | 31.2 | 49.6 | 24.0 | 3.0 |
| FUS | − | LPS | 41.1 | 29.7 | 50.0 | 28.1 | 4.2 |
| FUS | + | NaCl | 49.2 | 34.0 | 49.0 | 26.8 | 2.6 |
| FUS | + | LPS | 43.7 | 32.8 | 40.8 | 29.5 | 3.5 |
| ANOVA (p-value) | |||||||
| Maize | 0.583 | 0.438 | 0.153 | 0.259 | 0.305 | ||
| Treatment | 0.700 | 0.073 | 0.744 | 0.845 | 0.087 | ||
| Injection | 0.060 | 0.466 | 0.334 | 0.031 | 0.024 | ||
| Maize × treatment | 0.181 | 0.105 | 0.184 | 0.130 | 0.949 | ||
| Maize × injection | 0.220 | 0.363 | 0.028 | 0.594 | 0.345 | ||
| Treatment × injection | 0.755 | 0.713 | 0.481 | 0.130 | 0.949 | ||
| Maize × treatment × injection | 0.616 | 0.876 | 0.464 | 0.332 | 0.784 | ||
| PSEM # | 2.3 | 1.2 | 4.2 | 1.7 | 0.5 | ||
| Clinical Symptom | Grade | Score |
|---|---|---|
| Respiratory difficulties | None | 0 |
| Low labored breathing | 1 | |
| Medium labored breathing | 2 | |
| Severe labored breathing | 3 | |
| Open-mouth breathing | 4 | |
| Tremor | None | 0 |
| Low shivering | 1 | |
| Medium shivering | 2 | |
| Severe shivering | 3 | |
| Spasms | 4 | |
| Conjunctivae | Physiological (pale-rose) | 0 |
| (Rose) red | 1 | |
| Red | 2 | |
| Episcleral vessels | Physiological (not injected) | 0 |
| Slightly injected | 1 | |
| Medium injected | 2 | |
| Highly injected | 3 | |
| Cyanosis | None | 0 |
| Low cyanosis | 1 | |
| Medium cyanosis | 2 | |
| Severe cyanosis | 3 | |
| Vomiting, retching | None | 0 |
| Smacking, foam-forming, retching | 1 | |
| Vomiting of slime | 2 | |
| Vomiting of feed/digesta | 3 | |
| Vomiting of slime and feed/digesta | 4 | |
| Dermographism | None | 0 |
| Skin colouring pattern present | 1 | |
| Teeth gnashing | None | 0 |
| Teeth gnashing present | 1 | |
| Maximum clinical score (each animal each time point) | 22 | |
| Maximum clinical score (each animal for all time points) | 88 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, A.-T.; Kluess, J.; Berk, A.; Paulick, M.; Frahm, J.; Schatzmayr, D.; Kersten, S.; Dänicke, S. Effects of a Fusarium Toxin-Contaminated Maize Treated with Sodium Sulfite on Male Piglets in the Presence of an LPS-Induced Acute Inflammation. Toxins 2018, 10, 419. https://doi.org/10.3390/toxins10100419
Tran A-T, Kluess J, Berk A, Paulick M, Frahm J, Schatzmayr D, Kersten S, Dänicke S. Effects of a Fusarium Toxin-Contaminated Maize Treated with Sodium Sulfite on Male Piglets in the Presence of an LPS-Induced Acute Inflammation. Toxins. 2018; 10(10):419. https://doi.org/10.3390/toxins10100419
Chicago/Turabian StyleTran, Anh-Tuan, Jeannette Kluess, Andreas Berk, Marleen Paulick, Jana Frahm, Dian Schatzmayr, Susanne Kersten, and Sven Dänicke. 2018. "Effects of a Fusarium Toxin-Contaminated Maize Treated with Sodium Sulfite on Male Piglets in the Presence of an LPS-Induced Acute Inflammation" Toxins 10, no. 10: 419. https://doi.org/10.3390/toxins10100419
APA StyleTran, A.-T., Kluess, J., Berk, A., Paulick, M., Frahm, J., Schatzmayr, D., Kersten, S., & Dänicke, S. (2018). Effects of a Fusarium Toxin-Contaminated Maize Treated with Sodium Sulfite on Male Piglets in the Presence of an LPS-Induced Acute Inflammation. Toxins, 10(10), 419. https://doi.org/10.3390/toxins10100419





