Allelopathic and Bloom-Forming Picocyanobacteria in a Changing World
Abstract
1. Introduction
2. The Significance of Picocyanobacteria in Response to Global Change
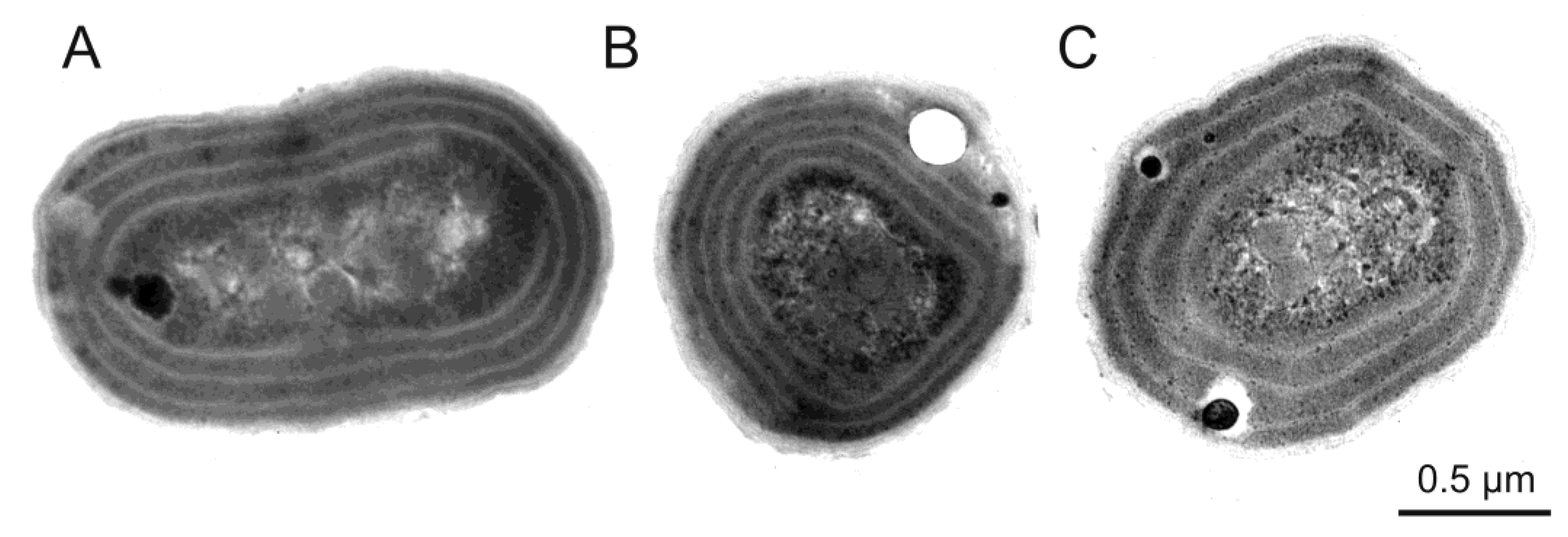
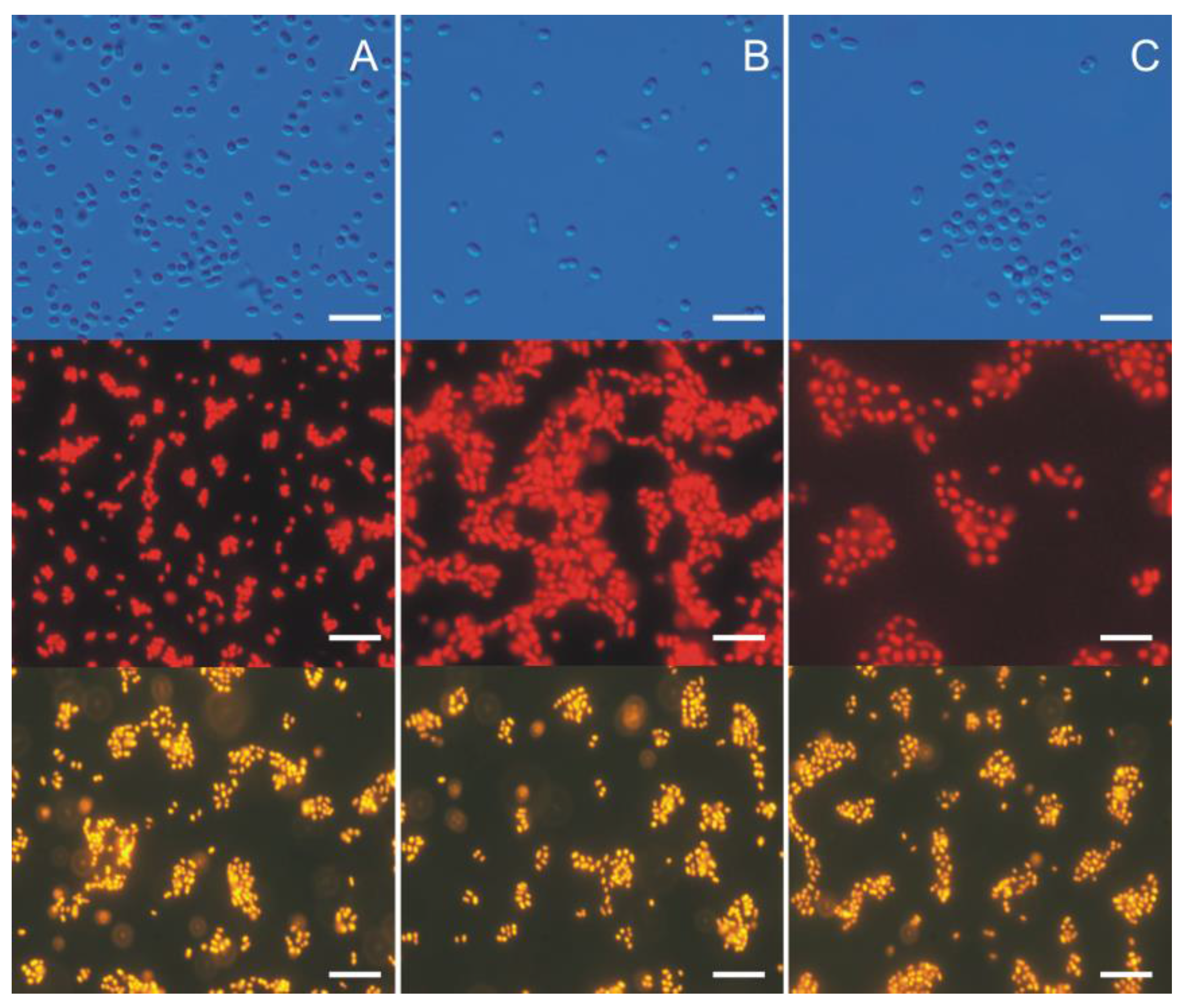
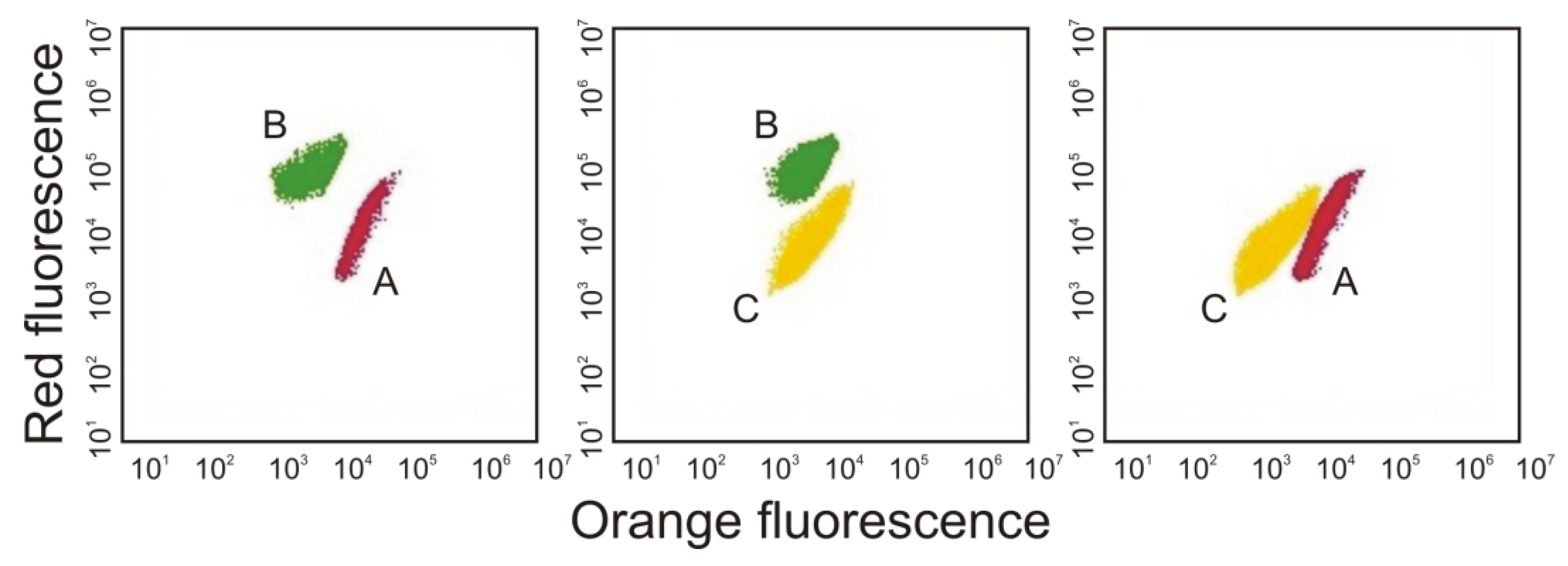
3. Morphological and Physiological Characteristics of Picoplanktonic Cyanobacteria
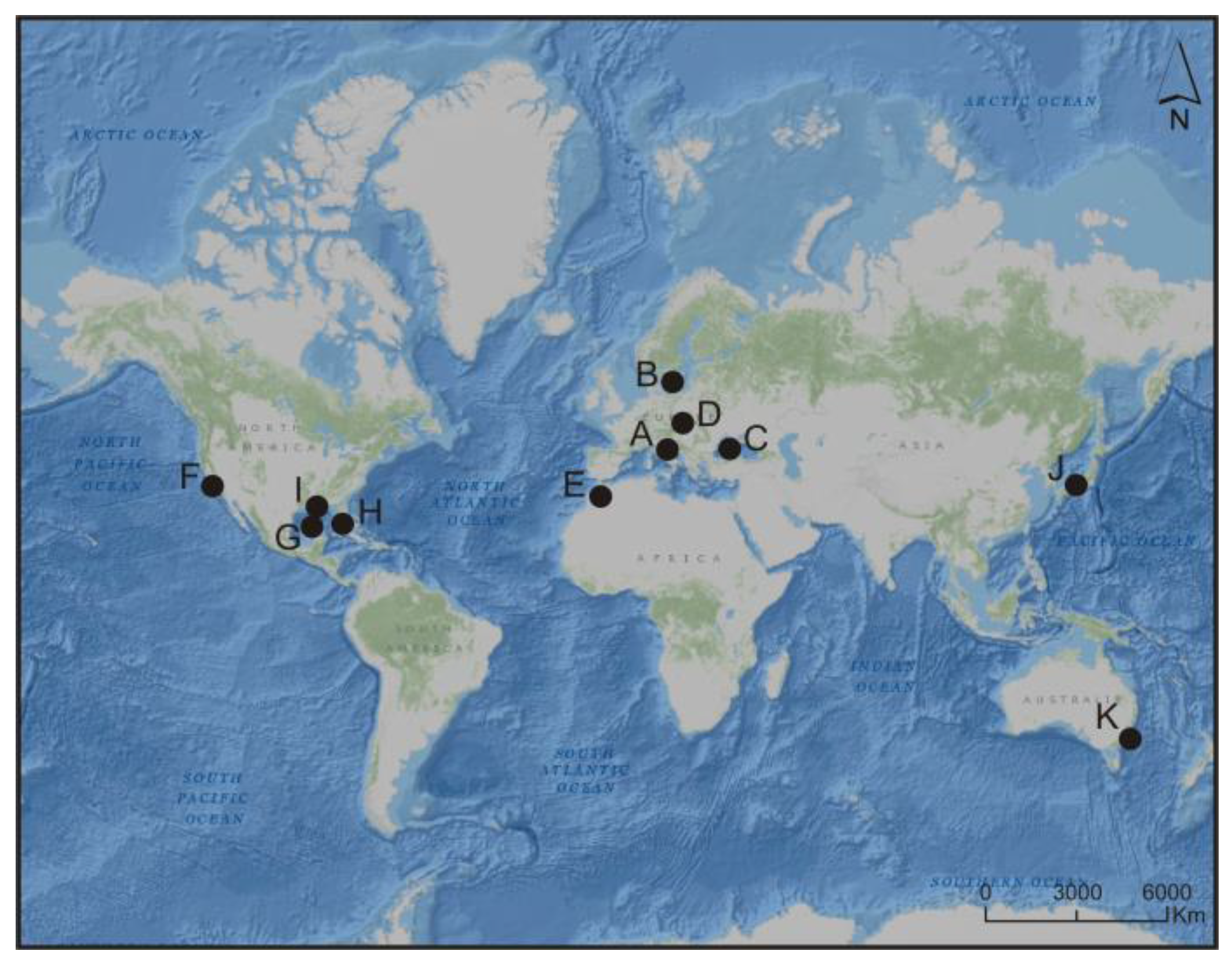
4. Blooms of Picocyanobacteria
5. Picocyanobacterial Secondary Metabolites
6. Allelopathic Activity of Picocyanobacteria and Their Impact on Aquatic Environment
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Callieri, C. Single cells and microcolonies of freshwater picocyanobacteria: A common ecology. J. Limnol. 2010, 69, 257–277. [Google Scholar] [CrossRef]
- Mazur-Marzec, H.; Sutryk, K.; Kobos, J.; Hebel, A.; Hohlfeld, N.; Błaszczyk, A.; Jasser, I. Occurrence of cyanobacteria and cyanotoxin in the Southern Baltic Proper. Filamentous cyanobacteria versus single-celled picocyanobacteria. Hydrobiologia 2013, 701, 235–252. [Google Scholar] [CrossRef]
- Worden, A.Z.; Wilken, S. A plankton bloom shifts as the ocean warms. Science 2016, 354, 287–288. [Google Scholar] [CrossRef] [PubMed]
- Flombaum, P.; Gallegos, J.L.; Gordillo, R.A.; Rincon, J.; Zabala, L.L.; Jiao, N.; Karl, D.M.; Li, W.K.; Lomas, M.W.; Veneziano, D. Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl. Acad. Sci. USA 2013, 110, 9824–9829. [Google Scholar] [CrossRef] [PubMed]
- Stockner, J.G. Phototrophic picoplankton: An overview from marine and freshwater ecosystems. Limnol. Oceanogr. 1988, 33, 765–775. [Google Scholar] [CrossRef]
- Suttle, C.A.; Harrison, P.J. Phosphate uptake rates of phytoplankton assemblages grown at different dilution rates in semi-continuous culture. Can. J. Fish Aquat. Sci. 1986, 43, 1474–1481. [Google Scholar] [CrossRef]
- Chow, T.J.; Tabita, F.R. Reciprocal light-dark transcriptional control of nif and rbc expression and light-dependent posttranslational control of nitrogenase activity in Synechococcus sp. strain RF-1. J. Bacteriol. 1994, 176, 6281–6285. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.L.; Jackson, G.A. Small phytoplankton and carbon export from the surface ocean. Science 2007, 315, 838–840. [Google Scholar] [CrossRef] [PubMed]
- Callieri, C.; Stockner, J.G. Freshwater autotrophic picoplankton: A review. J. Limnol. 2002, 61, 1–14. [Google Scholar] [CrossRef]
- Jasser, I.; Callieri, C. Picocyanobacteria. In Handbook on Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 19–27. [Google Scholar]
- Jyothibabu, R.; Mohan, A.P.; Jagadeeesan, L.; Anjusha, A.; Muraleedharan, K.R.; Lallu, K.R.; Kiran, K.; Ullas, N. Ecology and trophic preference of picoplankton and nanoplankton in the Gulf of Mannar and the Palk Bay, southeast coast of India. J. Mar. Syst. 2013, 111–112, 29–44. [Google Scholar] [CrossRef]
- Motwani, N.H.; Gorokhova, E. Mesozooplankton grazing on picocyanobacteria in the Baltic Sea as inferred from molecular diet analysis. PLoS ONE 2013, 8, e79230. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, P.Y.; Sorokin, Y.I.; Boscolo, R.; Giovanardi, O. Bloom of picocyanobacteria in the Venice lagoon during summer-autumn 2001: Ecological sequences. Hydrobiologia 2004, 523, 71–85. [Google Scholar] [CrossRef]
- Parvathi, A.; Zhong, X.; Ram, A.P.; Jacquet, S. Dynamics of auto- and heterotrophic picoplankton and associated viruses in Lake Geneva. Hydrol. Earth. Syst. Sci. 2014, 18, 1073–1087. [Google Scholar] [CrossRef]
- Sorokin, Y.I.; Dallocchio, F. Dynamics of phosphorus in the Venice lagoon during a picocyanobacteria bloom. J. Plankton Res. 2008, 30, 1019–1026. [Google Scholar] [CrossRef]
- Sánchez-Baracaldo, P.; Handley, B.A.; Hayes, P.K. Picocyanobacterial community structure of freshwater lakes and the Baltic Sea revealed by phylogenetic analyses and clade-specificquantitative PCR. Microbiology 2008, 154, 3347–3357. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, S.; Morris, J.J.; Follows, M.J.; Scott, J.; Levitan, O.; Dyhrman, S.T.; Berman-Frank, I. Impact of ocean acidification on the structure of future phytoplankton communities. Nat. Clim. Chang. 2015, 5, 1002–1006. [Google Scholar] [CrossRef]
- Beardall, J. Blooms of Synechococcus: An Analysis of the Problem Worldwide and Possible Causative Factors in Relation to Nuisance Blooms in the Gippsland Lakes; Monash University: Clayton, VIC, Australia, 2008; pp. 1–8. [Google Scholar]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Brutemark, A.; Vandelannoote, A.; Engström-Öst, J.; Suikkanen, S. A less saline Baltic Sea promotes cyanobacterial growth, hampers intracellular microcystin production and leads to strain-specific differences in allelopathy. PLoS ONE 2015, 10, e0128904. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Jodłowska, S.; Śliwińska, S. Effects of light intensity and temperature on the photosynthetic irradiance response curves and chlorophyll fluorescence in three picocyanobacterial strains of Synechococcus. Photosynthetica 2014, 52, 223–232. [Google Scholar] [CrossRef]
- Ibelings, B.W. Changes in photosynthesis in response to combined irradiance and temperature stress in cyanobacterial surface waterblooms. J. Phycol. 1996, 32, 549–557. [Google Scholar] [CrossRef]
- Antia, N.J.; Cheng, J.Y. The survival of axenic cultures of marine planktonic algae from prolonged exposure to darkness at 20 °C. Phycologia 1970, 9, 179–183. [Google Scholar] [CrossRef]
- Antia, N.J. Effects of temperature on the darkness survival of marine microplanktonic algae. Microb. Ecol. 1976, 3, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Kana, T.M.; Glibert, P.M. Effect of irradiances up to 2000 μE m−2 s−1 on marine Synechococcus WH7803-I. Growth, pigmentation and cell composition. Deep Sea Res. Part I 1987, 34, 479–495. [Google Scholar] [CrossRef]
- Stal, L.J.; Albertan, P.; Bergman, B.; Von Bröckel, K.; Gallon, J.R.; Hayes, P.K.; Walsby, A.E. BASIC: Baltic Sea cyanobacteria. An investigation of the structure and dynamics of water blooms of cyanobacteria in the Baltic Sea—Responses to a changing environment. Cont. Shelf Res. 2003, 23, 1695–1714. [Google Scholar] [CrossRef]
- Śliwińska-Wilczewska, S.; Pniewski, F.; Latała, A. Allelopathic activity of the picocyanobacterium Synechococcus sp. under varied light, temperature and salinity conditions. Int. Rev. Hydrobiol. 2016, 101, 69–77. [Google Scholar] [CrossRef]
- Śliwińska-Wilczewska, S.; Pniewski, F.; Latała, A. Allelopathic interactions between Synechococcus sp. and Nodularia spumigena under different light conditions. Allelopath. J. 2016, 37, 241–252. [Google Scholar]
- Murrell, M.C.; Lores, E.M. Phytoplankton and zooplankton seasonal dynamics in a subtropical estuary: Importance of cyanobacteria. J. Plankton Res. 2004, 26, 371–382. [Google Scholar] [CrossRef]
- Bec, B.; Husseini-Ratrema, J.; Collos, Y.; Souchu, P.; Vaquer, A. Phytoplankton seasonal dynamics in a Mediterranean coastal lagoon: Emphasis on the picoeukaryote community. J. Plankton Res. 2005, 27, 881–894. [Google Scholar] [CrossRef]
- Paul, V.J. Global warming and cyanobacterial harmful algal booms. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Advances in Experimental Medicine and Biology; Hudnell, K.H., Ed.; Springer: New York, NY, USA, 2008; pp. 239–257. [Google Scholar]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Eviron. Microb. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Houghton, J.T.; Ding, Y.; Griggs, D.J.; Noguer, M.; Van der Lin-den, P.J.; Dai, X.; Maskell, K.; Johnson, C.A. Climate Change 2001: The Scientific Basis; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Moore, L. Comparative physiology of Synechococcus and Prochlorococcus: Influence of light and temperature on growth, pigments, fluorescence and absorptive properties. Mar. Ecol. Prog. Ser. 1995, 116, 259–275. [Google Scholar] [CrossRef]
- Gazeau, F.; Sallon, A.; Maugendre, L.; Louis, J.; Dellisanti, W.; Gaubert, M.; Lejeune, P.; Gobert, S.; Borges, A.V.; Harlay, J.; et al. First mesocosm experiments to study the impacts of ocean acidification on plankton communities in the NW Mediterranean Sea (MedSeA project). Estuar. Coast. Shelf Sci. 2017, 186, 11–29. [Google Scholar] [CrossRef]
- Ahlgren, N.A.; Rocap, G. Diversity and distribution of marine Synechococcus: Multiple gene phylogenies for consensus classification and development of qPCR assays for sensitive measurement of clades in the ocean. Front. Microbiol. 2012, 3, 213. [Google Scholar] [CrossRef] [PubMed]
- Stomp, M.; Huisman, J.; Vörös, L.; Pick, F.R.; Laamanen, M.; Haverkamp, T.; Stal, L.J. Colourful coexistence of red and green picocyanobacteria in lakes and seas. Ecol. Lett. 2007, 10, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp, T.; Acinas, S.G.; Doeleman, M.; Stomp, M.; Huisman, J.; Stal, L.J. Diversity and phylogeny of Baltic Sea picocyanobacteria inferred from their ITS and phycobiliprotein operons. Environ. Microbiol. 2008, 10, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp, T.H.; Schouten, D.; Doeleman, M.; Wollenzien, U.; Huisman, J.; Stal, L.J. Colorful microdiversity of Synechococcus strains (picocyanobacteria) isolated from the Baltic Sea. ISME J. 2009, 3, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Everroad, R.C.; Wood, A.M. Comparative molecular evolution of newly discovered picocyanobacterial strains reveals a phylogenetically informative variable region of beta-phycoerythrin. J. Phycol. 2006, 42, 1300–1311. [Google Scholar] [CrossRef]
- Vörös, L.; Callieri, C.; Katalin, V.; Bertoni, R. Freshwater picocyanobacteria along a trophic gradient and light quality range. In Phytoplankton and Trophic Gradients; Springer: Dordrecht, The Netherlands, 1998; pp. 117–125. [Google Scholar]
- Rippka, R. Isolation and purification of cyanobacteria. Methods Enzymol. 1988, 167, 3–27. [Google Scholar] [PubMed]
- Larsson, J.; Celepli, N.; Ininbergs, K.; Dupont, C.L.; Yooseph, S.; Bergman, B.; Ekman, M. Picocyanobacteria containing a novel pigment gene cluster dominate the brackish water Baltic Sea. ISME J. 2014, 8, 1892–1903. [Google Scholar] [CrossRef] [PubMed]
- Paz-Yepes, J.; Brahamsha, B.; Palenik, B. Role of a Microcin-C-like biosynthetic gene cluster in allelopathic interactions in marine Synechococcus. Proc. Natl. Acad. Sci. USA 2013, 110, 12030–12035. [Google Scholar] [CrossRef] [PubMed]
- Stomp, M.; Huisman, J.; de Jongh, F.; Veraart, A.J.; Gerla, D.; Rijkeboer, M.; Ibelings, B.W.; Wollenzien, U.I.A.; Stal, L.J. Adaptive divergence in pigment composition promotes phytoplankton biodiversity. Nature 2004, 432, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in understanding harmful algal blooms: Paradigm shifts and new technologies for research, monitoring and management. Annu. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef] [PubMed]
- Stockner, J.; Callieri, C.; Cronberg, G. Picoplankton and other non-bloom forming cyanobacteria in lakes. In The Ecology of Cyanobacteria: Their Diversity in Time and Space; Whitton, B., Potts, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 195–238. [Google Scholar]
- Sorokin, Y.I.; Zakuskina, O.Y. Features of the Comacchio ecosystem transformed during persistent bloom of picocyanobacteria. J. Oceanogr. 2010, 66, 373–387. [Google Scholar] [CrossRef]
- Kuosa, H. Picoplanktonic algae in the northern Baltic Sea: Seasonal dynamics and flagellate grazing. Mar. Ecol. Prog. Ser. 1991, 73, 269–276. [Google Scholar] [CrossRef]
- Uysal, Z. Pigments, size and distribution of Synechococcus spp. in the Black Sea. J. Mar. Syst. 2000, 24, 313–326. [Google Scholar] [CrossRef]
- Somogyi, B.; Felföldi, T.; Vanyovszki, J.; Ágyi, Á.; Márialigeti, K.; Vörös, L. Winter bloom of picoeukaryotes in Hungarian shallow turbid soda pans and the role of light and temperature. Aquat. Ecol. 2009, 43, 735–744. [Google Scholar] [CrossRef]
- Mezrioui, N.; Oudra, B. Dynamics of picoplankton and microplankton flora in the experimental wastewater stabilisation ponds in the arid region of Marrakech, Morocco and Cyanobacteria effect on Escherichia coli and Vibrio cholerae survival. In Wastewater Treatment with Algae; Wong, Y.S., Tam, N.F.Y., Eds.; Springer-Verlag/Landes Bioscience: New York, NY, USA, 1998; pp. 165–188. [Google Scholar]
- Oudra, B.; El-Andaloussi-Dadi, M.; Franca, S.; Barros, P.; Martins, R.; Oufdou, K.; Sbiyyaa, B.; Loudiki, M.; Mezrioui, N.; Vasconcelos, V. Harmful cyanobacterial toxic blooms in waste stabilisation ponds. Water Sci. Technol. 2000, 42, 179–186. [Google Scholar]
- Ning, X.; Cloern, J.E.; Cole, B.E. Spatial and temporal variability of picocyanobacteria Synechococcus sp. in San Francisco Bay. Limnol. Oceanogr. 2000, 45, 695–702. [Google Scholar] [CrossRef]
- Wawrik, B.; Paul, J.H. Phytoplankton community structure and productivity along the axis of the Mississippi River plume in oligotrophic Gulf of Mexico waters. Aquat. Microb. Ecol. 2004, 35, 185–196. [Google Scholar] [CrossRef]
- Phlips, E.J.; Badylak, S.; Lynch, T.C. Blooms of the picoplanktonic cyanobacterium Synechococcus in Florida Bay, a subtropical inner-shelf lagoon. Limnol. Oceanogr. 1999, 14, 1166–1175. [Google Scholar] [CrossRef]
- Gardner, W.S.; McCarthy, M.J. Nitrogen dynamics at the sediment–water interface in shallow, sub-tropical Florida Bay: Why denitrification efficiency may decrease with increased eutrophication. Biogeochemistry 2009, 95, 185–198. [Google Scholar] [CrossRef]
- Nakamura, Y.; Suzuki, K.; Suzuki, S.Y.; Hiromi, J. Production of Oikopleura dioica (Appendicularia) following a picoplankton ‘bloom’ in a eutrophic coastal area. J. Plankton Res. 1997, 19, 113–124. [Google Scholar] [CrossRef]
- Passoni, S.; Callieri, C. Picocyanobacteria single forms aggregated and microcolonies. Verh. Internat. Verein Limnol. 2000, 27, 1879–1883. [Google Scholar]
- Komárek, J. Taxonomic and species delineation of picoplanktonic cyanoprocaryotes. Algol. Stud. 1996, 83, 119–179. [Google Scholar]
- Śliwińska-Wilczewska, S.; Maculewicz, J.; Barreiro Felpeto, A.; Vasconcelos, V.; Latała, A. Allelopathic activity of the picocyanobacterium Synechococcus sp. on filamentous cyanobacteria. J. Exp. Mar. Biol. Ecol. 2017, 496, 16–21. [Google Scholar] [CrossRef]
- Śliwińska-Wilczewska, S.; Maculewicz, J.; Tuszer, J.; Dobosz, K.; Kalusa, D.; Latała, A. First record of allelopathic activity of the picocyanobacterium Synechococcus sp. on a natural plankton community. Ecohydrol. Hydrobiol. 2017, 17, 227–234. [Google Scholar] [CrossRef]
- Mazur-Marzec, H. Characterization of phycotoxins produced by cyanobacteria. Oceanol. Hydrobiol. Stud. 2006, 35, 85–109. [Google Scholar]
- Vareli, K.; Jaeger, W.; Touka, A.; Frillingos, S.; Briasoulis, E.; Sainis, I. Hepatotoxic seafood poisoning (HSP) due to microcystins: A threat from the ocean? Mar. Drugs 2013, 11, 2751–2768. [Google Scholar] [CrossRef] [PubMed]
- Pearson, L.; Mihali, T.; Moffitt, M.; Kellmann, R.; Neilan, B. On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopsin. Mar. Drugs 2010, 8, 1650–1680. [Google Scholar] [CrossRef] [PubMed]
- Leão, P.N.; Engene, N.; Antunes, A.; Gerwick, W.H.; Vasconcelos, V. The chemical ecology of cyanobacteria. Nat. Prod. Rep. 2012, 29, 372–391. [Google Scholar] [CrossRef] [PubMed]
- Testai, E.; Scardala, S.; Vichi, S.; Buratti, F.M.; Funari, E. Risk to human health associated with the environmental occurrence of cyanobacterial neurotoxic alkaloids anatoxins and saxitoxins. Crit. Rev. Toxicol. 2016, 46, 1–35. [Google Scholar]
- Lincoln, E.P.; Carmichael, W.W. Preliminary tests of toxicity of Synechocystis sp. growth on wastewater medium. In The Water Environment: Algal Toxins and Health; Carmichael, W.W., Ed.; Plenum Press: New York, NY, USA, 1981; pp. 223–230. [Google Scholar]
- Mitsui, A.; Rosner, D.; Goodman, A.; Reyes-Vasquez, G.; Kusumi, T.; Kodama, T.; Nomoto, K. Hemolytic toxins in a marine cyanobacterium Synechococcus sp. In Proceedings of the International Red Tide Symposium, Takamatsu, Japan, 10–14 November 1987. [Google Scholar]
- Domingos, P.; Rubim, T.K.; Molica, R.J.R.; Azevedo, S.M.F.O.; Carmichael, W.W. First report of microcystin production by picoplanktonic cyanobacteria isolated from a northeast Brazilian drinking water supply. Environ. Toxicol. 1999, 14, 31–35. [Google Scholar] [CrossRef]
- Bláha, L.; Maršálek, B. Microcystin production and toxicity of picocyanobacteria as a risk factor for drinking water treatment plants. Algol. Stud. 1999, 92, 95–108. [Google Scholar]
- Carmichael, W.W.; Li, R. Cyanobacteria toxins in the Salton Sea. Saline Syst. 2006, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Gantar, M.; Sekar, R.; Richardson, L.L. Cyanotoxins from black band disease of corals and from other coral reef environments. Microb. Ecol. 2009, 58, 856–864. [Google Scholar] [PubMed]
- Martins, R.; Pereira, P.; Welker, M.; Fastner, J.; Vasconcelos, V.M. Toxicity of culturable cyanobacteria strains isolated from the Portuguese coast. Toxicon 2005, 46, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Furtado, A.L.F.F.; do Carmo Calijuri, M.; Lorenzi, A.S.; Honda, R.Y.; Genuário, D.B.; Fiore, M.F. Morphological and molecular characterization of cyanobacteria from a Brazilian facultative wastewater stabilization pond and evaluation of microcystin production. Hydrobiologia 2009, 627, 195–209. [Google Scholar] [CrossRef]
- Oudra, B.; Loudiki, M.; Vasconcelos, V.; Sabour, B.; Sbiyyaa, B.; Oufdou, K.; Mezrioui, N. Detection and quantification of microcystins from cyanobacteria strains isolated from reservoirs and ponds in Morocco. Environ. Toxicol. 2002, 17, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Vareli, K.; Zarali, E.; Zacharioudakis, G.S.A.; Vagenas, G.; Varelis, V.; Pilidis, G.; Briasoulis, E.; Sainis, I. Microcystin producing cyanobacterial communities in Amvrakikos Gulf (Mediterranean Sea, NW Greece) and toxin accumulation in mussels (Mytilus galloprovincialis). Harmful Algae 2012, 15, 109–118. [Google Scholar] [CrossRef]
- Jakubowska, N.; Szeląg-Wasielewska, E. Toxic Picoplanktonic Cyanobacteria-Review. Mar. Drugs 2015, 13, 1497–1518. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce β-N-methylamino-l-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [PubMed]
- Cianca, R.C.C.; Baptista, M.S.; Lopes, V.R.; Vasconcelos, V.M. The non-protein amino acid β-N-methylamino-l-alanine in Portuguese cyanobacterial isolates. Amino Acids 2012, 42, 2473–2479. [Google Scholar]
- Cox, P.A.; Banack, S.A.; Murch, S.J. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc. Natl. Acad. Sci. USA 2003, 100, 13380–13383. [Google Scholar] [PubMed]
- Graham, J.L.; Loftin, K.A.; Ziegler, A.C.; Meyer, M.T. Guidelines for Design and Sampling for Cyanobacterial Toxin and Taste-and-Odor Studies in Lakes and Reservoirs; U.S. Geological Survey: Reston, VA, USA, 2008.
- Journey, C.A.; Beaulieu, K.M.; Bradley, P.M. Environmental factors that influence cyanobacteria and geosmin occurrence in reservoirs. In Current Perspectives in Contaminant Hydrology and Water Resources Sustainability; Bradley, P.M., Ed.; Tech Online: Rijeka, Croatia, 2013. [Google Scholar]
- Snyder, D.S.; Brahamsha, B.; Azadi, P.; Palenik, B. Structure of compositionally simple lipopolysaccharide from marine Synechococcus. J. Bacteriol. 2009, 191, 5499–5509. [Google Scholar] [PubMed]
- Schmidt, W.; Drews, G.; Weckesser, J.; Fromme, I. Characterization of the lipopolysaccharides from eight strains of the cyanobacterium Synechococcus. Arch. Microbiol. 1980, 127, 209–215. [Google Scholar]
- Schmidt, W.; Drews, G.; Weckesser, J.; Mayer, H. Lipopolysaccharides in four strains of the unicellular cyanobacterium Synechocystis. Arch. Microbiol. 1980, 127, 217–222. [Google Scholar]
- Best, J.H.; Pflugmacher, S.; Wiegand, C.; Eddy, F.B.; Metcalf, J.S.; Codd, G.A. Effects of enteric bacterial and cyanobacterial lipopolysaccharides and of microcystin-LR, on glutathione S-transferase activities in zebra fish (Danio rerio). Aquat. Toxicol. 2002, 60, 223–231. [Google Scholar] [PubMed]
- Kunimitsu, K.; Tomoharu, S.; Fujio, S.; Hiroyasu, I. Thioic O-acid ester in sulfolipid isolated from freshwater picoplankton cyanobacterium, Synechococcus sp. Biochim. Biophys. Acta 1993, 1169, 39–45. [Google Scholar]
- Liu, H.; Mishima, Y.; Fujiwara, T.; Nagai, H.; Kitazawa, A.; Mine, Y.; Kobayashi, H.; Yao, X.; Yamada, J.; Oda, T.; et al. Isolation of araguspongine M, a new stereoisomer of an araguspongine/xestospongin alkaloid and dopamine from the marine sponge Neopetrosia exigua collected in Palau. Mar. Drugs 2004, 2, 154–163. [Google Scholar]
- Ito, Y.; Butler, A. Structure of synechobactins, new siderophores of the marine cyanobacterium Synechococcus sp. PCC 7002. Limnol. Oceanogr. 2005, 50, 1918–1923. [Google Scholar]
- Liu, X.; Sheng, J.; Curtiss, R., III. Fatty acid production in genetically modified cyanobacteria. Proc. Natl. Acad. Sci. USA 2011, 108, 6899–6904. [Google Scholar] [CrossRef] [PubMed]
- Figueredo, C.C.; Giani, A.; Bird, D.F. Does allelopathy contribute to Cylindrospermopsis raciborskii (cyanobacteria) bloom occurrence and geographic expansion? J. Phycol. 2007, 43, 256–265. [Google Scholar] [CrossRef]
- Żak, A.; Kosakowska, A. The influence of extracellular compounds produced by selected Baltic cyanobacteria, diatoms and dinoflagellates on growth of green algae Chlorella vulgaris. Estuar. Coast. Shelf. Sci. 2015, 167, 113–118. [Google Scholar] [CrossRef]
- Allen, J.L.; Ten-Hage, L.; Leflaive, J. Regulation of Fatty Acid Production and Release in Benthic Algae: Could Parallel Allelopathy Be Explained with Plant Defence Theories? Microb. Ecol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, A.; Roy, S.; Vasconcelos, V.M. Allelopathy prevents competitive exclusion and promotes phytoplankton biodiversity. Oikos 2017. [Google Scholar] [CrossRef]
- Dias, F.; Antunes, J.T.; Ribeiro, T.; Azevedo, J.; Vasconcelos, V.; Leão, P.N. Cyanobacterial Allelochemicals But Not Cyanobacterial Cells Markedly Reduce Microbial Community Diversity. Front. Microbiol. 2017, 8, 1495. [Google Scholar] [CrossRef] [PubMed]
- Pichierri, S.; Accoroni, S.; Pezzolesi, L.; Guerrini, F.; Romagnoli, T.; Pistocchi, R.; Totti, C. Allelopathic effects of diatom filtrates on the toxic benthic dinoflagellate Ostreopsis cf. ovata. Mar. Environ. Res. 2017, 131, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zi, J.; Xu, R.; Hilt, S.; Hou, X.; Chang, X. Allelopathic effects of Microcystis aeruginosa on green algae and a diatom: Evidence from exudates addition and co-culturing. Harmful Algae 2017, 61, 56–62. [Google Scholar] [CrossRef]
- Martins, R.F.; Ramos, M.F.; Herfindal, L.; Sousa, J.A.; Skærven, K.; Vasconcelos, V.M. Antimicrobial and cytotoxic assessment of marine cyanobacteria—Synechocystis and Synechococcus. Mar. Drugs 2008, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Garcia, M.; Costa-Rodrigues, J.; Costa, M.S.; Ribeiro, M.J.; Fernandes, M.H.; Barros, P.; Barreiro, A.; Vasconcelos, V.; Martins, R. Exploring bioactive properties of marine cyanobacteria isolated from the Portuguese coast: High potential as a source of anticancer compounds. Mar. Drugs 2014, 12, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Noaman, N.H.; Fattah, A.; Khaleafa, M.; Zaky, S.H. Factors affecting antimicrobial activity of Synechococcus leopoliensis. Microbiol. Res. 2004, 159, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.F.; Fernandez, N.; Beiras, R.; Vasconcelos, V.M. Toxicity assessment of crude and partially purified extracts of marine Synechocystis and Synechococcus cyanobacterial strains in marine invertebrates. Toxicon 2007, 50, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Frazão, B.; Martins, R.; Vasconcelos, V. Are known cyanotoxins involved in the toxicity of picoplanktonic and filamentous north atlantic marine cyanobacteria? Mar. Drugs 2010, 8, 1908–1919. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.S.; Costa, M.; Ramos, V.; Leão, P.N.; Barreiro, A.; Vasconcelos, V.; Martins, R. Picocyanobacteria from a clade of marine cyanobium revealed bioactive potential against microalgae, bacteria and marine invertebrates. Toxicol. Environ. Health Part A 2015, 78, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.J.; Paz-Yepes, J.; Morrison, R.A.; Palenik, B.; Tresguerres, M. Exposure to bloom-like concentrations of two marine Synechococcus cyanobacteria (strains CC9311 and CC9902) differentially alters fish behaviour. Conserv. Physiol. 2014, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Species (Strain) | Location/Habitat | Secondary Metabolites | Source |
|---|---|---|---|
| Aphanocapsa cumulus | Caruaru reservoirs (Brazil)/freshwater | MC | [71] |
| Synechococcus nidulans | Unknown/freshwater | MC | [72] |
| Cyanobium rubescens | Unknown/freshwater | MC | [72] |
| Cyanobacterium cedrorum | Unknown/freshwater | MC | [72] |
| Synechococcus sp. (SS-1) | Salton Sea (California)/marine | MC | [73] |
| Synechococcus sp. (63a-1 and 63a-3) | Florida Keys (Atlantic Ocean)/marine | MC | [74] |
| Synechococcus sp. | Portuguese coast (Atlantic Ocean)/marine | MC | [75] |
| Synechocystis sp. | Portuguese coast (Atlantic Ocean)/marine | MC | [75] |
| Synechococcus sp. (CENA108) | Cajati (Brazil)/freshwater | MC | [76] |
| Merismopedia sp. (CENA106) | Cajati (Brazil)/freshwater | MC | [76] |
| Synechocystis sp. (Syn-WTP93 and Syn-WTP97) | Biological wastewater treatment plant (Morocco)/freshwater | MC | [77] |
| Synechococcus sp. | Amvrakikos Gulf (Mediterranean Sea)/marine | MC | [78] |
| Synechocystis sp. | Amvrakikos Gulf (Mediterranean Sea)/marine | MC | [78] |
| Synechococcus sp. (PCC 6301) | USA/freshwater | BMAA | [80] |
| Prochlorococcus marinus (CCMP1377) | Sargasso Sea (Atlantic Ocean)/marine | BMAA | [80] |
| Cyanobium sp. (LEGE 06068) | Douro estuary/brackish | BMAA | [81] |
| Synechocystis salina (LEGE 06079) | Douro estuary/brackish | BMAA | [81] |
| Synechocystis cf. salina (LEGE 06083) | Douro estuary/brackish | BMAA | [81] |
| Synechocystis cf. salina (LEGE 07073) | Vouga estuary/brackish | BMAA | [81] |
| Synechococcus sp. (LEGE 07074) | Douro estuary/brackish | BMAA | [81] |
| Synechococcus sp. | Lake Bowen and Municipal Reservoir #1 (USA)/freshwater | MIB | [83,84] |
| Synechococcus sp. | Lake Bowen and Municipal Reservoir #1 (USA)/freshwater | GSM | [83,84] |
| Synechococcus sp. (PCC 6907, 6307, 6911, 6603, 6908, 6311, 6312, 6910) | France/freshwater | LPS | [86] |
| Synechocystis sp. (PCC 6714, 6803, 6807, 6308) | France/freshwater | LPS | [87] |
| Synechococcus sp. (WH8102 and CC9311) | Carribean Sea (Atlantic Ocean) and Pacific Ocean/marine | LPS | [85] |
| Synechococcus sp. | Unknown | thionsulfolipid | [89] |
| endosymbiotic Synechococcus-like cyanobacterium | marine sponge Neopetrosia exigua, Palau (Pacific Ocean)/marine | araguspongine M | [90] |
| Synechococcus sp. (PCC 7002) | USA/marine | synechobactins A–C | [91] |
| Synechocystis sp. (PCC6803/SD100) | France/freshwater | fatty acids | [92] |
| Donor Species (Strain) | Target Species | Effect | Source |
|---|---|---|---|
| Synechococcus sp. (CC9311) | Synechococcus sp. (WH8102) | − | [45] |
| Synechococcus sp. (WH8102) | Synechococcus sp. (CC9311) | 0 | [45] |
| Synechococcus sp. (CC9605) | Synechococcus sp. (CC9311), Synechococcus sp. (WH8102) | − | [45] |
| Synechococcus sp. (CC9311), Synechococcus sp. (WH8102) | Synechococcus sp. (CC9605) | 0 | [45] |
| Synechococcus sp. (CCBA-124) | Navicula perminuta | − | [28] |
| Synechococcus sp. (CCBA-124) | Nodularia spumigena | − | [29] |
| Synechococcus sp. (CCBA-124) | Nostoc sp., Phormidium sp. | − | [62] |
| Synechococcus sp. (CCBA-124) | Aphanizomenon flos-aquae | + | [62] |
| Synechococcus sp. (CCBA-124) | Rivularia sp. | 0 | [62] |
| Synechococcus sp. (CCBA-124) | Navicula sp., Chaetoceros sp., Amphora sp., Coscinodiscus sp., Grammatophora sp., Nitzschia sp. | − | [63] |
| Synechocystis sp. (LEANCYA 1, 5, 13, 15, 17, 20, 21) and Synechococcus sp. (LEANCYA 7, 10, 11, 16, 18, 19, 22) | Candida albicans | 0 | [100] |
| Synechocystis sp. (LEANCYA 1, 5, 13, 15, 17, 20, 21) and Synechococcus sp. (LEANCYA 7, 10, 11, 16, 18, 19, 22) | Cellulomonas uda, Clavibacter michiganensis subsp. insidiosum | − | [100] |
| Synechocystis sp. (LEANCYA 1, 5, 13, 15, 17, 20, 21) and Synechococcus sp. (LEANCYA 7, 10, 11, 16, 18, 19, 22) | Aeromonas hydrophila, A. salmonicida subsp. salmonicida, Bacillus cereus, B. megaterium, Enterobacter cloacae, Escherichia coli, Halomonas aquamarina, H. pacifica, Micrococcus luteus, Photobacterium damselae subsp. piscicida, Proteus vulgaris, Pseudomonas doudoroff, Staphylococcus epidermidis, S. aureus, S. parauberis, Thiobacillus thioparus, Vibrio compbelli, V. harveyi, V. natriegens, V. parahemolyticus, V. fluvialis, V. tubiashii, V. vulnificus, Yersinia ruckeri | 0 | [100] |
| Synechocystis sp. (LEANCYA 5, 13, 17, 20, 21) and Synechococcus sp. (LEANCYA 11, 16, 18, 19) | primary rat hepatocytes and HL-60 cells | − | [100] |
| Cyanobium sp. (LEGE 06098, 06134, 07175, 07186, 06113, 06137, 006097, 06139) | human cancer cell lines | − | [101] |
| Synechococcus nidulans (LEGE 07171) | human cancer cell lines | − | [101] |
| Synechococcus sp. (LEGE 07172, 06005, 06026), | human cancer cell lines | − | [101] |
| Synechocystis salina (LEGE 06099, 06155, 07173) | human cancer cell lines | − | [101] |
| endosymbiotic Synechococcus-like cyanobacterium | human leukemia cell line HL-60 | − | [90] |
| endosymbiotic Synechococcus-like cyanobacterium | Escherichia coli, Staphylococcus aureus, Saccharomyces cerevisiae, Mucor hiemalis, Ruegeria atlantica | 0 | [90] |
| Synechococcus leopoliensis (Utex 625) | Staphylococcus aureus | − | [102] |
| Synechocystis sp. (LEANCYA 1, 5, 13, 17, 20, 21) and Synechococcus sp. (LEANCYA 7, 10, 11, 16, 18, 19) | Artemia salina | − | [103] |
| Synechocystis sp. (LEANCYA 1, 5, 13, 17, 20, 21) and Synechococcus sp. (LEANCYA 7, 10, 11, 16, 18, 19) | Brachionus plicatillis | 0 | [103] |
| Synechocystis sp. (LEANCYA 1, 5, 17, 20, 21) and Synechococcus sp. (LEANCYA 7, 11, 16, 18, 19) | Paracentrotus lividus | − | [103] |
| Synechocystis sp. (LEANCYA 21) and Synechococcus sp. (LEANCYA 16) | Mytilus galloprovincialis | − | [103] |
| Cyanobium sp. (LEGE 06008, LEGE 06011 and LEGE 06015) | Artemia salina | − | [104] |
| Synechococcus sp. (LEGE 06005) | Artemia salina | − | [104] |
| Cyanobium sp. (LEGE 06098, 06134, 06139, 07175, 07186) | Artemia salina | 0 | [105] |
| Cyanobium sp. (LEGE 06098, 06134, 06139, 07175, 07186) | eggs of the sea urchin Paracentrotus lividus | − | [105] |
| Cyanobium sp. (LEGE 06098, 06134, 06139, 07175, 07186) | Pseudomonas sp. | − | [105] |
| Cyanobium sp. (LEGE 06098, 06134, 06139, 07175, 07186) | Nannochloropsis sp. | − | [105] |
| Synechococcus sp. (CC9311) | Embiotoca jacksoni | − | [106] |
| Synechococcus sp. (CC9902) | Embiotoca jacksoni | 0 | [106] |
| Cyanobacterium sp., Synechococcus sp., Synechocystis sp. | liver, kidney, small intestine and lungs of mice | − | [75] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śliwińska-Wilczewska, S.; Maculewicz, J.; Barreiro Felpeto, A.; Latała, A. Allelopathic and Bloom-Forming Picocyanobacteria in a Changing World. Toxins 2018, 10, 48. https://doi.org/10.3390/toxins10010048
Śliwińska-Wilczewska S, Maculewicz J, Barreiro Felpeto A, Latała A. Allelopathic and Bloom-Forming Picocyanobacteria in a Changing World. Toxins. 2018; 10(1):48. https://doi.org/10.3390/toxins10010048
Chicago/Turabian StyleŚliwińska-Wilczewska, Sylwia, Jakub Maculewicz, Aldo Barreiro Felpeto, and Adam Latała. 2018. "Allelopathic and Bloom-Forming Picocyanobacteria in a Changing World" Toxins 10, no. 1: 48. https://doi.org/10.3390/toxins10010048
APA StyleŚliwińska-Wilczewska, S., Maculewicz, J., Barreiro Felpeto, A., & Latała, A. (2018). Allelopathic and Bloom-Forming Picocyanobacteria in a Changing World. Toxins, 10(1), 48. https://doi.org/10.3390/toxins10010048





