Molecular and Physiological Effects on the Small Intestine of Weaner Pigs Following Feeding with Deoxynivalenol-Contaminated Feed
Abstract
1. Introduction
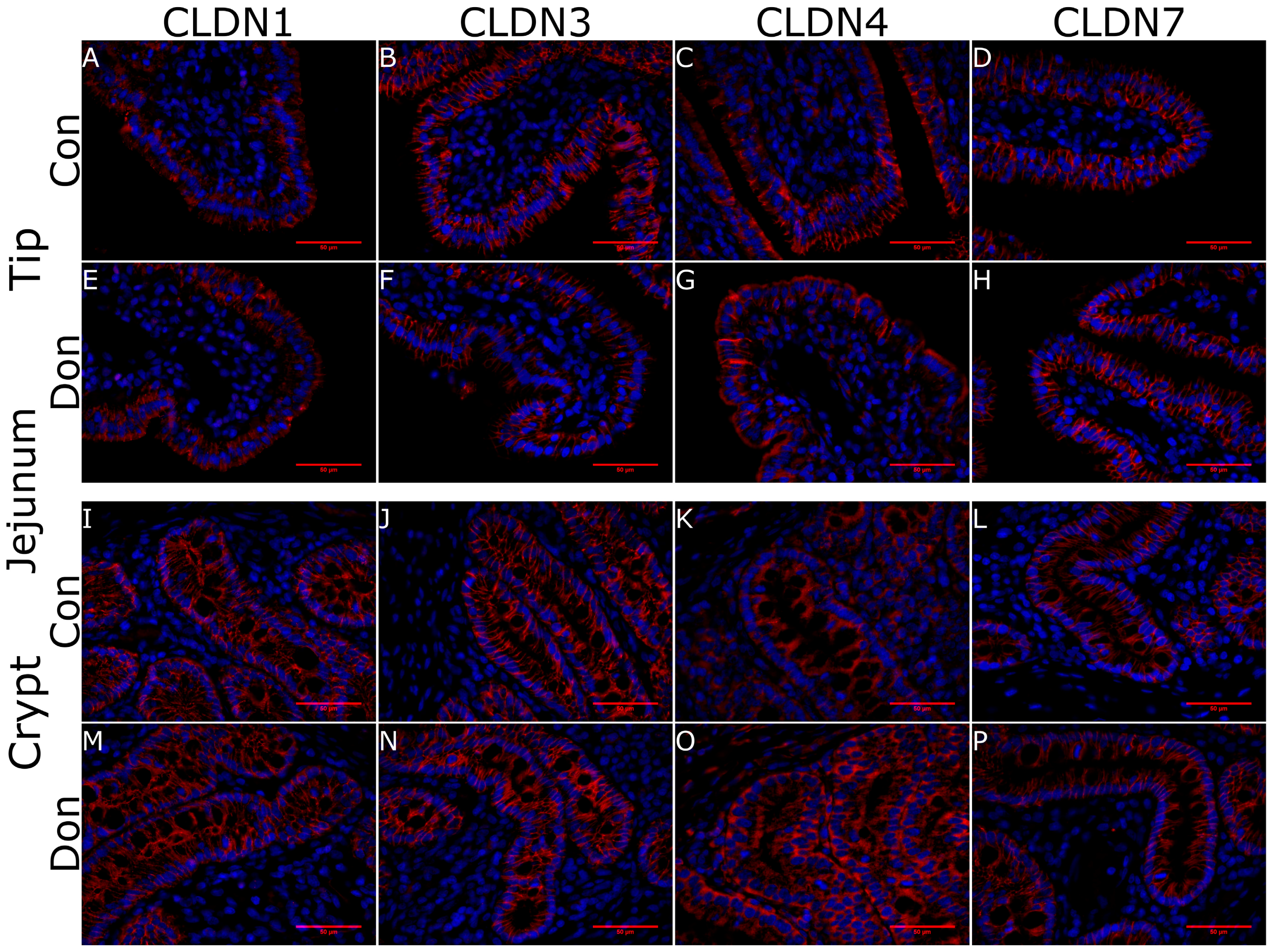
2. Results
2.1. Feed Intake and Growth Performance
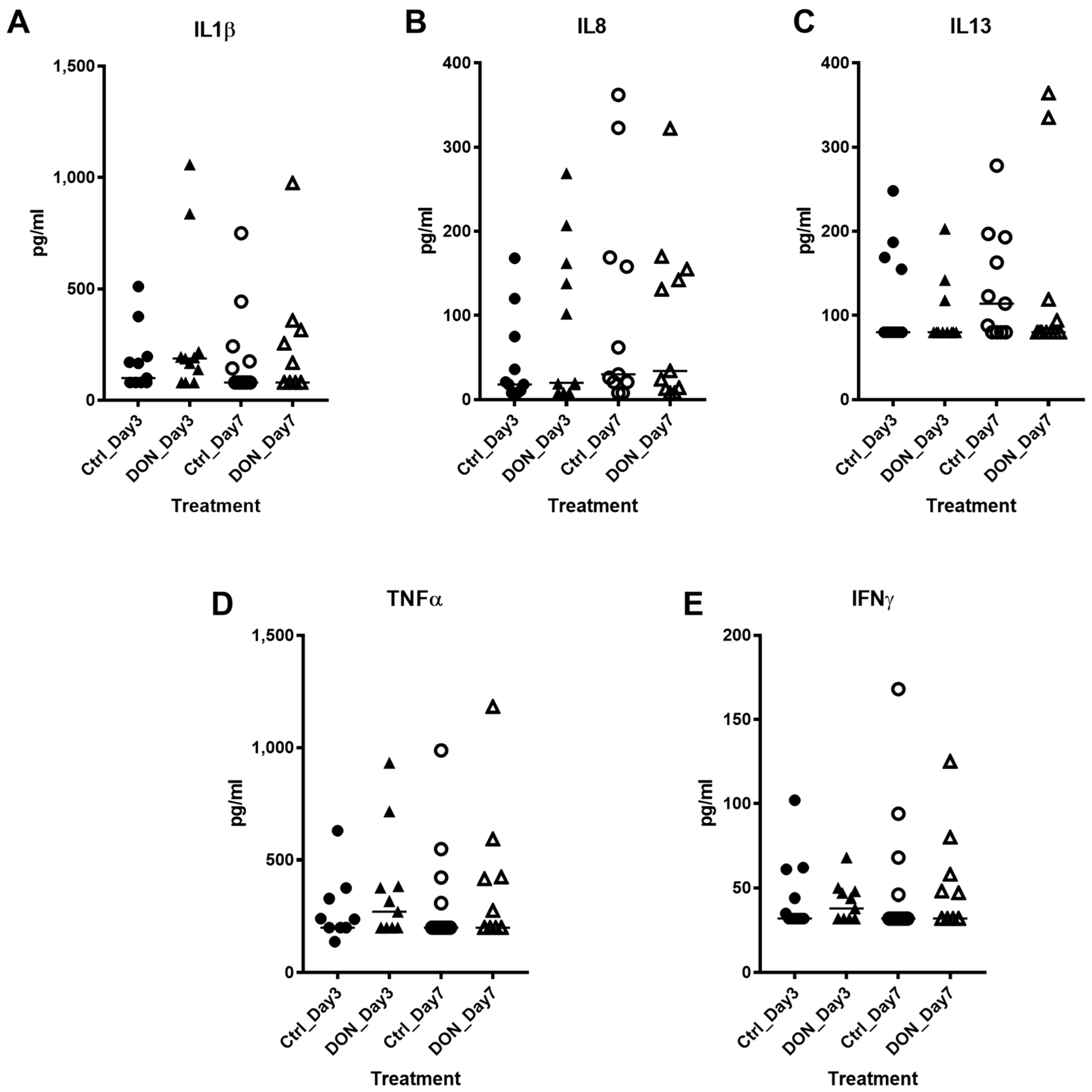
2.2. Serum Cytokine Analysis in Acute Period after DON Exposure
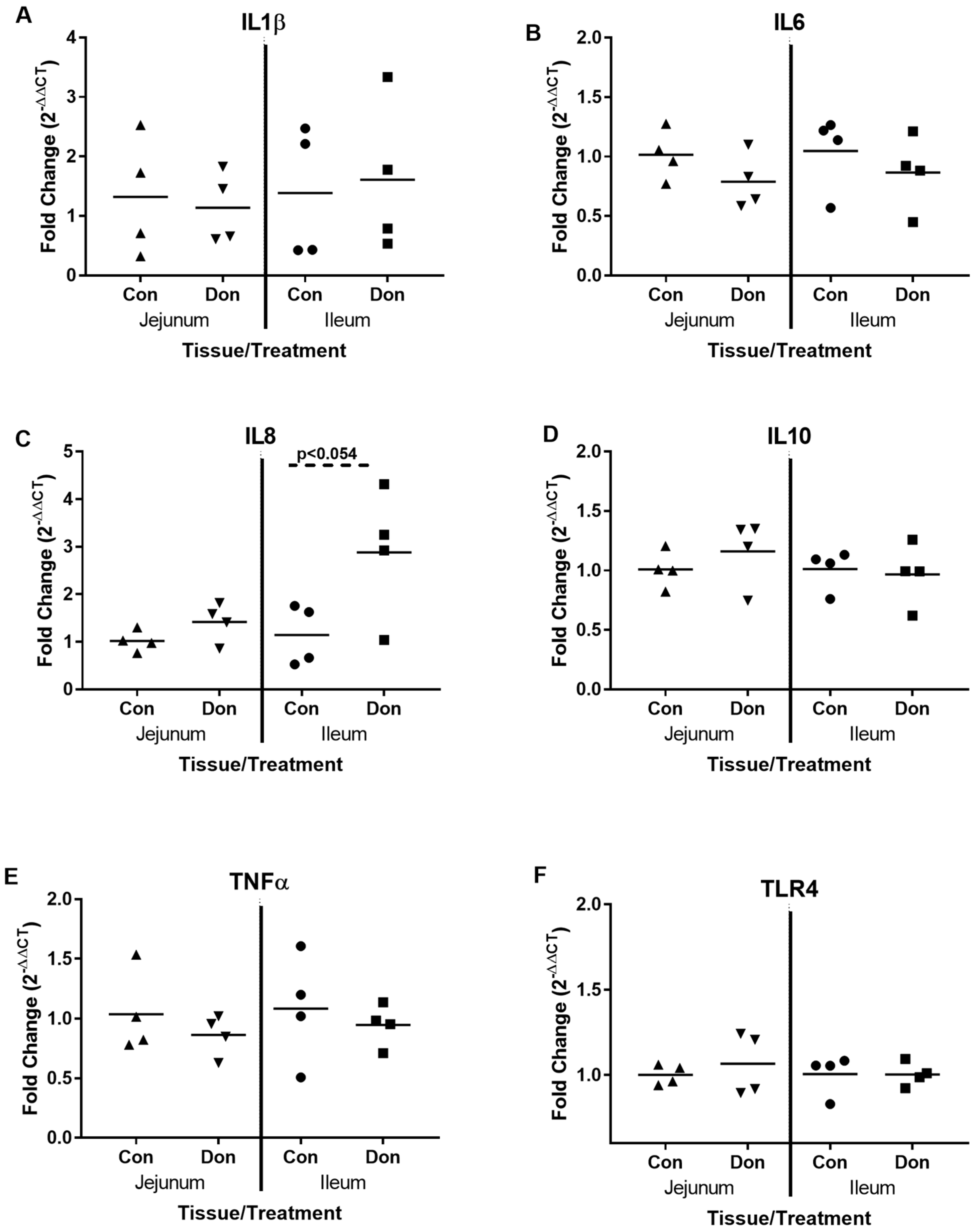
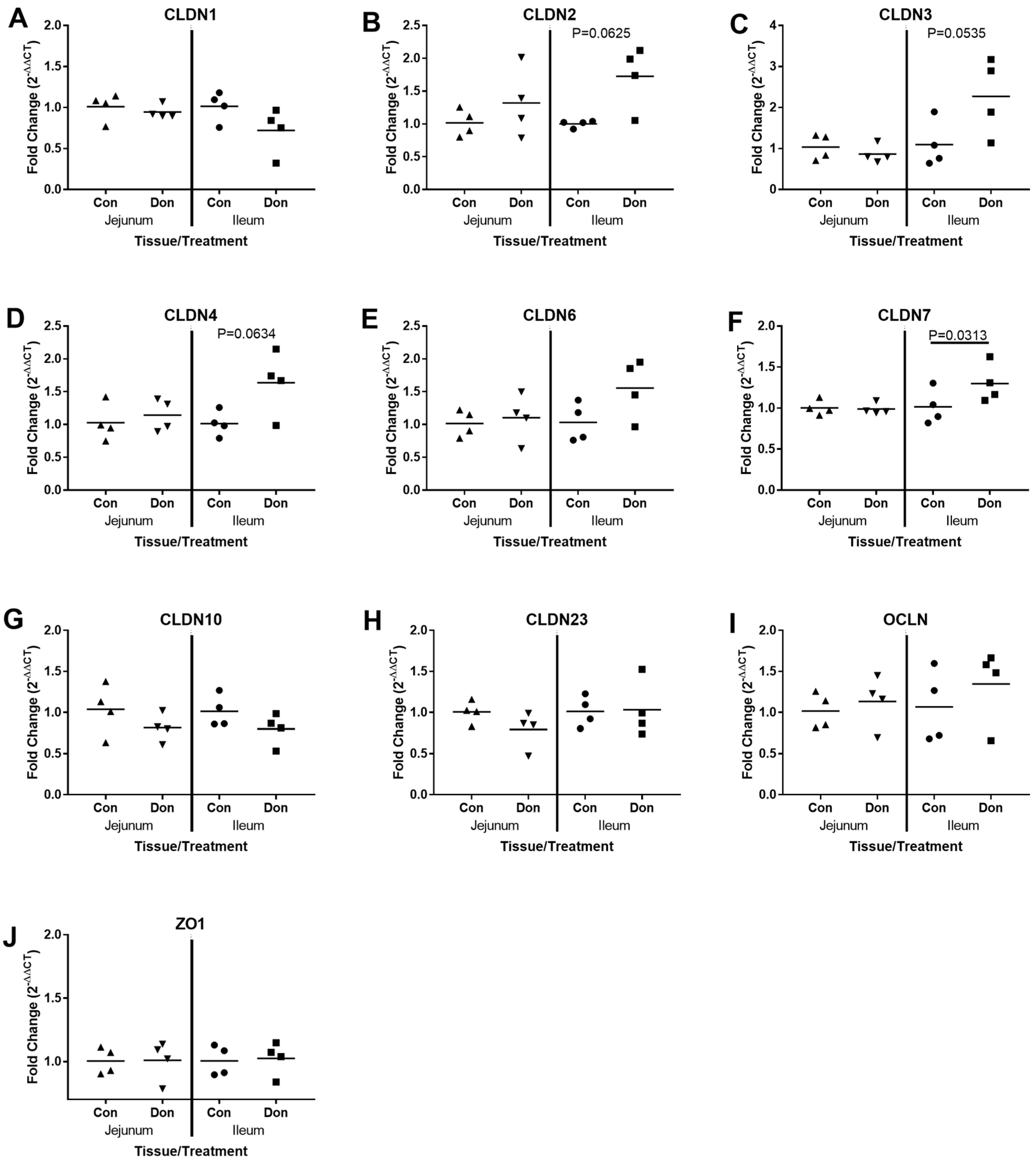
2.3. Jejunal and Ileal Immune Response Gene Profile after Exposure to DON
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animal Care and Selection
5.2. Dietary Treatments and Preparation
5.3. Animal Sampling and Weight Calculations
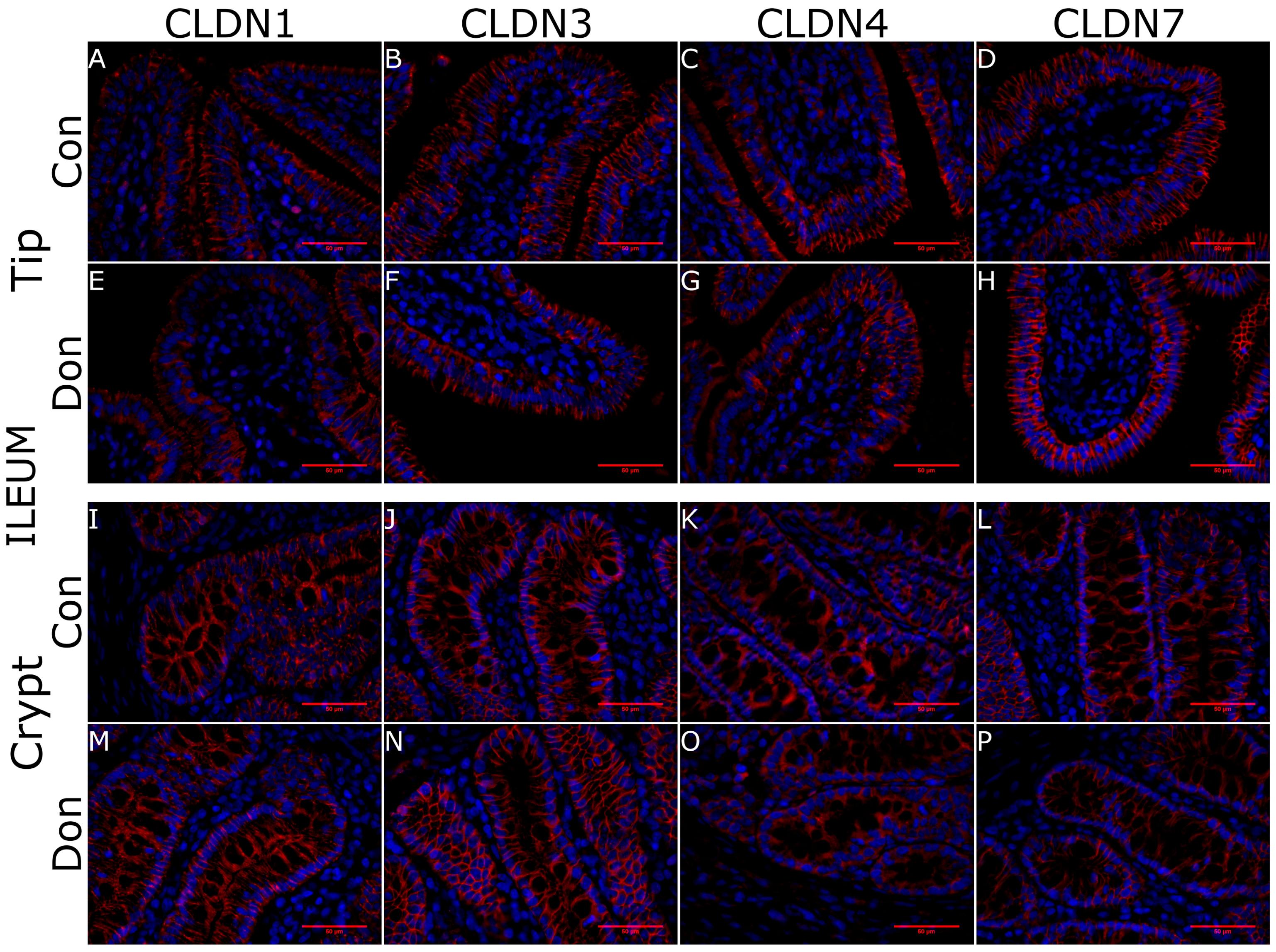
5.4. Histology and Immunohistoflourescence
5.5. Bioplex Cytokine Assays
5.6. Quantitative Gene Expression Analysis
5.7. Statistics
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of two mycotoxins deoxynivalenol and fumonisin on pig intestinal health. Porcine Health Manag. 2016, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Prelusky, D.B. A study on the effect of deoxynivalenol on serotonin receptor binding in pig brain membranes. J. Environ. Sci. Health B 1996, 31, 1103–1117. [Google Scholar] [CrossRef] [PubMed]
- House, J.D.; Abramson, D.; Crow, G.H.; Nyachoti, C.M. Feed intake, growth and carcass parameters of swine consuming diets containing low levels of deoxynivalenol from naturally contaminated barley. Can. J. Anim. Sci. 2002, 82, 559–565. [Google Scholar] [CrossRef]
- Goyarts, T.; Dänicke, S. Effects of deoxynivalenol (DON) on growth performance, nutrient digestibility and DON metabolism in pigs. Mycotoxin Res. 2005, 21, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Swamy, H.V.; Smith, T.K.; MacDonald, E.J.; Boermans, H.J.; Squires, E.J. Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on swine performance, brain regional neurochemistry, and serum chemistry and the efficacy of a polymeric glucomannan mycotoxin adsorbent. J. Anim. Sci. 2002, 80, 3257–3267. [Google Scholar] [CrossRef] [PubMed]
- Charmley, L.L.; Trenholme, H.L. RG-8 Regulatory Guidance: Contaminants in Feed. Canadian Food Inspection Agency. Available online: http://www.inspection.gc.ca/animals/feeds/regulatory-guidance/rg-8/eng/1347383943203/1347384015909 (accessed on 8 January 2018).
- FDA. Guidance for Industry and FDAi Advisory Levels for Deoxynivalenol (DON) in Finished Wheat Products for Human Consumption and Grains and Grain By-Products Used for Animal Feed. 2010. Available online: http://www.fda.gov/downloads/Food/GuidanceRegulation/UCM217558.pdf (accessed on 8 January 2018).
- Commission_Recommendation. On the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding (2006/576/EC). J. Eur. Union 2006. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32006H0576 (accessed on 8 January 2018).
- Alizadeh, A.; Braber, S.; Akbari, P.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol Impairs Weight Gain and Affects Markers of Gut Health after Low-Dose, Short-Term Exposure of Growing Pigs. Toxins 2015, 7, 2071–2095. [Google Scholar] [CrossRef] [PubMed]
- Bracarense, A.-P.F.L.; Lucioli, J.; Grenier, B.; Drociunas Pacheco, G.; Moll, W.-D.; Schatzmayr, G.; Oswald, I.P. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. Br. J. Nutr. 2012, 107, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Accensi, F.; Beauchamp, E.; Cossalter, A.-M.; Callu, P.; Grosjean, F.; Oswald, I.P. Ingestion of deoxynivalenol (DON) contaminated feed alters the pig vaccinal immune responses. Toxicol. Lett. 2008, 177, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Reiter, M.; Pfaffl, M.W.; Meyer, H.H.D.; Bauer, J.; Meyer, K.H.D. Expression of immune relevant genes in pigs under the influence of low doses of deoxynivalenol (DON). Mycotoxin Res. 2011, 27, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Van Itallie, C.M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 1995, 269 Pt 1, G467–G475. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, E.E.; Lynch, R.D. The tight junction: A multifunctional complex. Am. J. Physiol. Cell Physiol. 2004, 286, C1213–C1228. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Furuse, M.; Itoh, M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001, 2, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Berkes, J.; Viswanathan, V.K.; Savkovic, S.D.; Hecht, G. Intestinal epithelial responses to enteric pathogens: Effects on the tight junction barrier, ion transport, and inflammation. Gut 2003, 52, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Fujita, K.; Hiiragi, T.; Fujimoto, K.; Tsukita, S. Claudin-1 and -2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 1998, 141, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Nougayrede, J.P.; Del Rio, J.C.; Moreno, C.; Marin, D.E.; Ferrier, L.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol. 2009, 237, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lagunas, M.J.; Storniolo, C.E.; Ferrer, R.; Moreno, J.J. 5-Hydroxyeicosatetraenoic acid and leukotriene D4 increase intestinal epithelial paracellular permeability. Int. J. Biochem. Cell Biol. 2013, 45, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Kolf-Clauw, M.; Castellote, J.; Joly, B.; Bourges-Abella, N.; Raymond-Letron, I.; Pinton, P.; Oswald, I.P. Development of a pig jejunal explant culture for studying the gastrointestinal toxicity of the mycotoxin deoxynivalenol: Histopathological analysis. Toxicol. In Vitro 2009, 23, 1580–1584. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Tsybulskyy, D.; Lucioli, J.; Laffitte, J.; Callu, P.; Lyazhri, F.; Grosjean, F.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: differential effects on morphology, barrier function, tight junction proteins, and mitogen-activated protein kinases. Toxicol. Sci. 2012, 130, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Halawa, A.; Danicke, S.; Kersten, S.; Breves, G. Effects of deoxynivalenol and lipopolysaccharide on electrophysiological parameters in growing pigs. Mycotoxin Res. 2012, 28, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Lessard, M.; Savard, C.; Deschene, K.; Lauzon, K.; Pinilla, V.A.; Gagnon, C.A.; Lapointe, J.; Guay, F.; Chorfi, Y. Impact of deoxynivalenol (DON) contaminated feed on intestinal integrity and immune response in swine. Food Chem. Toxicol. 2015, 80, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
| Time | Control Diet (0.20 to 0.40 ppm DON) | DON-Contaminated Diet (3.30 to 3.80 ppm DON) |
|---|---|---|
| Day 0 | 5.80 | 5.79 |
| Day 3 | 5.85 | 5.77 |
| Day 7 | 6.09 | 5.82 |
| Day 14 | 7.43 | 6.94 |
| Day 21 | 9.96 | 9.28 |
| Day 24 | 11.43 * | 10.39 * |
| Interval | Control Diet (0.20 to 0.40 ppm DON) | DON-Contaminated Diet (3.30 to 3.80 ppm DON) |
|---|---|---|
| Average daily gain (d/g) | ||
| Day 3–7 | 63.3 | 6.4 |
| Day 7–14 | 190.8 | 158.3 |
| Day 14–21 | 358.9 | 331.6 |
| Day 21–24 | 367.0 | 273.6 * |
| Average daily feed intake (g/d) | ||
| Day 3–7 | 137.6 | 90.4 |
| Day 7–14 | 236.9 | 202.4 |
| Day 14–21 | 519.7 | 457.9 |
| Day 21–24 | 700.8 * | 602.3 * |
| Ingredient | Phase I | Phase II | Phase III | |||
|---|---|---|---|---|---|---|
| Control Diet | DON-Contaminated Diet | Control Diet | DON-Contaminated Diet | Control Diet | DON-Contaminated Diet | |
| Wheat (clean) | 58.1 | 20.3 | 42.6 | 4.3 | 44.4 | 6.2 |
| Wheat (DON) | - | 34.8 | - | 34.8 | - | 34.8 |
| Soybean meal | 22.0 | 25.0 | 21.0 | 24.6 | 18.6 | 22.1 |
| Barley | - | - | 27.9 | 27.9 | 31.9 | 31.9 |
| Whey | 11.4 | 11.4 | - | - | - | - |
| Fish meal | 3.9 | 3.9 | 3.2 | 3.2 | - | - |
| Canola oil | 1.9 | 1.9 | 2.4 | 2.4 | 2.0 | 2.0 |
| Limestone | 1.05 | 1.05 | 1.30 | 1.30 | 1.55 | 1.55 |
| Salt | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| L-Lys, HCl | 0.615 | 0.568 | 0.573 | 0.508 | 0.637 | 0.575 |
| DL-Met | 0.125 | 0.180 | 0.105 | 0.105 | 0.050 | 0.050 |
| L-Thr | 0.180 | 0.125 | 0.175 | 0.175 | 0.130 | 0.130 |
| L-Trp | 0.057 | 0.057 | 0.004 | 0.004 | 0.021 | 0.021 |
| Choline chloride | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 |
| Copper sulfate | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| Vit/min premix 1 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Calculated nutrient content | ||||||
| DM (%) | 88.7 | 88.8 | 87.6 | 87.7 | 87.8 | 87.9 |
| CP (%) | 23.5 | 23.1 | 22.1 | 21.8 | 19.7 | 19.4 |
| ME (kcal/kg) | 3323 | 3323 | 3270 | 3273 | 3225 | 3228 |
| Lys (% SID) | 1.50 | 1.50 | 1.35 | 1.35 | 1.23 | 1.23 |
| Ca (%) | 0.73 | 0.74 | 0.72 | 0.73 | 0.66 | 0.67 |
| P (%) | 0.58 | 0.59 | 0.51 | 0.52 | 0.42 | 0.43 |
| DON (ppm) | 0.00 | 4.00 | 0.00 | 4.00 | 0.00 | 4.00 |
| Analyzed nutrient content | ||||||
| DM (%) | 89.2 | 89.2 | 89.5 | 89.2 | 89.1 | 89.4 |
| CP (%) | 22.4 | 23.4 | 21.8 | 22.7 | 19.6 | 20.8 |
| Ca (%) | 0.80 | 0.88 | 0.82 | 1.00 | 0.86 | 0.94 |
| P (%) | 0.61 | 0.61 | 0.53 | 0.51 | 0.45 | 0.46 |
| DON (ppm) | 0.30 | 3.30 | 0.20 | 3.80 | 0.40 | 3.80 |
| Mycotoxin | Level (ppb) 2 |
|---|---|
| Deoxynivalenol | 11,470 |
| 3-acetyl-deoxynivalenol | 763.9 |
| 15-acetyl-deoxynivalenol | <25.0 |
| α-zearalenol | <66.0 |
| Diacetoxyscirpenol | <25.0 |
| HT-2 toxin | 107 |
| Nivalenol | 59.2 |
| Ochratoxin A | <25.0 |
| T-2 toxin | <25.0 |
| β-zearalenol | <66.0 |
| Zeralenone | <25.0 |
| Aflatoxin B1 | <25.0 |
| Cytokine | Capture Antibody; Supplier | Detection Antibody; Supplier; Dilution | Standard; Supplier; Initial Concentration | Bead; Supplier |
|---|---|---|---|---|
| IL1β | MAb anti porc IL1β/IF2; R & D MAB6811 | Goat anti porc IL1β/IF2 biotin; R & D BAF681; 0.5 μg/mL | recombinant porc IL1β/IF2; R & D 681-PI-10; 5000 pg/mL | Region 26; BioRad MC10026-01 |
| IL8 | MAb anti sheep IL8 (86.9% homology); AbD Serotec MCA1660 | MAb anti porc CXCL8/IL8; R & D MAB5351; biotinylated in house; 1/400 dilution | Recombinant porc IL-8; Kingfisher RP0109S-005; 200 pg/mL | Region 27; BioRad MC10027-01 |
| IL13 | Goat anti swine IL-13; Kingfisher PB0094S-100 | Goat anti swine IL-13 biotin; Kingfisher PBB0096S-050; 0.5 μg/mL | Recombinant swine IL-13; Kingfisher RP0007S-005; 5000 pg/mL | Region 52 ; BioRad MC10052-01 |
| TNFα | MAb anti porcine TNFα; R&D MAB6902 | Goat anti porcine TNF α biotin; R & D BAF690; 0.5 μg/mL | Recombinant porcine TNFα; R & D 690-PT-025; 5000 pg/mL | Region 34; BioRad MC10034-01 |
| IFNγ | MAb anti-porcine IFNγ; Fisher ENMP700 | MAb anti-porc IFNγ; Fisher ENPP700; biotinylated in-house; 1/400 dilution | Recombinant porcine IFNγ; Ceiba Geigy (gift); 2000 pg/mL | Region 43; BioRad MC10043-01 |
| Target | Source | Forward Primer | Reverse Primer | Amplicon Length (bp) | Annealing Temp (°C) |
|---|---|---|---|---|---|
| Actin B | Nygard et al., 2007 | 5′-CACGCCATCCTGCGTCTGGA-3′ | 5′-AGCACCGTGTTGGCGTAGAG-3′ | 100 | 63 |
| ALOX5 | XM_001927671.3 | 5′-TGGCTTCCCCTTGAGTATTG-3′ | 5′-CAGGTTCTCCATCGCTTTTG-3′ | 118 | 62 |
| ALOX5AP | NM_001164001.1 | 5′-TGGAGCACGAAAGCAAGAC-3′ | 5′-CACAGTTCTGGTTGGCAGTG-3′ | 93 | 60 |
| B2MI | Nygard et al., 2007 | 5′-CAAGATAGTTAAGTGGGATCG-AGAC-3′ | 5′-TGGTAACATCAATACGATTT-CTGA-3′ | 161 | 58 |
| CLDN1 | NM_001244539.1 | 5′-TCCTTGCTGAATCTGAACACC-3′ | 5′-ACACTTCATGCCAACAGTGG-3′ | 108 | 60 |
| CLDN2 | NM_001161638.1 | 5′-CGTTGCGTGGAATCTTCAT-3′ | 5′-GGGAGAACAGGGAGGAAATG-3′ | 119 | 60 |
| CLDN3 | NM_001160075.1 | 5′-GCCAAAGCCAAGATCCTCTAC-3′ | 5′-AGCATCTGGGTGGACTGGT-3′ | 190 | 60 |
| CLDN4 | NM_001161637.1 | 5′-CAACTGCGTGGATGATGAGA-3′ | 5′-CCAGGGGATTGTAGAAGTCG-3′ | 140 | 62 |
| CLDN6 | NM_001161645.1 | 5′-CTTCATCGGCAACAGCATC-3′ | 5′-CAGCAGCGAGTCATACACCT-3′ | 112 | 60 |
| CLDN7 | NM_001160076.1 | 5′-ATCGTGGCAGGTCTTTGTG-3′ | 5′-CTCACTCCCAGGACAAGAGC-3′ | 192 | 60 |
| CLDN8 | NM_001161646.1 | 5′-GGAGTGCTCTTCGTCCTCAC-3′ | 5′-CTGCCGTCCAGCCTATGTA-3′ | 148 | 62 |
| CLDN10 | NM_001243444.1 | 5′-GCCCTGTTTGGAATGAAATG-3′ | 5′-AGCACAGCCCTGACAGTATG-3′ | 103 | 62 |
| CLDN14 | NM_001161642.1 | 5′-ACGCCTACAAGGACAATCG-3′ | 5′-AATGAACTCGGTGTGGGAAC-3′ | 168 | 62 |
| CLDN23 | NM_001159778.1 | 5′-TGTCTGGCTGAAGGACTCG-3′ | 5′-CCACAGGAAAGGAAGGTCAC-3′ | 112 | 60 |
| IL1b | NM_001005149 | 5′-AGAAGAGCCCATCGTCCTTG-3′ | 5′-GAGAGCCTTCAGCTCATGTG-3′ | 139 | 62 |
| IL6 | NM_214399 | 5′-ATCAGGAGACCTGCTTGATG-3′ | 5′-TGGTGGCTTTGTCTGGATTC-3′ | 177 | 60 |
| IL8 | NM_213867 | 5′-TCCTGCTTTCTGCAGCTCTC-3′ | 5′-GGGTGGAAAGGTGTGGAATG-3′ | 100 | 62 |
| IL10 | NM_214041 | 5′-GGTTGCCAAGCCTTGTCAG-3′ | 5′-AGGCACTCTTCACCTCCTC-3′ | 202 | 60 |
| LTA4H | NM_001185132.1 | 5′-CTGGGAAGGAACACCCCTAT-3′ | 5′-GGGACAGACACCTCTGCACT-3′ | 118 | 60 |
| LTC4S | XM_003123645.4 | 5′-CTACCGAGCCCAAGTAAACTG-3′ | 5′-GCGTGCGTACAGGTAGATGA-3′ | 124 | 60 |
| OCCLN | NM_001163647.2 | 5′-GAGTACATGGCTGCTGCTGA-3′ | 5′-TTTGCTCTTCAACTGCTTGC-3′ | 102 | 62 |
| TLR2 | NM_213761 | 5′-ACGGACTGTGGTGCATGAAG-3′ | 5′-GGACACGAAAGCGTCATAGC-3′ | 101 | 62 |
| TLR4 | NM_001113039 | 5′-TGTGCGTGTGAACACCAGAC-3′ | 5′-AGGTGGCGTTCCTGAAACTC-3′ | 136 | 60 |
| TNFa | NM_214022 | 5′-CCAATGGCAGAGTGGGTATG-3′ | 5′-TGAAGAGGACCTGGGAGTAG-3′ | 116 | 60 |
| ZO1 | XM_003353439.2 | 5′-ACGGCGAAGGTAATTCAGTG-3′ | 5′-CTTCTCGGTTTGGTGGTCTG-3′ | 111 | 62 |
| GAPDH | AF017079 | 5′-CTTCACGACCATGGAGAAGG-3′ | 5′-CCAAGCAGTTGGTGGTACAG-3′ | 170 | 63 |
| HPRT | Nygard et al., 2007 | 5′-GGACTTGAATCATGTTTGTG-3′ | 5′-CAGATGTTTCCAAACTCAAC-3′ | 91 | 60 |
| RPL19 | AF_435591 | 5′-AACTCCCGTCAGCAGATCC-3′ | 5′-AGTACCCTTCCGCTTACCG-3′ | 147 | 60 |
| SDHA | Nygard et al., 2007 | 5′-CTACAAGGGGCAGGTTCTGA-3′ | 5′-AAGACAACGAGGTCCAGGAG-3′ | 141 | 58 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasternak, J.A.; Aiyer, V.I.A.; Hamonic, G.; Beaulieu, A.D.; Columbus, D.A.; Wilson, H.L. Molecular and Physiological Effects on the Small Intestine of Weaner Pigs Following Feeding with Deoxynivalenol-Contaminated Feed. Toxins 2018, 10, 40. https://doi.org/10.3390/toxins10010040
Pasternak JA, Aiyer VIA, Hamonic G, Beaulieu AD, Columbus DA, Wilson HL. Molecular and Physiological Effects on the Small Intestine of Weaner Pigs Following Feeding with Deoxynivalenol-Contaminated Feed. Toxins. 2018; 10(1):40. https://doi.org/10.3390/toxins10010040
Chicago/Turabian StylePasternak, J. Alex, Vaishnavi Iyer Aka Aiyer, Glenn Hamonic, A. Denise Beaulieu, Daniel A. Columbus, and Heather L. Wilson. 2018. "Molecular and Physiological Effects on the Small Intestine of Weaner Pigs Following Feeding with Deoxynivalenol-Contaminated Feed" Toxins 10, no. 1: 40. https://doi.org/10.3390/toxins10010040
APA StylePasternak, J. A., Aiyer, V. I. A., Hamonic, G., Beaulieu, A. D., Columbus, D. A., & Wilson, H. L. (2018). Molecular and Physiological Effects on the Small Intestine of Weaner Pigs Following Feeding with Deoxynivalenol-Contaminated Feed. Toxins, 10(1), 40. https://doi.org/10.3390/toxins10010040






