The Effects of Autophagy and PI3K/AKT/m-TOR Signaling Pathway on the Cell-Cycle Arrest of Rats Primary Sertoli Cells Induced by Zearalenone
Abstract
:1. Introduction
2. Results
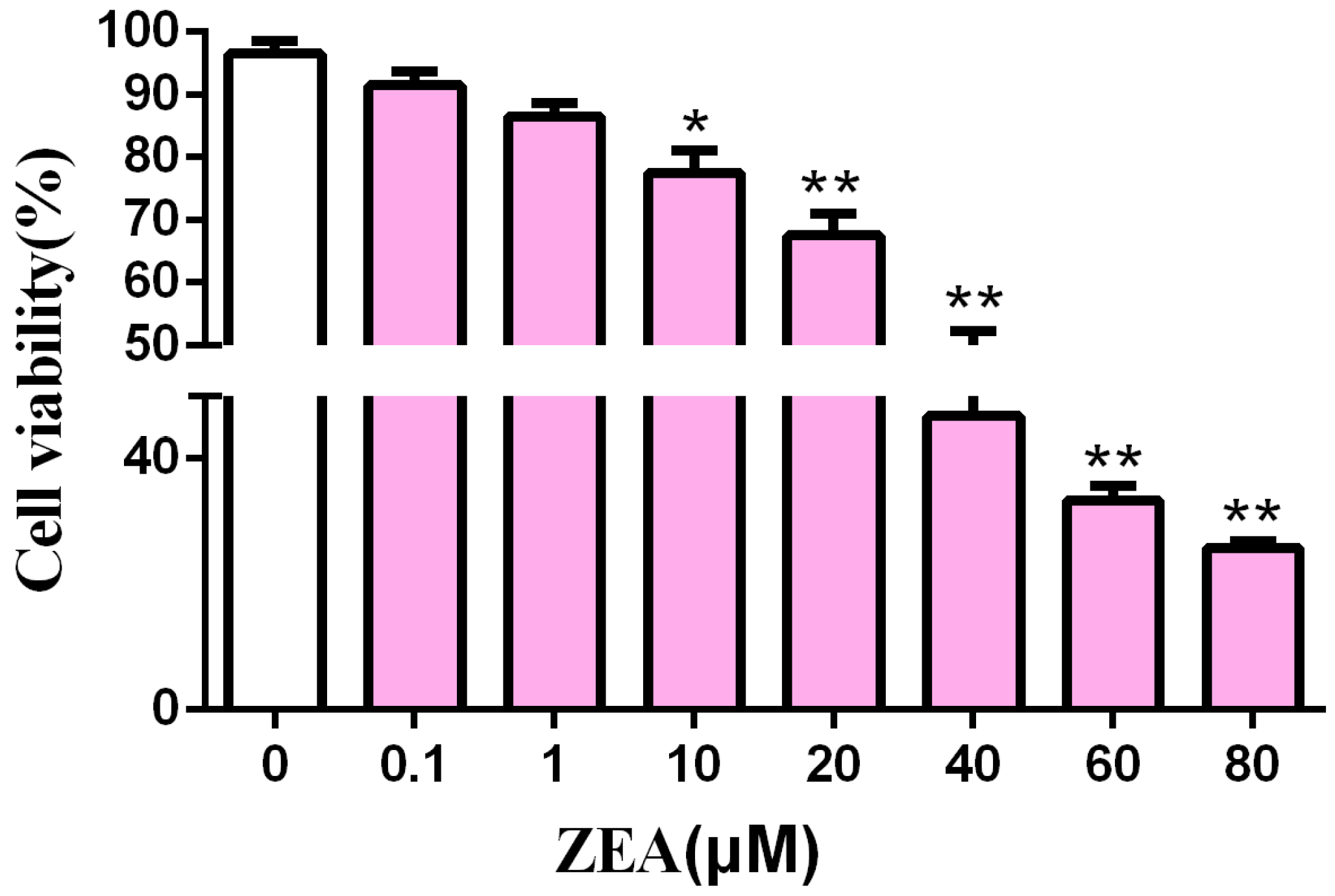
2.1. Cytotoxic Effect of ZEA in SCs
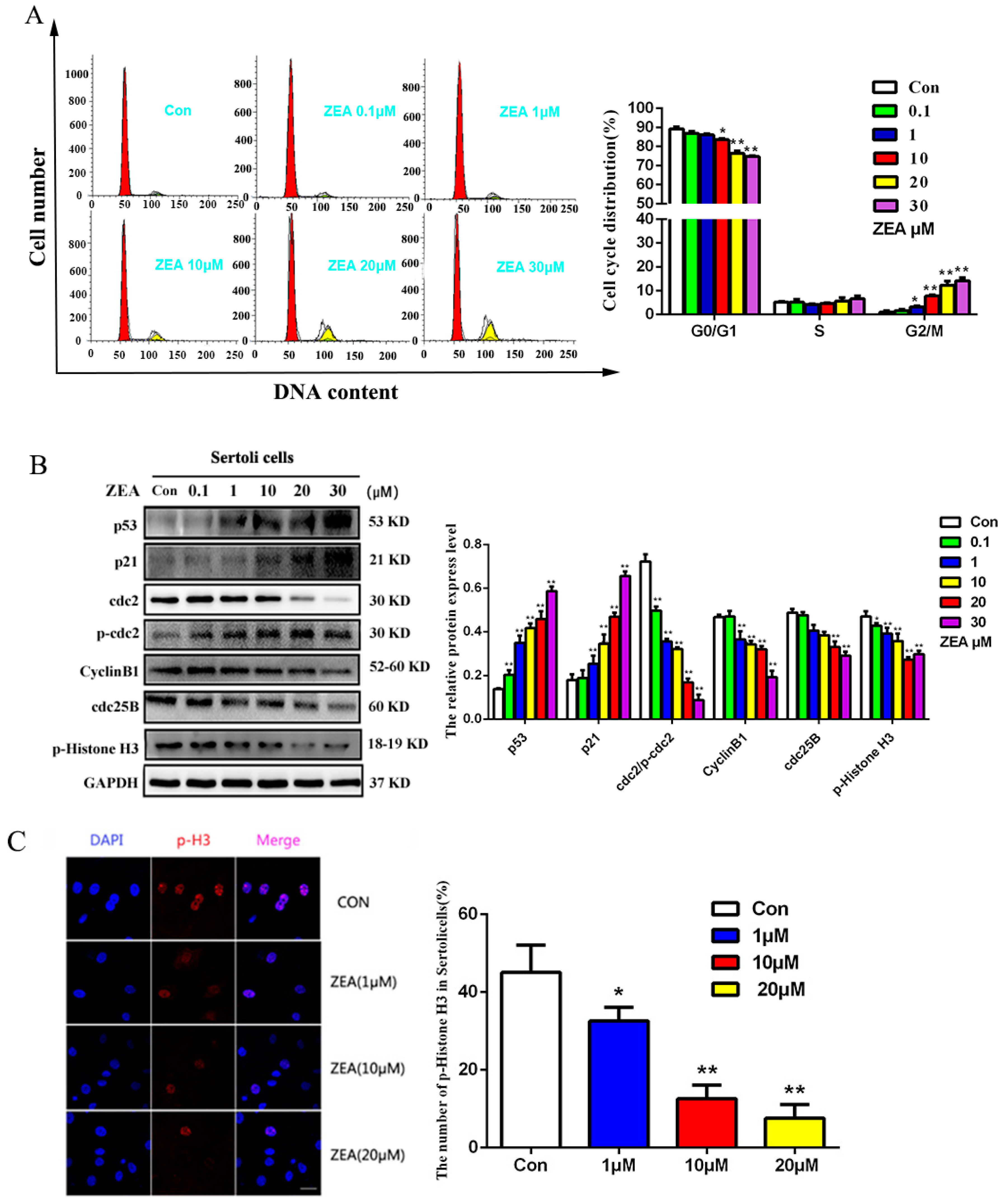
2.2. The Effects of ZEA on the Cell Cycle Distribution and the Cell Cycle Associated Proteins in SCs
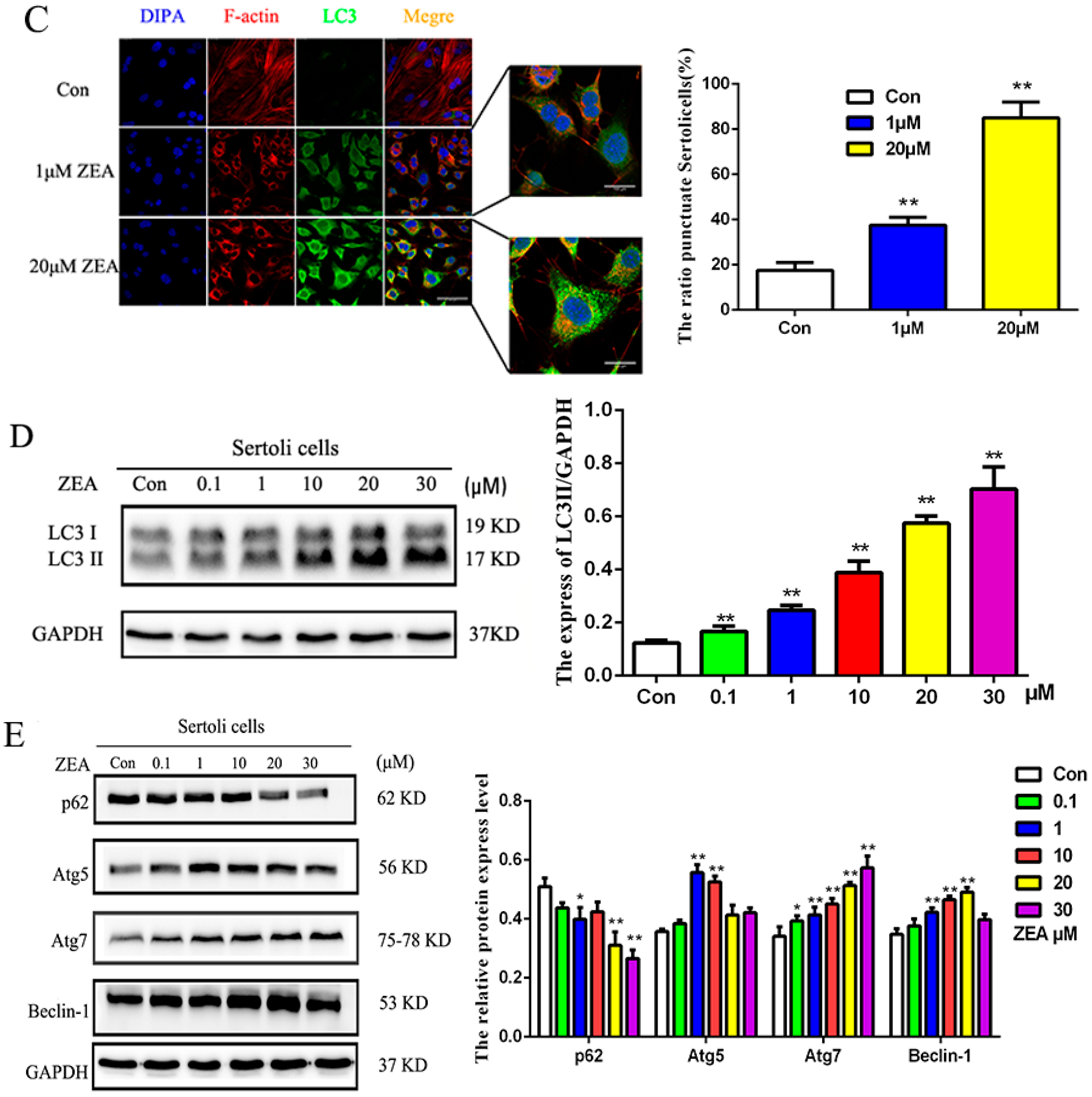
2.3. ZEA Could Trigger the Autophagy in SCs
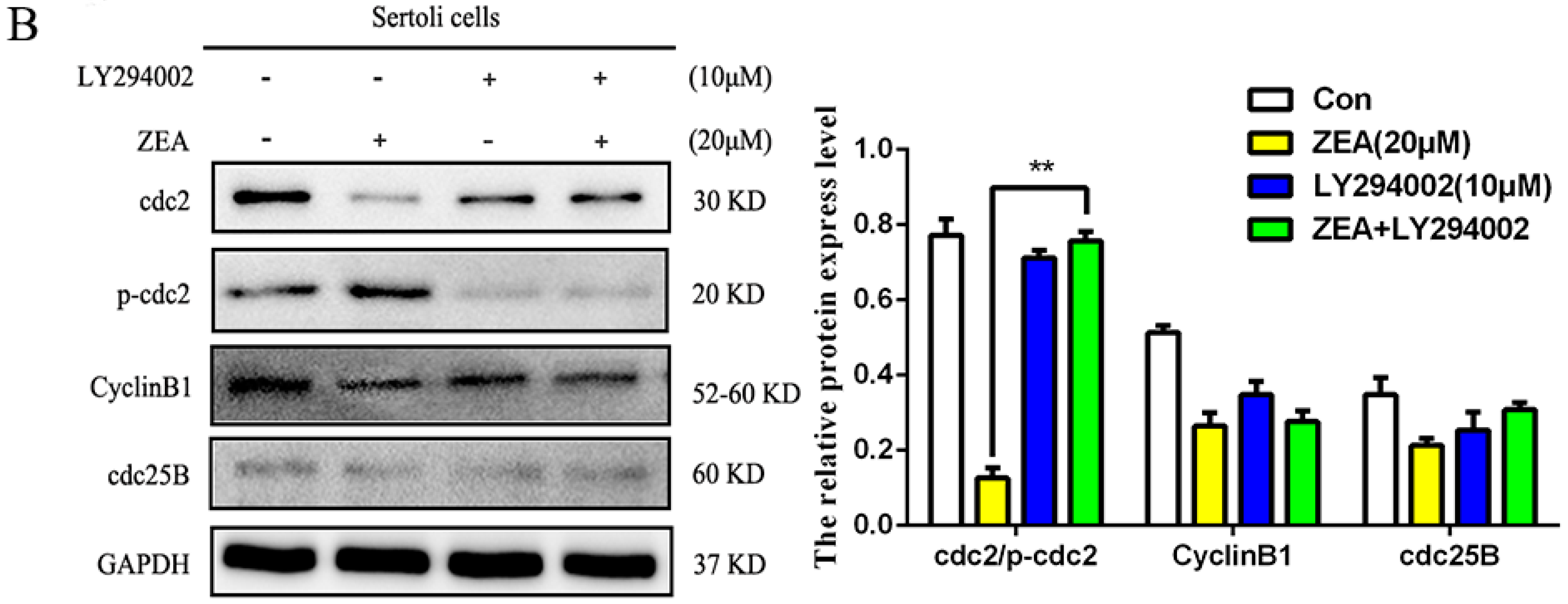
2.4. ZEA Induced Autophagy through Inhibiting the PI3K/Akt/m TOR Signaling Pathway in SCs
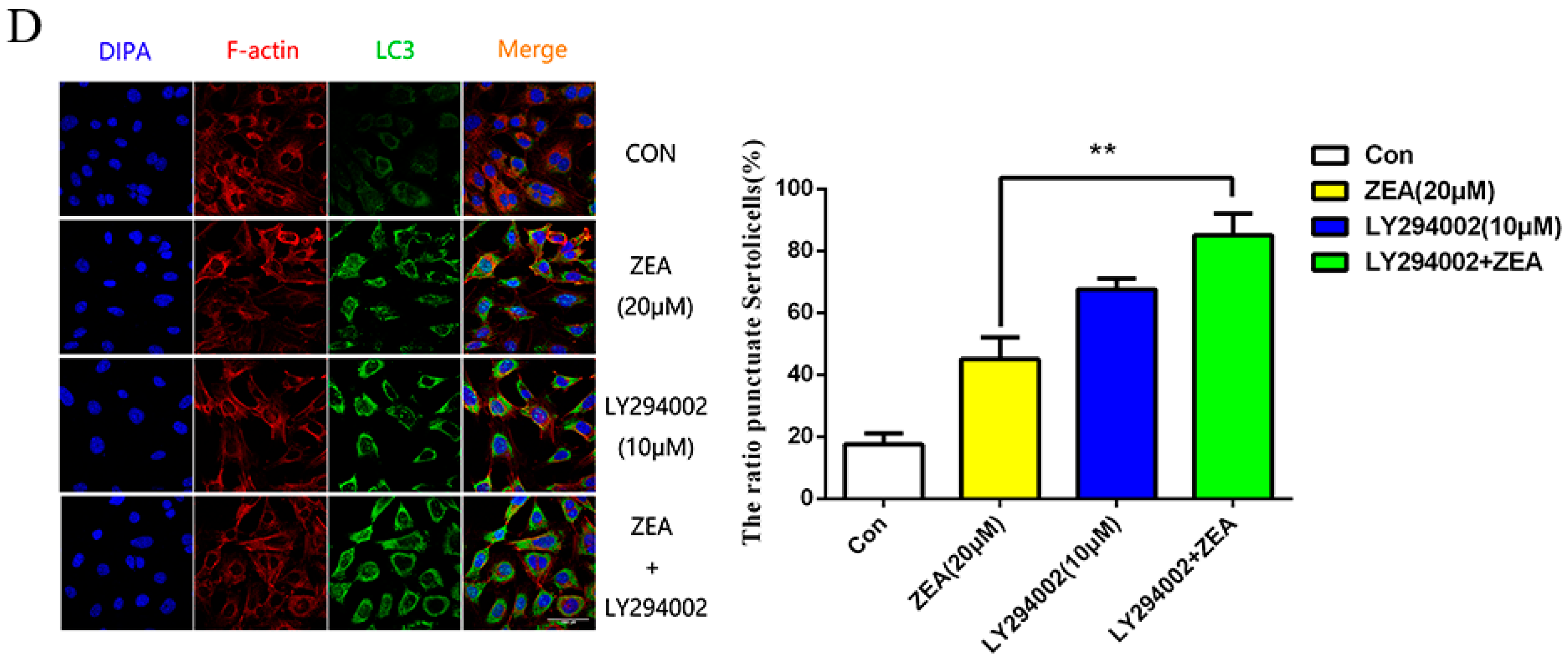
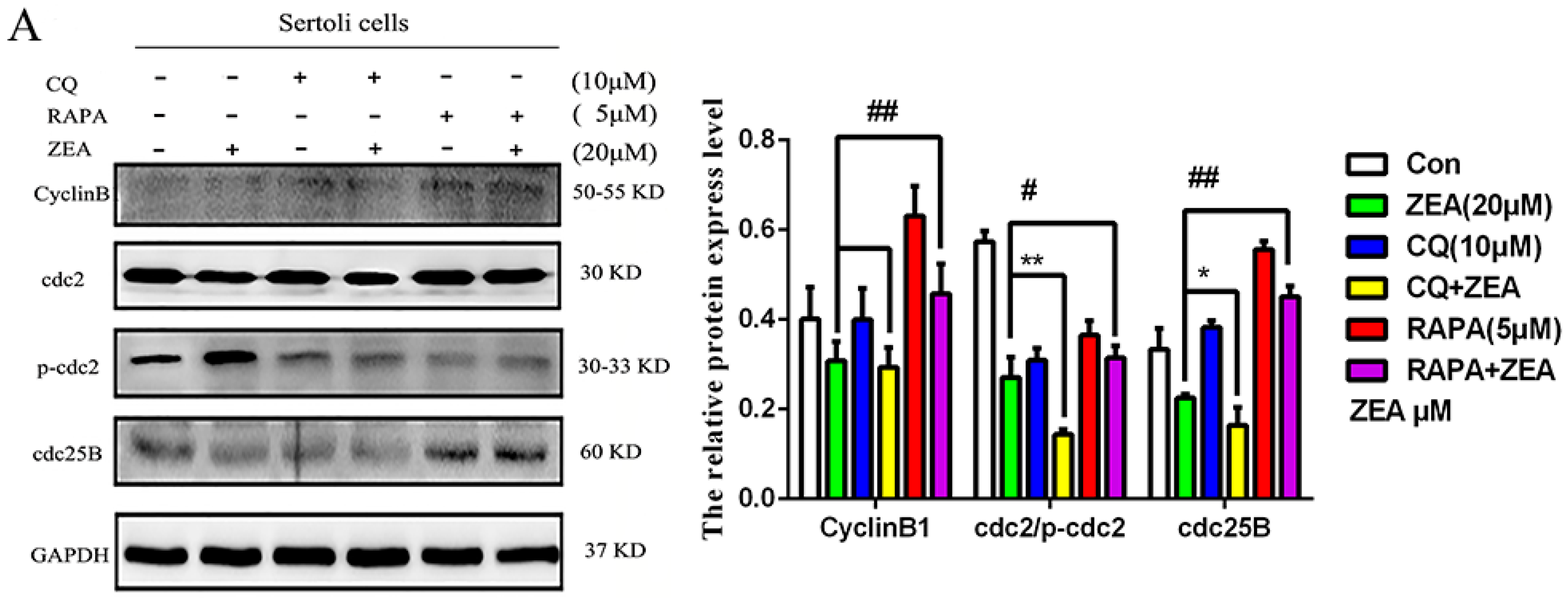
2.5. The Role of Autophagy in ZEA-Induced G2/M Arrest in SCs
2.6. The Role of Autophagy in the Expressions of Cell Cycle Regulatory Protein in SCs
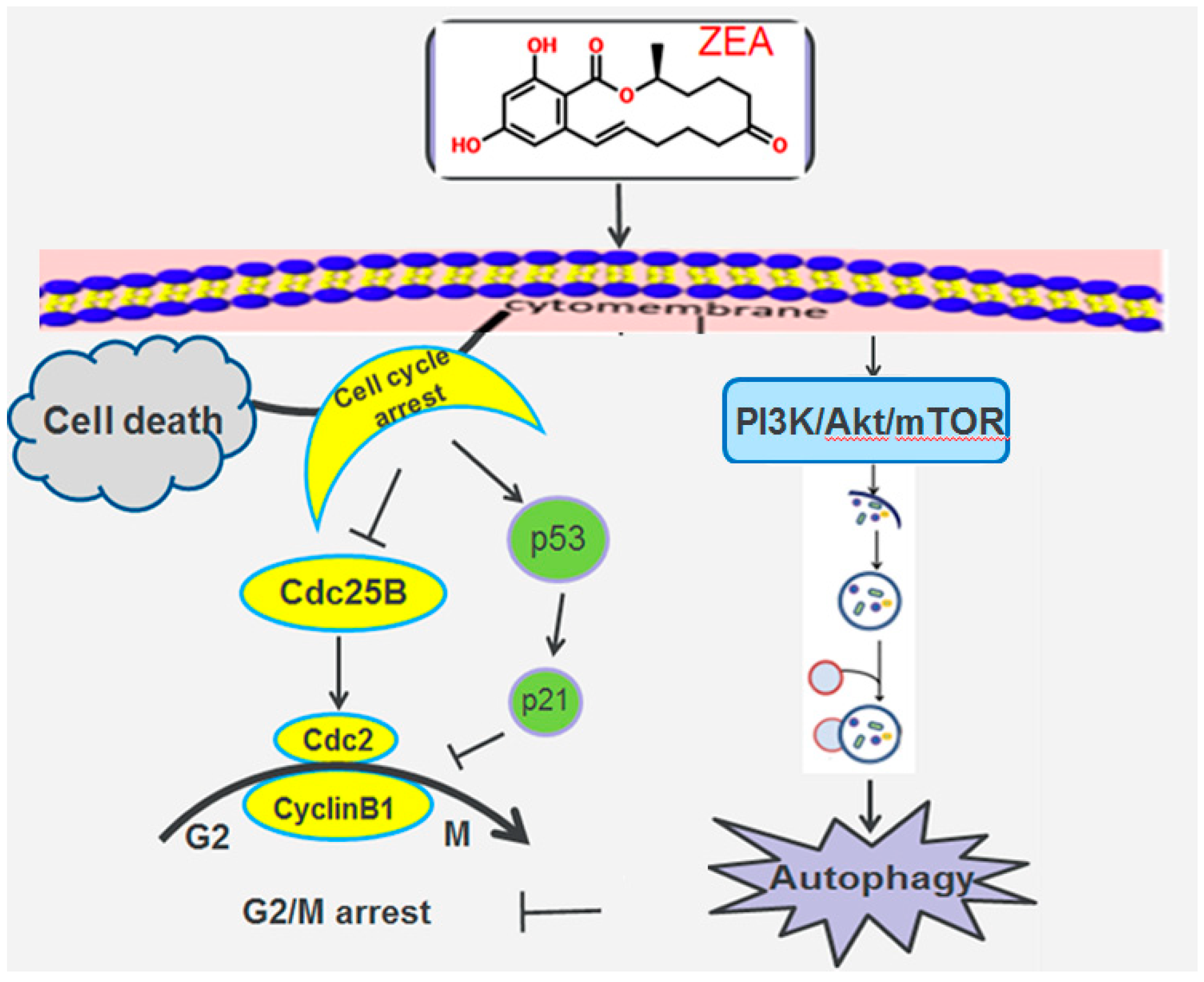
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Reagents
5.2. Cell Cultures
5.3. Cell Proliferation Assay
5.4. Cell Cycle Assay
5.5. Western Blotting Analysis
5.6. Transmission Electron Microscopy (TEM)
5.7. Immunofluorescence Microscopy
5.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Fu, G.; Ma, J.; Wang, L.; Yang, X.; Liu, J.; Zhao, X. Effect of degradation of zearalenone-contaminated feed by bacillus licheniformis CK1 on postweaning female piglets. Toxins 2016, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhang, L.; Liu, M.; Su, Y.T.; Xie, W.M.; Zhang, N.Y.; Dai, J.F.; Wang, Y.; Rajput, S.A.; Qi, D.S. Individual and combined occurrence of mycotoxins in feed ingredients and complete feeds in China. Toxins 2018, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Hueza, I.M.; Raspantini, P.C.; Raspantini, L.E.; Latorre, A.O.; Górniak, S.L. Zearalenone, an estrogenic mycotoxin, is an immunotoxic compound. Toxins 2014, 6, 1080–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constanze, P.; Susanne, K.; Patricia, B.H.; Hana, V.; Sven, D. Occurrence of deoxynivalenol and zearalenone in commercial fish feed: An initial study. Toxins 2013, 5, 184–192. [Google Scholar]
- Shi, B.; Su, Y.; Chang, S.; Sun, Y.; Meng, X.; Shan, A. Vitamin C protects piglet liver against zearalenone-induced oxidative stress by modulating expression of nuclear receptors PXR and CAR and their target genes. Food Funct. 2017, 8, 3675–3687. [Google Scholar] [CrossRef] [PubMed]
- Moltó, J.C. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar]
- Jiang, S.; Yang, W.; Yang, Z. The contamination, residue and mechanism of zearalenone. China Feed 2011, 2. [Google Scholar]
- Wang, Y.J.; Liu, Q.; Zheng, W.L.; Bian, X.J.; Jian-Hong, G.U.; Yuan, Y.; Liu, X.Z.; Liu, Z.P.; Bian, J.C. Effect of zearalenone on apoptosis and expression of caspase-3 and caspase-9 in leydig cells. Chin. J. Veter. Sci. 2014, 34, 131–135. [Google Scholar]
- Chang, H.J.; Ro, S.H.; Jing, C.; Otto, N.M.; Kim, D.H. mTOR regulation of autophagy. FEBS Lett. 2010, 584, 1287–1295. [Google Scholar] [Green Version]
- Jiang, S.Z.; Yang, Z.B.; Yang, W.R.; Wang, S.J.; Liu, F.X.; Johnston, L.A.; Chi, F.; Wang, Y. Effect of purified zearalenone with or without modified montmorillonite on nutrient availability, genital organs and serum hormones in post-weaning piglets. Livest. Sci. 2012, 144, 110–118. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.J.; Hui, X.U.; Huang, Q.Y.; Jian-Hong, G.U.; Yuan, Y.; Liu, X.Z.; Liu, Z.P.; Bian, J.C. The role of LH/hCG receptor and camp in zearalenone inhibiting testosterone secretion in mouse leydig cells. Chin. J. Anim. Veter. Sci. 2013, 44, 1147–1154. [Google Scholar]
- Wang, Y.; Zheng, W.; Bian, X.; Yuan, Y.; Gu, J.; Liu, X.; Liu, Z.; Bian, J. Zearalenone induces apoptosis and cytoprotective autophagy in primary leydig cells. Toxicol. Lett. 2014, 226, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.D. The central role of sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998, 9, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Steinberger, A.; Klinefelter, G. Sensitivity of sertoli and leydig cells to xenobiotics in in vitro models. Reprod. Toxicol. 1993, 7, 23. [Google Scholar] [CrossRef]
- Franca, L.R.; Silva, V.A., Jr.; Chiarini-Garcia, H.; Garcia, S.K.; Debeljuk, L. Cell proliferation and hormonal changes during postnatal development of the testis in the pig. Biol. Reprod. 2000, 63, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Xu, B.; Yao, M.; Mao, Z.; Zhang, Y.; Xu, G.; Tang, Q.; Wang, X.; Xia, Y. Graphene oxide quantum dots disrupt autophagic flux by inhibiting lysosome activity in GC-2 and TM4 cell lines. Toxicology 2016, 374, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Pan, S.; Wang, G.; Wang, Y.J.; Liu, Q.; Gu, J.H.; Yuan, Y.; Liu, X.Z.; Liu, Z.P.; Bian, J.C. Zearalenone impairs the male reproductive system functions via inducing structural and functional alterations of sertoli cells. Environ. Toxicol. Pharmacol. 2016, 42, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, B.; Si, M.; Zou, H.; Song, R.; Gu, J.; Yuan, Y.; Liu, X.; Zhu, G.; Bai, J. Zearalenone altered the cytoskeletal structure via ER stress-autophagy-oxidative stress pathway in mouse TM4 sertoli cells. Sci. Rep. 2018, 8, 3320. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.C.; Levine, B. Autophagy in cellular growth control. FEBS Lett. 2010, 584, 1417–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.C.; Wu, W.K.; Li, Y.; Yu, L.; Li, Z.J.; Wong, C.C.; Li, H.T.; Sung, J.J.; Cho, C.H. Inhibition of macroautophagy by bafilomycin A1 lowers proliferation and induces apoptosis in colon cancer cells. Biochem. Biophys. Res. Commun. 2009, 382, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Levine, B. Cell biology: Autophagy and cancer. Nature 2007, 446, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Klionsky, D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell 2004, 6, 463. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.M.; Evangelisti, C.; Follo, M.Y.; Ramazzotti, G.; Fini, M.; Giardino, R.; Manzoli, L.; McCubrey, J.A.; Cocco, L. Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling network in cancer stem cells. Curr. Med. Chem. 2011, 18, 2715–2726. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, E.; Maruki, H.; Minato, Y.; Doki, Y.; Weinstein, I.B.; Imoto, M. Overexpression of cyclin D1 contributes to malignancy by up-regulation of fibroblast growth factor receptor 1 via the pRB/E2F pathway. Cancer Res. 2003, 63, 424–431. [Google Scholar] [PubMed]
- Simon, P. Ochratoxin and kidney disease in the human. Toxin Rev. 1996, 15, 239–249. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; He, X.; Cheng, W.H.; Xu, W.; Luo, Y.; Liang, R.; Luo, H.; Huang, K. Analysis of individual and combined effects of ochratoxin A and zearalenone on HepG2 and KK-1 cells with mathematical models. Toxins 2014, 6, 1177–1192. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Mcarthur, G. Cell cycle regulation and melanoma. Curr. Oncol. Rep. 2016, 18, 34. [Google Scholar]
- Terzoudi, G.I.; Jung, T.; Hain, J.; Vrouvas, J.; Margaritis, K.; Donta-Bakoyianni, C.; Makropoulos, V.; Angelakis, P.; Pantelias, G.E. Increased G2 chromosomal radiosensitivity in cancer patients: The role of cdk1/cyclin-B activity level in the mechanisms involved. Int. J. Radiat. Biol. 2000, 76, 607–615. [Google Scholar] [PubMed]
- Ji, J.Y.; Crest, J.; Schubiger, G. Genetic interactions between cdk1-cyclinb and the separase complex in drosophila. Development 2005, 132, 1875. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.W.; Jeong, D.W.; Won, J.Y.; Choi, E.J.; Choi, Y.H.; Kim, I.Y. H2O2-induced AP-1 activation and its effect on p21WAF1/CIP1)-mediated G2/M arrest in a p53-deficient human lung cancer cell. Biochem. Biophys. Res. Commun. 2002, 293, 1248–1253. [Google Scholar] [CrossRef]
- O’Reilly, M.A. Redox activation of p21Cip1/WAF1/Sdi1: A multifunctional regulator of cell survival and death. Antioxid. Redox Sign. 2005, 7, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Emr, S.D. Autophagy as a regulated pathway of cellular degradation. Science 2000, 290, 1717–1721. [Google Scholar] [PubMed]
- Durlacher, C.T.; Chow, K.; Chen, X.W.; He, Z.X.; Zhang, X.; Yang, T.; Zhou, S.F. Targeting Na+/K+-translocating adenosine triphosphatase in cancer treatment. Clin. Exp. Pharmacol. Physiol. 2015, 42, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B. Autophagy in mammalian development and differentiation. Nat. Cell Biol. 2010, 12, 823. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Hu, C.; Quan, C.; Yu, T.; Huang, W.; Chen, W.; Tang, S.; Shi, Y.; Martin, F.L.; Yang, K. 4-Nonylphenol induces autophagy and attenuates mTOR-p70S6K/4EBP1 signaling by modulating ampk activation in sertoli cells. Toxicol. Lett. 2016, 267, 21. [Google Scholar] [CrossRef] [PubMed]
- Morel, J.; Palao, J.C.; Castells, J.; Desgeorges, M.; Busso, T.; Molliex, S.; Jahnke, V.; Carmine, P.; Gondin, J.; Arnould, D. Regulation of Akt-mTOR, ubiquitin-proteasome and autophagy-lysosome pathways in locomotor and respiratory muscles during experimental sepsis in mice. Sci. Rep. 2017, 7, 10866. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Matsui, Y.; Sadoshima, J. The role of autophagy in mediating cell survival and death during ischemia and reperfusion in the heart. Antioxid. Redox Sign. 2007, 9, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Nie, T.; Yang, S.; Ma, H.; Zhang, L.; Lu, F.; Tao, K.; Wang, R.; Yang, R.; Huang, L.; Mao, Z. Regulation of er stress-induced autophagy by GSKβ3-TIP60-ULK1 pathway. Cell Death Dis. 2016, 7, e2563. [Google Scholar] [PubMed]
- Follo, M.Y.; Manzoli, L.; Poli, A.; Mccubrey, J.A.; Cocco, L. PLC and PI3K/Akt/mTOR signalling in disease and cancer. Adv. Biol. Regul. 2015, 57, 10. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, A.R.; Larrick, J.W. Rapamycin as an antiaging therapeutic?: Targeting mammalian target of rapamycin to treat hutchinson-gilford progeria and neurodegenerative diseases. Rejuv. Res. 2011, 14, 437–441. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.-j.; Zheng, W.-l.; Feng, N.-n.; Wang, T.; Zou, H.; Gu, J.-h.; Yuan, Y.; Liu, X.-z.; Liu, Z.-p.; Bian, J.-c. The Effects of Autophagy and PI3K/AKT/m-TOR Signaling Pathway on the Cell-Cycle Arrest of Rats Primary Sertoli Cells Induced by Zearalenone. Toxins 2018, 10, 398. https://doi.org/10.3390/toxins10100398
Wang B-j, Zheng W-l, Feng N-n, Wang T, Zou H, Gu J-h, Yuan Y, Liu X-z, Liu Z-p, Bian J-c. The Effects of Autophagy and PI3K/AKT/m-TOR Signaling Pathway on the Cell-Cycle Arrest of Rats Primary Sertoli Cells Induced by Zearalenone. Toxins. 2018; 10(10):398. https://doi.org/10.3390/toxins10100398
Chicago/Turabian StyleWang, Bing-jie, Wang-long Zheng, Nan-nan Feng, Tao Wang, Hui Zou, Jian-hong Gu, Yan Yuan, Xue-zhong Liu, Zong-ping Liu, and Jian-chun Bian. 2018. "The Effects of Autophagy and PI3K/AKT/m-TOR Signaling Pathway on the Cell-Cycle Arrest of Rats Primary Sertoli Cells Induced by Zearalenone" Toxins 10, no. 10: 398. https://doi.org/10.3390/toxins10100398
APA StyleWang, B.-j., Zheng, W.-l., Feng, N.-n., Wang, T., Zou, H., Gu, J.-h., Yuan, Y., Liu, X.-z., Liu, Z.-p., & Bian, J.-c. (2018). The Effects of Autophagy and PI3K/AKT/m-TOR Signaling Pathway on the Cell-Cycle Arrest of Rats Primary Sertoli Cells Induced by Zearalenone. Toxins, 10(10), 398. https://doi.org/10.3390/toxins10100398




