Multi-Targeted Molecular Effects of Hibiscus sabdariffa Polyphenols: An Opportunity for a Global Approach to Obesity
Abstract
1. Introduction
2. Hibiscus sabdariffa Polyphenols as Xenohormetic Agents
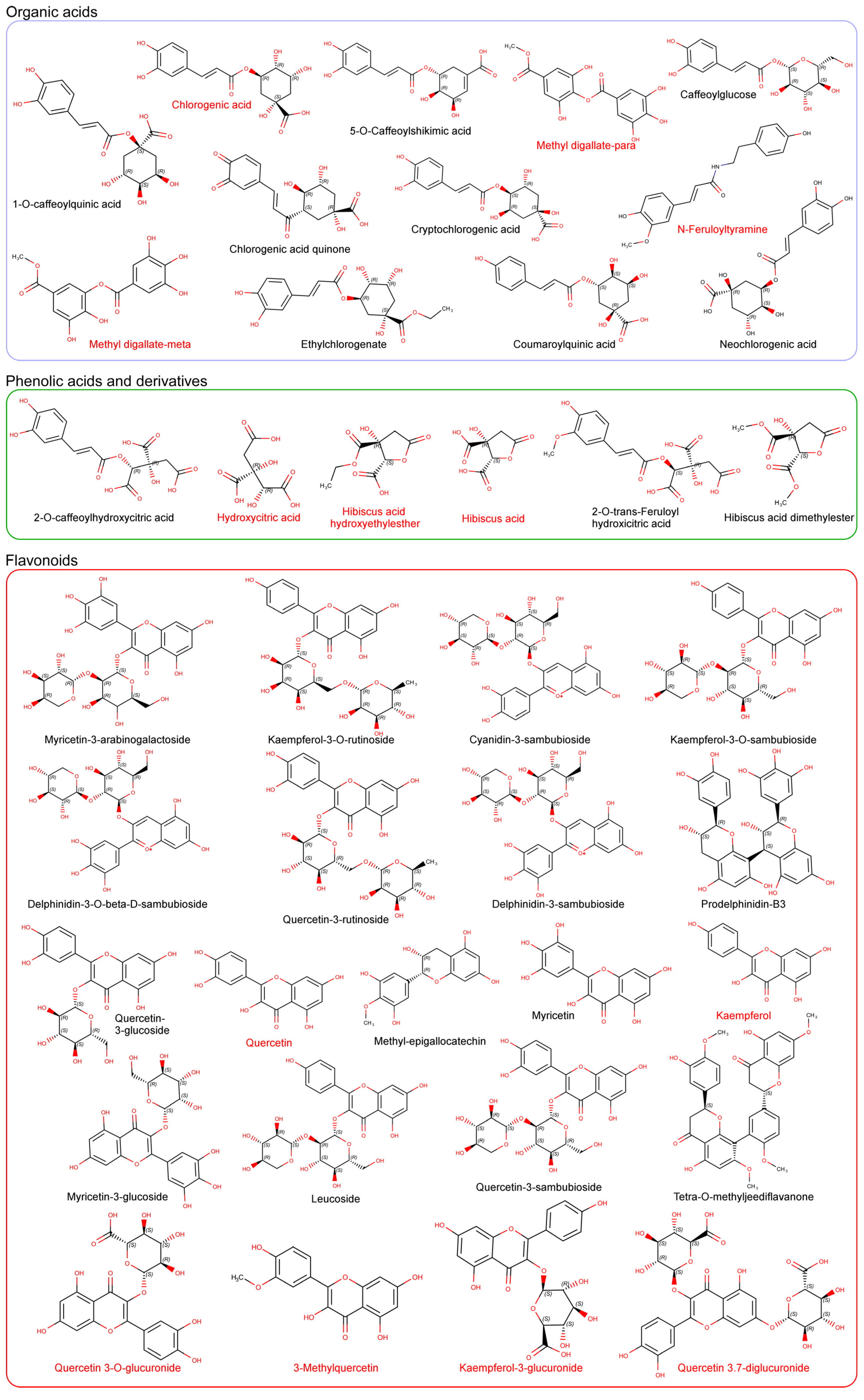
3. Characterization and Synergy of Hibiscus sabdariffa Bioactive Compounds
4. Bioavailability, Tissue Distribution and Cellular Metabolites Derived from Hibiscus sabdariffa Bioactive Compounds
5. Effect of Hibiscus sabdariffa Compounds on Selected Digestive Enzymes
6. Molecular Effects of Hibiscus sabdariffa Polyphenols
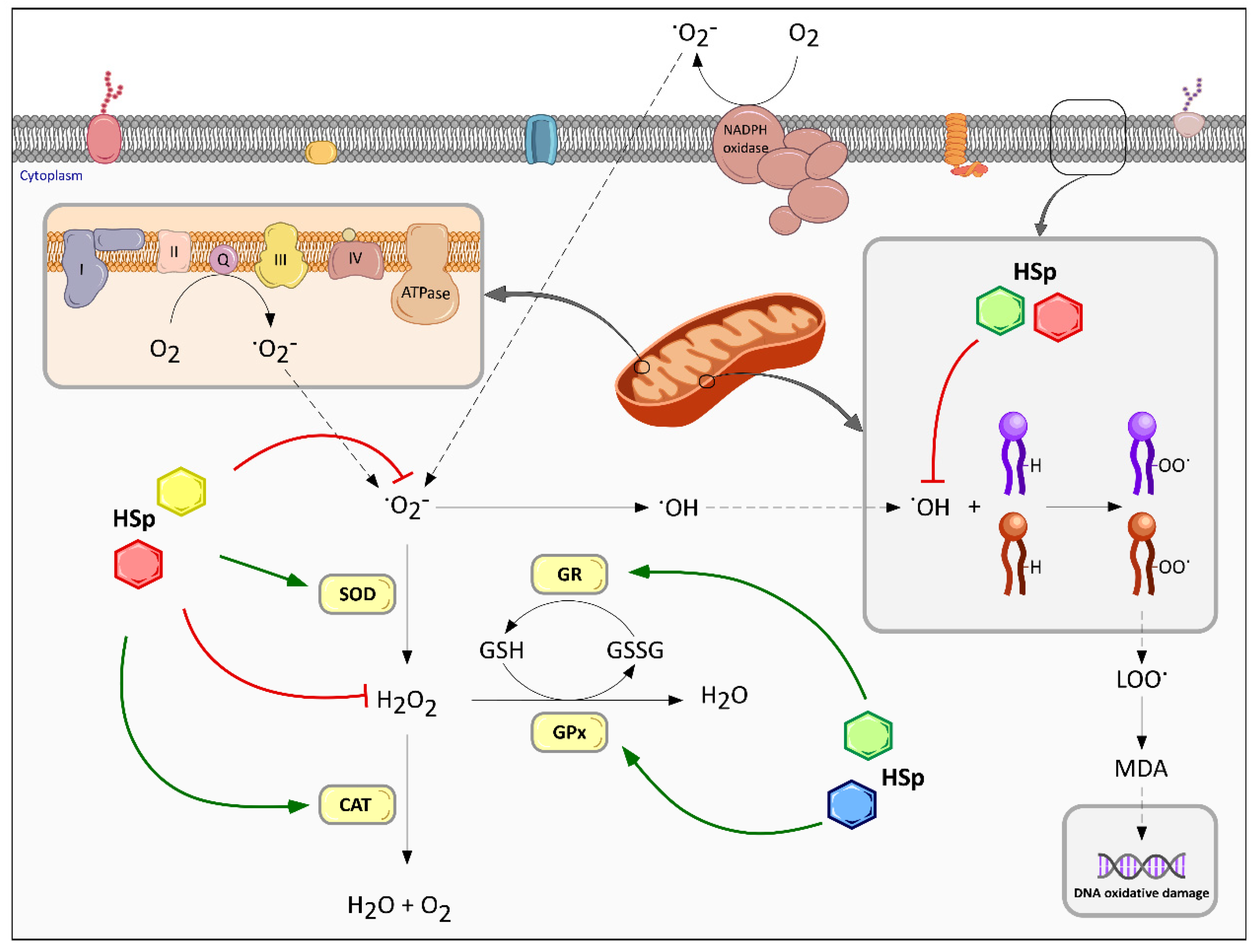
6.1. Effect on Redox Homeostasis
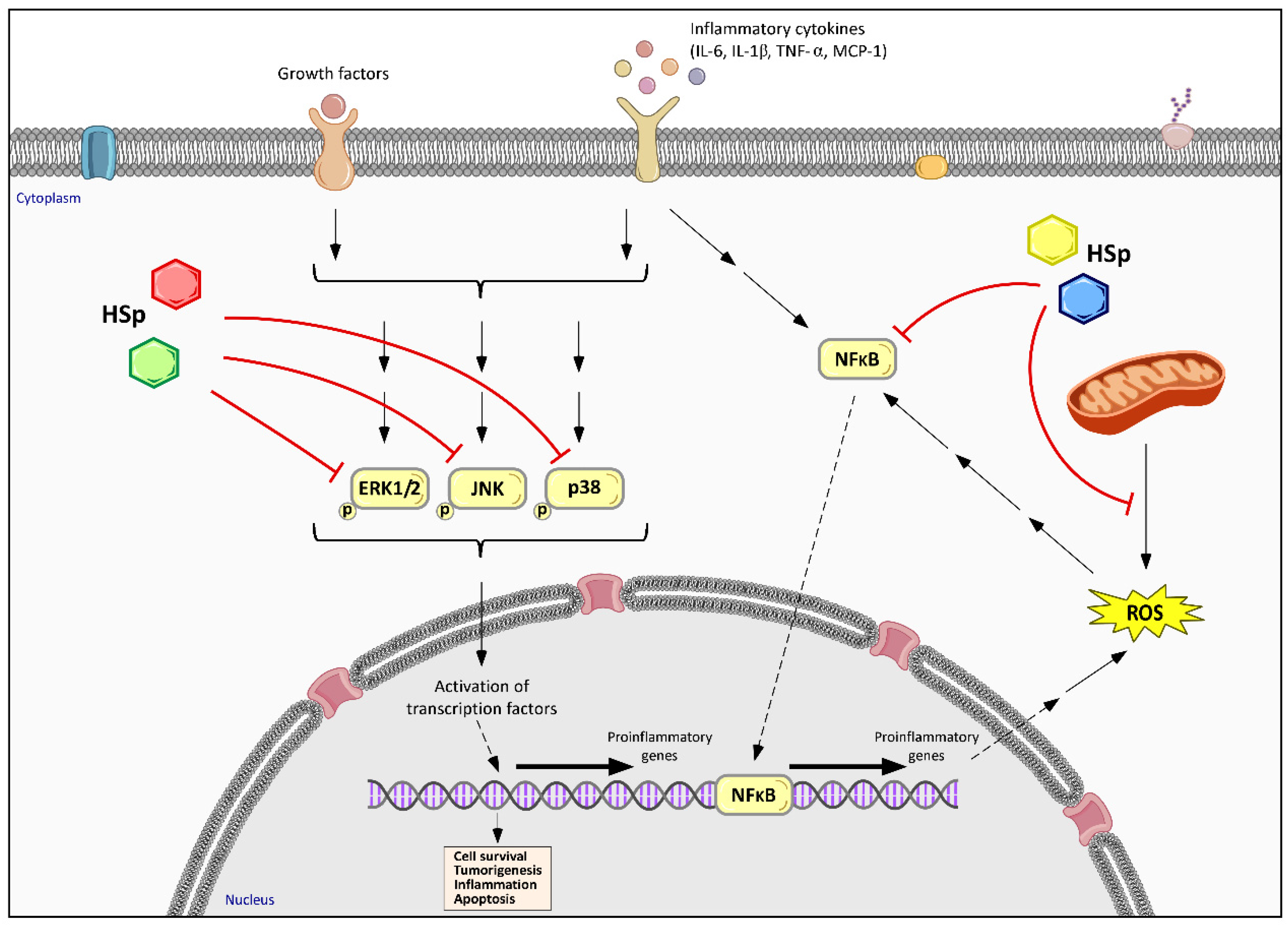
6.2. Hibiscus sabdariffa Effects on Inflammatory and Immune Response
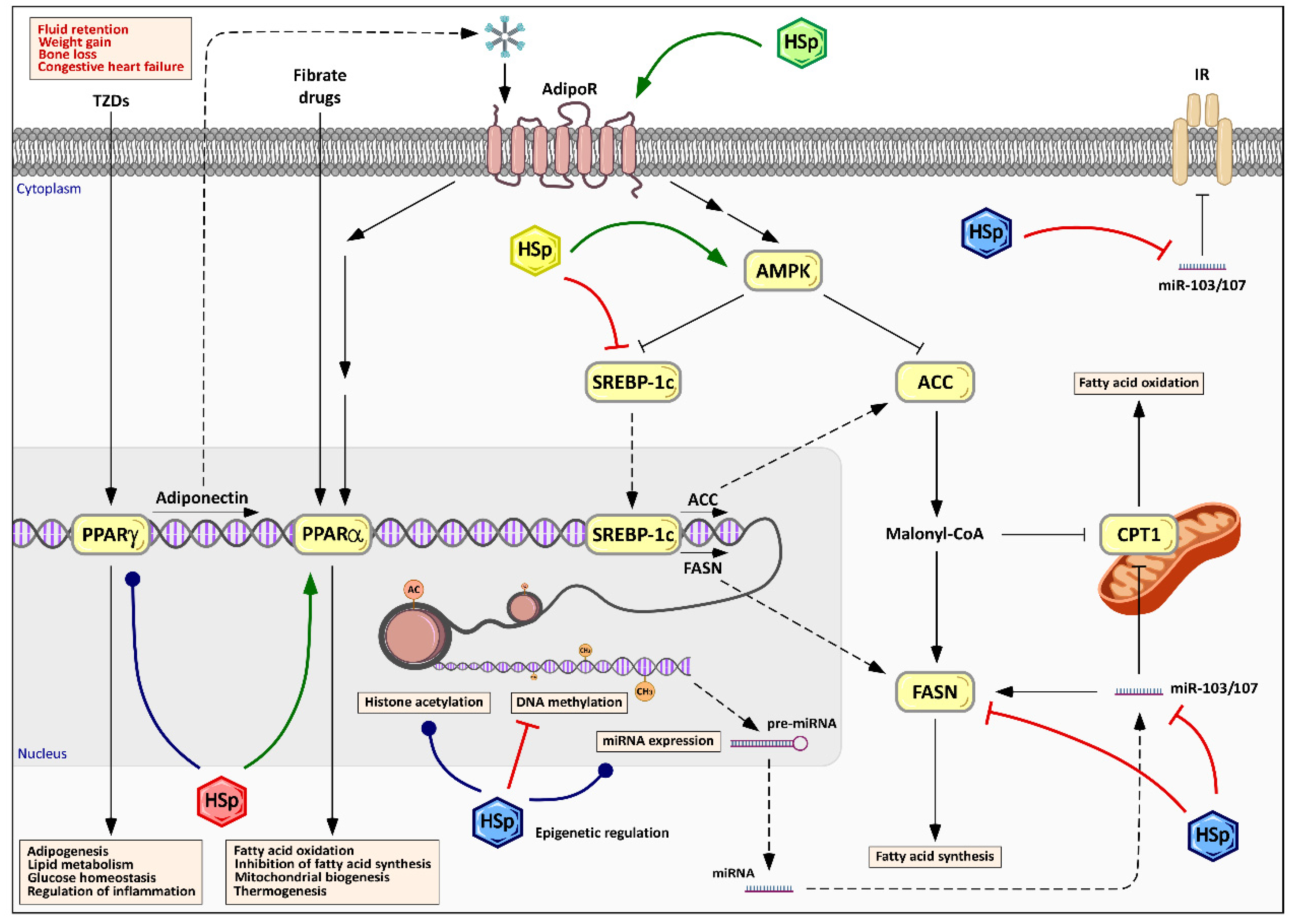
6.3. Modulation of Energy Metabolism and Lipid Management by Hibiscus sabdariffa Polyphenols
6.4. Epigenetic Effects of Hibiscus sabdariffa Polyphenols
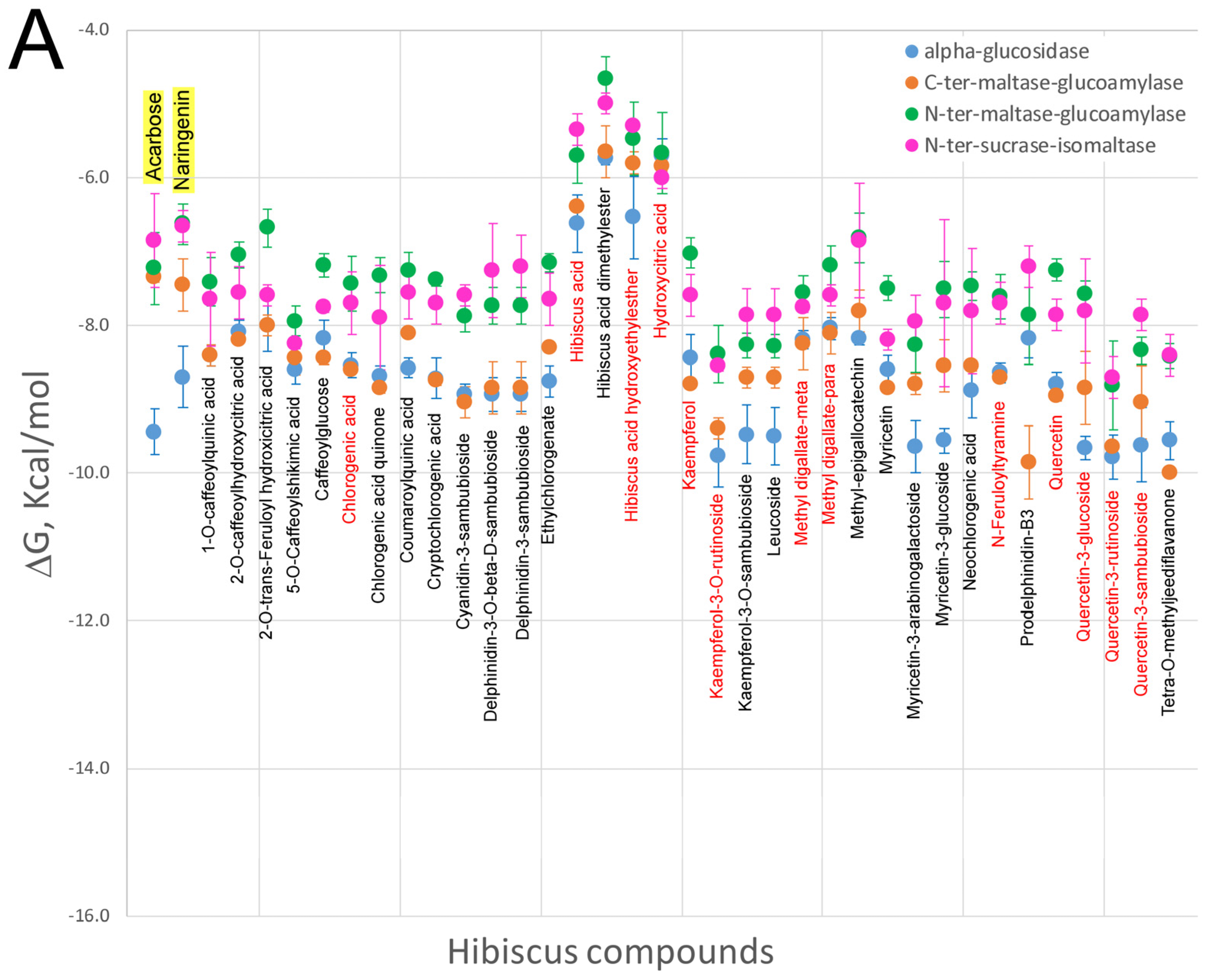
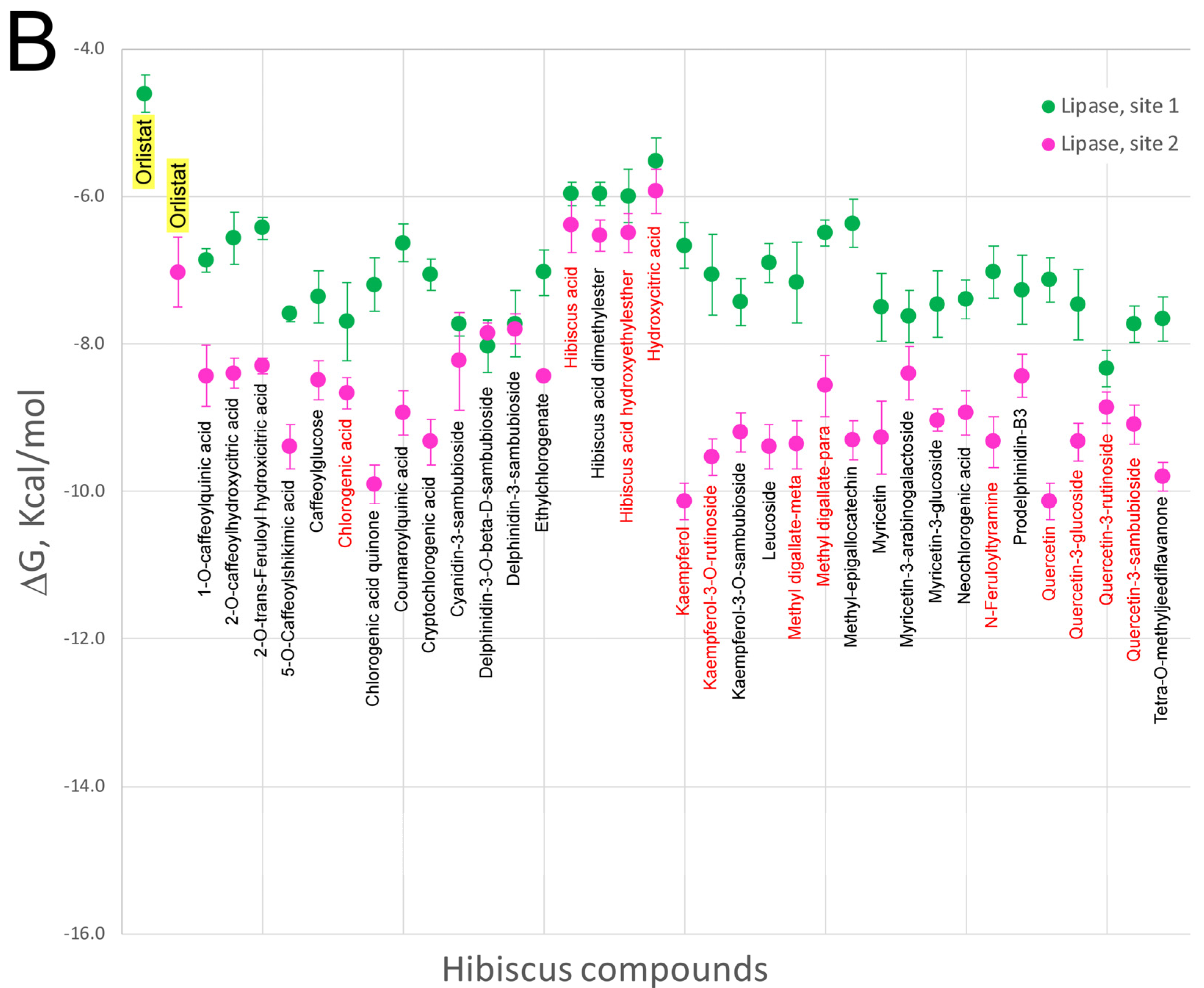
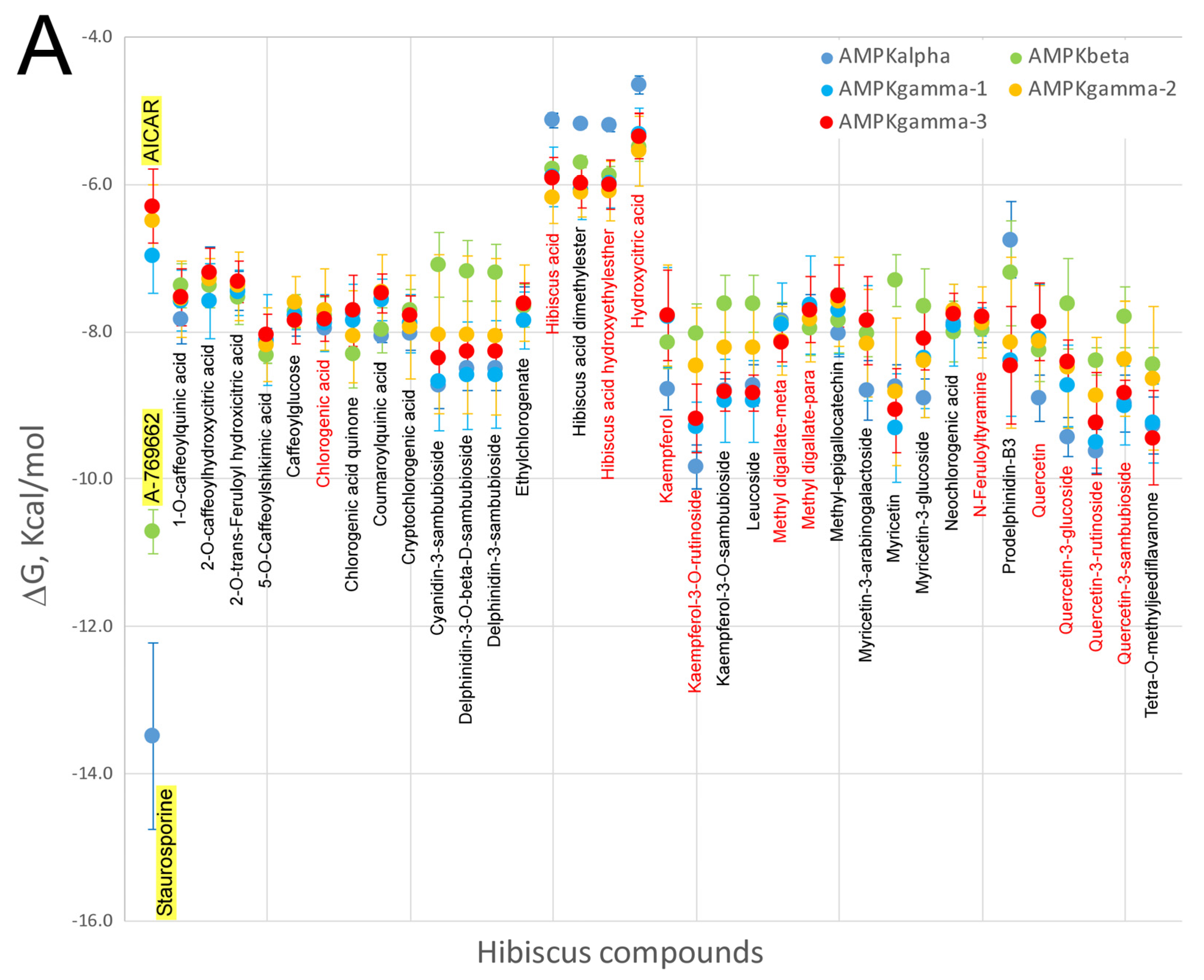
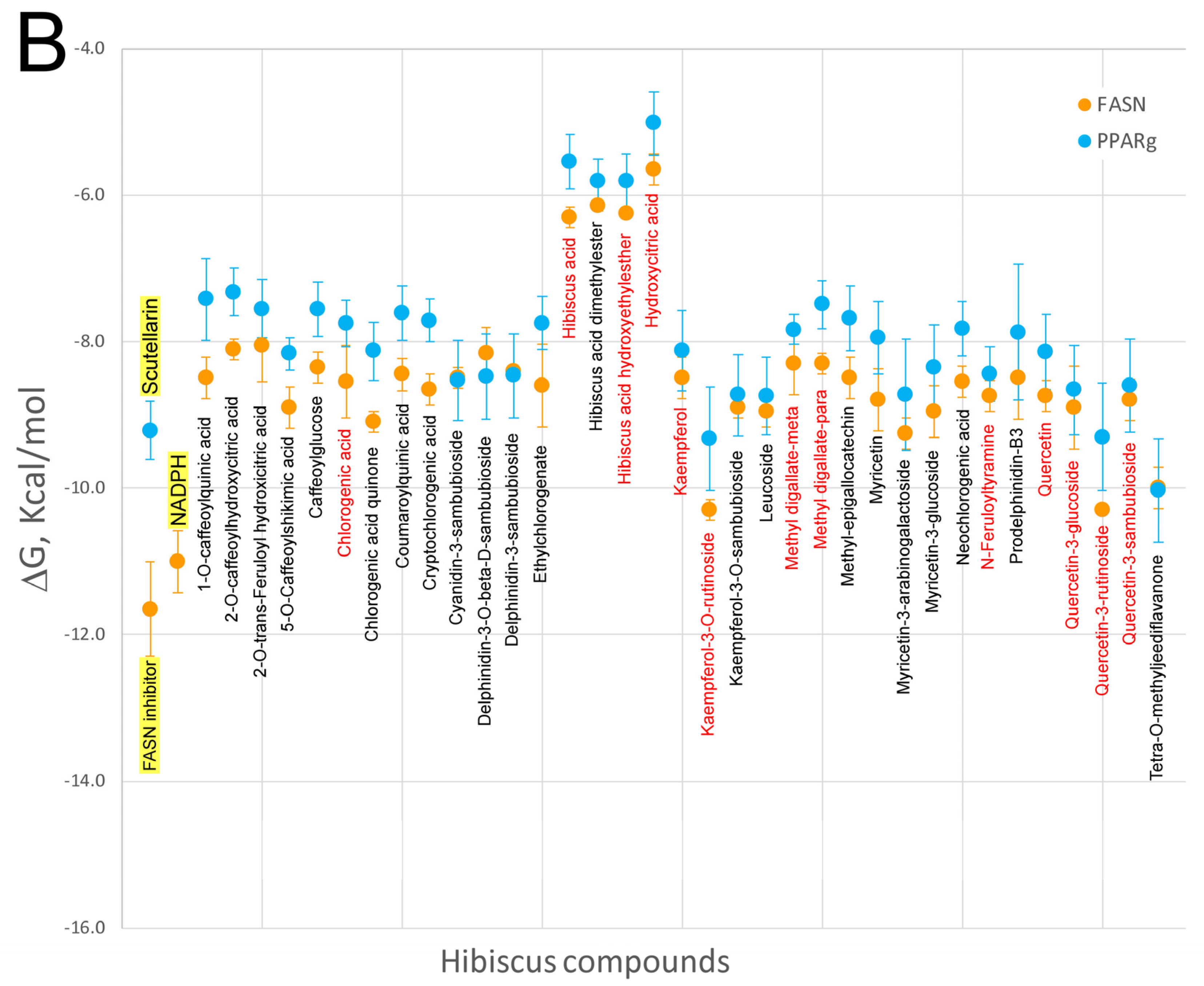
7. Virtual Screening of Hibiscus sabdariffa Polyphenols on Selected Protein Targets
8. Potential Molecular Mechanism of Hibiscus sabdariffa in Obesity: A Global Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Hibiscus sabdariffa | HS |
| low-density lipoprotein | LDL |
| LDL receptor | LDLr |
| reactive oxygen species | ROS |
| Sterol regulatory element-binding proteins | SREBP-1c |
| AMP-activated protein kinase | AMPK |
| fatty acid synthase | FASN |
| peroxisome proliferator-activated receptor | PPAR |
| superoxide dismutase | SOD |
| catalase | CAT |
| glutathione peroxidase | GPx |
| glutathione reductase | GR |
| diode array detection | DAD |
| electrospray | ESI |
| high-performance liquid chromatography | HPLC |
References
- Luna-Luna, M.; Medina-Urrutia, A.; Vargas-Alarcon, G.; Coss-Rovirosa, F.; Vargas-Barron, J.; Perez-Mendez, O. Adipose tissue in metabolic syndrome: Onset and progression of atherosclerosis. Arch. Med. Res. 2015, 46. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Metabolic syndrome: Connecting and reconciling cardiovascular and diabetes worlds. J. Am. Coll. Cardiol. 2006, 47, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Okada-Iwabu, M.; Yamauchi, T.; Iwabu, M.; Honma, T.; Hamagami, K.; Matsuda, K.; Yamaguchi, M.; Tanabe, H.; Kimura-Someya, T.; Shirouzu, M.; et al. A small-molecule adipor agonist for type 2 diabetes and short life in obesity. Nature 2013, 503, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Rise, M.B.; Pellerud, A.; Rygg, L.Ø.; Steinsbekk, A. Making and maintaining lifestyle changes after participating in group based type 2 diabetes self-management educations: A qualitative study. PLoS ONE 2013, 8, e64009. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Alberti, K.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. Ppargamma signaling and metabolism: The good, the bad and the future. Nature 2013, 19, 557–566. [Google Scholar]
- Leite, C.E.; Mocelin, C.A.; Petersen, G.O.; Leal, M.B.; Thiesen, F.V. Rimonabant: An antagonist drug of the endocannabinoid system for the treatment of obesity. Pharmacol Rep. 2009, 61, 217–224. [Google Scholar] [CrossRef]
- Heck, A.M.; Yanovski, J.A.; Calis, K.A. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy 2000, 20, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Joven, J.; Aragones, G.; Barrajon-Catalan, E.; Beltran-Debon, R.; Borras-Linares, I.; Camps, J.; Corominas-Faja, B.; Cufi, S.; Fernandez-Arroyo, S.; et al. Xenohormetic and anti-aging activity of secoiridoid polyphenols present in extra virgin olive oil: A new family of gerosuppressant agents. Cell Cycle 2013, 12, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Barrajon-Catalan, E.; Herranz-Lopez, M.; Joven, J.; Segura-Carretero, A.; Alonso-Villaverde, C.; Menendez, J.A.; Micol, V. Molecular promiscuity of plant polyphenols in the management of age-related diseases: Far beyond their antioxidant properties. Adv. Exp. Med. Biol. 2014, 824, 141–159. [Google Scholar] [PubMed]
- Beltran-Debon, R.; Rull, A.; Rodriguez-Sanabria, F.; Iswaldi, I.; Herranz-Lopez, M.; Aragones, G.; Camps, J.; Alonso-Villaverde, C.; Menendez, J.A.; Micol, V.; et al. Continuous administration of polyphenols from aqueous rooibos (Aspalathus linearis) extract ameliorates dietary-induced metabolic disturbances in hyperlipidemic mice. Phytomedicine 2011, 18, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Herranz-López, M.; Barrajón-Catalán, E.; Segura-Carretero, A.; Menéndez, J.A.; Joven, J.; Micol, V. Lemon verbena (Lippia citriodora) polyphenols alleviate obesity-related disturbances in hypertrophic adipocytes through ampk-dependent mechanisms. Phytomedicine 2015, 22, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Ismail, A.; Kersten, S. Molecular mechanisms underlying the potential antiobesity-related diseases effect of cocoa polyphenols. Mol. Nutr. Food Res. 2014, 58, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Rafter, J.; Jenner, A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: Direct or indirect effects? Antioxidant or not? Am. J. Clin. Nutr. 2005, 81, 268S–276S. [Google Scholar] [PubMed]
- Beltran-Debon, R.; Alonso-Villaverde, C.; Aragones, G.; Rodriguez-Medina, I.; Rull, A.; Micol, V.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Camps, J.; Joven, J. The aqueous extract of hibiscus sabdariffa calices modulates the production of monocyte chemoattractant protein-1 in humans. Phytomedicine 2010, 17, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Arroyo, S.; Rodríguez-Medina, I.C.; Beltrán-Debón, R.; Pasini, F.; Joven, J.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Quantification of the polyphenolic fraction and in vitro antioxidant and in vivo anti-hyperlipemic activities of hibiscus sabdariffa aqueous extract. Food Res. Int. 2011, 44, 1490–1495. [Google Scholar] [CrossRef]
- Fernandez-Arroyo, S.; Herranz-Lopez, M.; Beltran-Debon, R.; Borras-Linares, I.; Barrajon-Catalan, E.; Joven, J.; Fernandez-Gutierrez, A.; Segura-Carretero, A.; Micol, V. Bioavailability study of a polyphenol-enriched extract from hibiscus sabdariffa in rats and associated antioxidant status. Mol. Nutr. Food Res. 2012, 56, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Herranz-Lopez, M.; Fernandez-Arroyo, S.; Perez-Sanchez, A.; Barrajon-Catalan, E.; Beltran-Debon, R.; Menendez, J.A.; Alonso-Villaverde, C.; Segura-Carretero, A.; Joven, J.; Micol, V. Synergism of plant-derived polyphenols in adipogenesis: Perspectives and implications. Phytomedicine 2012, 19, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Joven, J.; Espinel, E.; Rull, A.; Aragones, G.; Rodriguez-Gallego, E.; Camps, J.; Micol, V.; Herranz-Lopez, M.; Menendez, J.A.; Borras, I.; et al. Plant-derived polyphenols regulate expression of mirna paralogs mir-103/107 and mir-122 and prevent diet-induced fatty liver disease in hyperlipidemic mice. Biochim. Biophys. Acta. 2012, 1820, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Joven, J.; March, I.; Espinel, E.; Fernandez-Arroyo, S.; Rodriguez-Gallego, E.; Aragones, G.; Beltran-Debon, R.; Alonso-Villaverde, C.; Rios, L.; Martin-Paredero, V.; et al. Hibiscus sabdariffa extract lowers blood pressure and improves endothelial function. Mol. Nutr. Food Res. 2014, 58, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Villalpando-Arteaga, E.V.; Mendieta-Condado, E.; Esquivel-Solis, H.; Canales-Aguirre, A.A.; Galvez-Gastelum, F.J.; Mateos-Diaz, J.C.; Rodriguez-Gonzalez, J.A.; Marquez-Aguirre, A.L. Hibiscus sabdariffa l. Aqueous extract attenuates hepatic steatosis through down-regulation of ppar-gamma and srebp-1c in diet-induced obese mice. Food Funct. 2013, 4, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Y.; Peng, C.H.; Chan, K.C.; Yang, Y.S.; Huang, C.N.; Wang, C.J. The hypolipidemic effect of hibiscus sabdariffa polyphenols via inhibiting lipogenesis and promoting hepatic lipid clearance. J. Agric. Food Chem. 2010, 58, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Sinclair, D.A. Xenohormesis: Sensing the chemical cues of other species. Cell 2008, 133, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Lamming, D.W.; Wood, J.G.; Sinclair, D.A. Small molecules that regulate lifespan: Evidence for xenohormesis. Mol. Microbiol. 2004, 53, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef] [PubMed]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Hooper, P.L.; Hooper, P.L.; Tytell, M.; Vígh, L. Xenohormesis: Health benefits from an eon of plant stress response evolution. Cell Stress Chaperones 2010, 15, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.; Vianello, F.; Corrêa, C.; Campos, R.; Borguini, M. Polyphenols in fruits and vegetables and its effect on human health. Food Nutr. Sci. 2014, 5, 1065–1082. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073s–2085s. [Google Scholar] [PubMed]
- Devalaraja, S.; Jain, S.; Yadav, H. Exotic fruits as therapeutic complements for diabetes, obesity and metabolic syndrome. Food Res. Int. 2011, 44, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Tufts, H.R.; Harris, C.S.; Bukania, Z.N.; Johns, T. Antioxidant and anti-inflammatory activities of kenyan leafy green vegetables, wild fruits, and medicinal plants with potential relevance for kwashiorkor. Evid. Based Complement Alternat. Med. 2015, 2015, 807158. [Google Scholar] [CrossRef] [PubMed]
- Brglez Mojzer, E.; Knez Hrncic, M.; Skerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Medina, I.C.; Beltran-Debon, R.; Molina, V.M.; Alonso-Villaverde, C.; Joven, J.; Menendez, J.A.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Direct characterization of aqueous extract of hibiscus sabdariffa using HPLC with diode array detection coupled to ESI and ion trap MS. J. Sep. Sci. 2009, 32, 3441–3448. [Google Scholar] [CrossRef] [PubMed]
- Serban, C.; Sahebkar, A.; Ursoniu, S.; Andrica, F.; Banach, M. Effect of sour tea (Hibiscus sabdariffa L.) on arterial hypertension: A systematic review and meta-analysis of randomized controlled trials. J. Hypertens. 2015, 33, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.L.; Zhen, J.; Qi, Y.; Chin, S.L.; Breithaupt, M.; Wu, Q.L.; Simon, J.; Henson, J.; Ferchaud, V. A comparative evaluation: Phytochemical composition and antioxidant capacity of three roselle (Hibiscus sabdariffa L.) accessions. Acta. Hortic. 2016, 1125, 99–107. [Google Scholar] [CrossRef]
- Sindi, H.A.; Marshall, L.J.; Morgan, M.R.A. Comparative chemical and biochemical analysis of extracts of hibiscus sabdariffa. Food Chem. 2014, 164, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Villani, T.; Juliani, H.R.; Simon, J.E.; Wu, Q.L. Hibiscus sabdariffa: Phytochemistry, quality control and health properties. ACS Symp. Ser. 2013, 1127, 209–230. [Google Scholar]
- Huang, W.Y.; Zhang, H.C.; Liu, W.X.; Li, C.Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. Food Comp. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Chang, Y.C.; Huang, K.X.; Huang, A.C.; Ho, Y.C.; Wang, C.J. Hibiscus anthocyanins-rich extract inhibited LDL oxidation and oxLDL-mediated macrophages apoptosis. Food Chem. Toxicol. 2006, 44, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.J.; McIntosh, J.; Pearce, P.; Camden, B.; Jordan, B.R. Anthocyanin and antioxidant capacity in roselle (Hhibiscus sabdariffa L.) extract. Food Res. Int. 2002, 35, 351–356. [Google Scholar] [CrossRef]
- Mahdavi, S.A.; Jafari, S.M.; Ghorbani, M.; Assadpoor, E. Spray-drying microencapsulation of anthocyanins by natural biopolymers: A review. Drying Technol. 2014, 32, 509–518. [Google Scholar] [CrossRef]
- Avior, Y.; Bomze, D.; Ramon, O.; Nahmias, Y. Flavonoids as dietary regulators of nuclear receptor activity. Food Funct. 2013, 4, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Joven, J.; Micol, V.; Segura-Carretero, A.; Alonso-Villaverde, C.; Menéndez, J.A.; Aragonès, G.; Barrajón-Catalán, E.; Beltrán-Debón, R.; Camps, J.; Cufí, S.; et al. Polyphenols and the modulation of gene expression pathways: Can we eat our way out of the danger of chronic disease? Crit. Rev. Food Sci. Nutr. 2014, 54, 985–1001. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, S.M.; Salama, M.M.; Seif El-Din, S.H.; Saleh, S.; El-Lakkany, N.M.; Hammam, O.A.; Salem, M.B.; Botros, S.S. Metabolic profile and hepatoprotective activity of the anthocyanin-rich extract of Hibiscus sabdariffa calyces. Pharm. Biol. 2016, 54, 3172–3181. [Google Scholar] [CrossRef] [PubMed]
- Borrás-Linares, I.; Herranz-López, M.; Barrajón-Catalán, E.; Arráez-Román, D.; González-Álvarez, I.; Bermejo, M.; Gutiérrez, A.F.; Micol, V.; Segura-Carretero, A. Permeability study of polyphenols derived from a phenolic-enriched hibiscus sabdariffa extract by UHPLC-ESI-UHR-QQ-TOF-MS. Int. J. Mol. Sci. 2015, 16, 18396–18411. [Google Scholar] [CrossRef] [PubMed]
- Muller, B.M.; Franz, G. Chemical structure and biological activity of polysaccharides from Hibiscus sabdariffa. Planta Med. 1992, 58, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Sayago-Ayerdi, S.G.; Arranz, S.; Serrano, J.; Goni, I. Dietary fiber content and associated antioxidant compounds in roselle flower (Hibiscus sabdariffa L.) beverage. J. Agric. Food Chem. 2007, 55, 7886–7890. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Debon, R.; Rodriguez-Gallego, E.; Fernandez-Arroyo, S.; Senan-Campos, O.; Massucci, F.A.; Hernandez-Aguilera, A.; Sales-Pardo, M.; Guimera, R.; Camps, J.; Menendez, J.A.; et al. The acute impact of polyphenols from hibiscus sabdariffa in metabolic homeostasis: An approach combining metabolomics and gene-expression analyses. Food Funct. 2015, 6, 2957–2966. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Z.; Li, S.; Ye, X.; Li, X.; He, K. Synergy effects of herb extracts: Pharmacokinetics and pharmacodynamic basis. Fitoterapia 2014, 92, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J. Botanical drugs, synergy, and network pharmacology: Forth and back to intelligent mixtures. Planta Med. 2011, 77, 1086–1098. [Google Scholar] [CrossRef] [PubMed]
- Keith, C.T.; Borisy, A.A.; Stockwell, B.R. Multicomponent therapeutics for networked systems. Nat. Rev. Drug Discov. 2005, 4, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.K.; Søderholm, K.L.; Isaksson, J.; Andersen, J.H.; Hansen, E. Metabolomic profiling reveals the n-acyl-taurine geodiataurine in extracts from the marine sponge Geodia macandrewii (Bowerbank). J. Nat. Prod. 2016, 79, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Frank, T.; Janssen, M.; Netzel, M.; Strass, G.; Kler, A.; Kriesl, E.; Bitsch, I. Pharmacokinetics of anthocyanidin-3-glycosides following consumption of Hibiscus sabdariffa L. Extract. J. Clin. Pharmacol. 2005, 45, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.; Piskula, M.K. Food content, processing, absorption and metabolism of onion flavonoids. Crit. Rev. Food Sci. Nutr. 2007, 47, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Del Mar Contreras, M.; Borras-Linares, I.; Herranz-Lopez, M.; Micol, V.; Segura-Carretero, A. Further exploring the absorption and enterocyte metabolism of quercetin forms in the caco-2 model using NANO-LC-TOF-MS. Electrophoresis 2016, 37, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Herranz-López, M.; Borrás-Linares, I.; Olivares-Vicente, M.; Gálvez, J.; Segura-Carretero, A.; Micol, V. Correlation between the cellular metabolism of quercetin and its glucuronide metabolite and oxidative stress in hypertrophied 3t3-l1 adipocytes. Phytomedicine 2017, 25, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, P.; Ghosh, A.K.; Ghosh, C. Recent developments on polyphenol-protein interactions: Effects on tea and coffee taste, antioxidant properties and the digestive system. Food Funct. 2012, 3, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Hansawasdi, C.; Kawabata, J.; Kasai, T. Α-amylase inhibitors from roselle (Hibiscus sabdariffa L.) tea. Biosci. Biotechnol. Biochem. 2000, 64, 1041–1043. [Google Scholar] [CrossRef] [PubMed]
- Hansawasdi, C.; Kawabata, J.; Kasai, T. Hibiscus acid as an inhibitor of starch digestion in the caco-2 cell model system. Biosci. Biotechnol. Biochem. 2001, 65, 2087–2089. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Zarrabal, O.; Hayward-Jones, P.M.; Orta-Flores, Z.; Nolasco-Hipolito, C.; Barradas-Dermitz, D.M.; Aguilar-Uscanga, M.G.; Pedroza-Hernandez, M.F.; et al. Effect of hibiscus sabdariffa l. Dried calyx ethanol extract on fat absorption-excretion, and body weight implication in rats. J. Biomed. Biotechnol. 2009, 2009, 5. [Google Scholar] [CrossRef] [PubMed]
- Rodina, A.V.; Severin, S.E. The role of adiponectin in the pathogenesis of the metabolic syndrome and approach to therapy. Patol. Fiziol. Eksp. Ter. 2013, 15–26. [Google Scholar]
- Encinar, J.A.; Fernandez-Ballester, G.; Galiano-Ibarra, V.; Micol, V. In silico approach for the discovery of new ppargamma modulators among plant-derived polyphenols. Drug Des. Devel. Ther. 2015, 9, 5877–5895. [Google Scholar] [CrossRef] [PubMed]
- Galiano, V.; Garcia-Valtanen, P.; Micol, V.; Encinar, J.A. Looking for inhibitors of the dengue virus ns5 rna-dependent rna-polymerase using a molecular docking approach. Drug Des. Devel. Ther. 2016, 10, 3163–3181. [Google Scholar] [CrossRef] [PubMed]
- Priscilla, D.H.; Roy, D.; Suresh, A.; Kumar, V.; Thirumurugan, K. Naringenin inhibits alpha-glucosidase activity: A promising strategy for the regulation of postprandial hyperglycemia in high fat diet fed streptozotocin induced diabetic rats. Chem. Biol. Interact. 2014, 210, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna, M.; Hejmo, T.; Poterala-Hejmo, A.; Cieslar-Pobuda, A.; Buldak, R.J. Nadph oxidases: Insights into selected functions and mechanisms of action in cancer and stem cells. Oxid. Med. Cell. Longev. 2017, 2017, 9420539. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Yeop Han, C.; Kargi, A.Y.; Omer, M.; Chan, C.K.; Wabitsch, M.; O'Brien, K.D.; Wight, T.N.; Chait, A. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: Dissociation of adipocyte hypertrophy from inflammation. Diabetes 2010, 59, 386–396. [Google Scholar] [PubMed]
- Sakurai, T.; Ogasawara, J.; Shirato, K.; Izawa, T.; Oh-Ishi, S.; Ishibashi, Y.; Radak, Z.; Ohno, H.; Kizaki, T. Exercise training attenuates the dysregulated expression of adipokines and oxidative stress in white adipose tissue. Oxid. Med. Cell. Longev. 2017, 2017, 9410954. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gou, Y.; Zhao, T.; Zhao, J.; Li, F.; Zhang, B.; Wu, X. Antioxidant capacity of extracts from calyx fruits of roselle (Hibiscus sabdariffa l.). Afr. J. Biotechnol. 2012, 11, 4063–4068. [Google Scholar]
- Tseng, T.H.; Kao, E.S.; Chu, C.Y.; Chou, F.P.; Lin Wu, H.W.; Wang, C.J. Protective effects of dried flower extracts of hibiscus sabdariffa l. Against oxidative stress in rat primary hepatocytes. Food Chem. Toxicol. 1997, 35, 1159–1164. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Salawu, N.A.; Yakubu, M.T.; Oladiji, A.T.; Akanji, M.A.; Okogun, J.I. Antioxidant and drug detoxification potentials of hibiscus sabdariffa anthocyanin extract. Drug Chem. Toxicol. 2011, 34, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Farombi, E.O.; Fakoya, A. Free radical scavenging and antigenotoxic activities of natural phenolic compounds in dried flowers of Hibiscus sabdariffa l. Mol. Nutr. Food Res. 2005, 49, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Essa, M.M.; Subramanian, P. Hibiscus sabdariffa affects ammonium chloride-induced hyperammonemic rats. Evid. Based Complement Alternat. Med. 2007, 4, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Farombi, E.O.; Ige, O.O. Hypolipidemic and antioxidant effects of ethanolic extract from dried calyx of Hibiscus sabdariffa in alloxan-induced diabetic rats. Fundam. Clin. Pharmacol. 2007, 21, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M.; Adesanoye, O.A.; Udo, I.E.; Adegoke, O.A.; Raji, J.; Farombi, E.O. Hibiscus sabdariffa ethanolic extract protects against dyslipidemia and oxidative stress induced by chronic cholesterol administration in rabbits. Afr. J. Med. Med. Sci. 2010, 39, 161–170. [Google Scholar] [PubMed]
- Adeyemi, D.O.; Ukwenya, V.O.; Obuotor, E.M.; Adewole, S.O. Anti-hepatotoxic activities of hibiscus sabdariffa l. In animal model of streptozotocin diabetes-induced liver damage. BMC Complement Altern. Med. 2014, 14, 277. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Raji, H.O.; Adeleye, A.O.; Adigun, N.S.; Giwa, O.B.; Ojewuyi, O.B.; Oladiji, A.T. Hibiscus sabdariffa calyx palliates insulin resistance, hyperglycemia, dyslipidemia and oxidative rout in fructose-induced metabolic syndrome rats. J. Sci. Food Agric. 2016, 96, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Zuniga-Munoz, A.; Guarner, V.; Diaz-Cruz, A.; Diaz-Diaz, E.; Beltran-Rodriguez, U.; Perez-Torres, I. Modulation of oxidative stress in fatty liver of rat with metabolic syndrome by Hibiscus sabdariffa. Immunol. Endocr. Metab. Agents Med. Chem 2013, 13, 196–205. [Google Scholar]
- Greenberg, A.S.; Obin, M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006, 83, 461s–465s. [Google Scholar] [PubMed]
- Kang, Y.E.; Kim, J.M.; Joung, K.H.; Lee, J.H.; You, B.R.; Choi, M.J.; Ryu, M.J.; Ko, Y.B.; Lee, M.A.; Lee, J.; et al. The roles of adipokines, proinflammatory cytokines, and adipose tissue macrophages in obesity-associated insulin resistance in modest obesity and early metabolic dysfunction. PLoS ONE 2016, 11, e0154003. [Google Scholar] [CrossRef] [PubMed]
- O'Neill, S.; O'Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cancello, R.; Clement, K. Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. Bjog 2006, 113, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; Manti, S.; D'Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef] [PubMed]
- Kao, E.S.; Hsu, J.D.; Wang, C.J.; Yang, S.H.; Cheng, S.Y.; Lee, H.J. Polyphenols extracted from Hibiscus sabdariffa l. Inhibited lipopolysaccharide-induced inflammation by improving antioxidative conditions and regulating cyclooxygenase-2 expression. Biosci. Biotechnol. Biochem. 2009, 73, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Ichiyama, T.; Hasegawa, M.; Hasegawa, S.; Matsubara, T.; Furukawa, S. Cysteinyl leukotrienes induce monocyte chemoattractant protein-1 in human monocyte/macrophages via mitogen-activated protein kinase and nuclear factor-kappab pathways. Int. Arch. Allergy. Immunol. 2009, 149, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L.; Lamm, M.G.; Funk, J.L.; Ritenbaugh, C. Hibiscus sabdariffa l. In the treatment of hypertension and hyperlipidemia: A comprehensive review of animal and human studies. Fitoterapia 2013, 85, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Arellano, A.; Miranda-Sanchez, J.; Avila-Castro, P.; Herrera-Alvarez, S.; Jimenez-Ferrer, J.E.; Zamilpa, A.; Roman-Ramos, R.; Ponce-Monter, H.; Tortoriello, J. Clinical effects produced by a standardized herbal medicinal product of hibiscus sabdariffa on patients with hypertension. A randomized, double-blind, lisinopril-controlled clinical trial. Planta Med. 2007, 73, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, D.C.; Aneke, E.; Nwachukwu, N.Z.; Obika, L.F.; Nwagha, U.I.; Eze, A.A. Effect of hibiscus sabdariffaon blood pressure and electrolyte profile of mild to moderate hypertensive nigerians: A comparative study with hydrochlorothiazide. Niger. J. Clin. Pract. 2015, 18, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Perez-Torres, I.; Ruiz-Ramirez, A.; Banos, G.; El-Hafidi, M. Hibiscus sabdariffa Linnaeus (Malvaceae), curcumin and resveratrol as alternative medicinal agents against metabolic syndrome. Cardiovasc. Hematol. Agents Med. Chem. 2013, 11, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Aguilar, F.J.; Zamilpa, A.; Perez-Garcia, M.D.; Almanza-Perez, J.C.; Romero-Nunez, E.; Campos-Sepulveda, E.A.; Vazquez-Carrillo, L.I.; Roman-Ramos, R. Effect of Hibiscus sabdariffa on obesity in msg mice. J. Ethnopharmacol. 2007, 114, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Gurrola-Díaz, C.M.; García-López, P.M.; Sánchez-Enríquez, S.; Troyo-Sanromán, R.; Andrade-González, I.; Gómez-Leyva, J.F. Effects of hibiscus sabdariffa extract powder and preventive treatment (diet) on the lipid profiles of patients with metabolic syndrome (mesy). Phytomedicine 2010, 17, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Gosain, S.; Ircchiaya, R.; Sharma, P.C.; Thareja, S.; Kalra, A.; Deep, A.; Bhardwaj, T.R. Hypolipidemic effect of ethanolic extract from the leaves of Hibiscus sabdariffa l. In hyperlipidemic rats. Acta. Pol. Pharm. 2010, 67, 179–184. [Google Scholar] [PubMed]
- Ochani, P.C.; D'Mello, P. Antioxidant and antihyperlipidemic activity of Hibiscus sabdariffa linn. Leaves and calyces extracts in rats. Indian. J. Exp. Biol. 2009, 47, 276–282. [Google Scholar] [PubMed]
- Srivastava, R.A.K.; Pinkosky, S.L.; Filippov, S.; Hanselman, J.C.; Cramer, C.T.; Newton, R.S. Amp-activated protein kinase: An emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases: Thematic review series: New lipid and lipoprotein targets for the treatment of cardiometabolic diseases. J. Lipid. Res. 2012, 53, 2490–2514. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating amp-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Calleri, E.; Pochetti, G.; Dossou, K.S.S.; Laghezza, A.; Montanari, R.; Capelli, D.; Prada, E.; Loiodice, F.; Massolini, G.; Bernier, M.; et al. Resveratrol and its metabolites bind to ppars. Chembiochem 2014, 15, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Han, M.; Ting, H.L.; Liu, Z.; Zhang, D. Scutellarin from scutellaria baicalensis suppresses adipogenesis by upregulating pparalpha in 3T3-L1 cells. J. Nat. Prod. 2013, 76, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Laghezza, A.; Montanari, R.; Lavecchia, A.; Piemontese, L.; Pochetti, G.; Iacobazzi, V.; Infantino, V.; Capelli, D.; De Bellis, M.; Liantonio, A.; et al. On the metabolically active form of metaglidasen: Improved synthesis and investigation of its peculiar activity on peroxisome proliferator-activated receptors and skeletal muscles. ChemMedChem 2015, 10, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Vanden Berghe, W. Epigenetic impact of dietary polyphenols in cancer chemoprevention: Lifelong remodeling of our epigenomes. Pharmacol. Res. 2012, 65, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guo, Y.; Zhang, C.; Wu, R.; Yang, A.Y.; Gaspar, J.; Kong, A.N.T. Dietary phytochemicals and cancer chemoprevention: A perspective on oxidative stress, inflammation, and epigenetics. Chem. Res. Toxicol. 2016, 29, 2071–2095. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.S.; Deb, G.; Babcook, M.A.; Gupta, S. Plant phytochemicals as epigenetic modulators: Role in cancer chemoprevention. AAPS J. 2014, 16, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Lai, C.S.; Wu, J.C.; Ho, C.T. Epigenetic and disease targets by polyphenols. Curr. Pharm. Des. 2013, 19, 6156–6185. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.I.; Kapila, R. Dietary metabolites derived from gut microbiota: Critical modulators of epigenetic changes in mammals. Nutr. Rev. 2017, 75, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Stilling, R.M.; Dinan, T.G.; Cryan, J.F. Microbial genes, brain & behaviour-epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014, 13, 69–86. [Google Scholar] [PubMed]
- Ruiz, P.A.; Braune, A.; Hölzlwimmer, G.; Quintanilla-Fend, L.; Haller, D. Quercetin inhibits tnf-induced nf-κb transcription factor recruitment to proinflammatory gene promoters in murine intestinal epithelial cells. J. Nutr. 2007, 137, 1208–1215. [Google Scholar] [PubMed]
- Rottiers, V.; Naar, A.M. Micrornas in metabolism and metabolic disorders. Nat. Rev. Mol. Cell Biol. 2012, 13, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, S.; Mach, N. Therapeutic potential of hibiscus sabdariffa: A review of the scientific evidence. Endocrinol. Nutr. 2014, 63, 274–295. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. Amp-activated protein kinase: An energy sensor that regulates all aspects of cell function. Genes Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Sanchez, C.; Olivares-Vicente, M.; Rodriguez-Perez, C.; Herranz-Lopez, M.; Lozano-Sanchez, J.; Segura Carretero, A.; Fernandez-Gutierrez, A.; Micol, V. Ampk modulatory activity of olive–tree leaves phenolic compounds: Bioassay-guided isolation on adipocyte model and in silico approach. PLoS ONE 2017. [Google Scholar] [CrossRef] [PubMed]
- Hardwicke, M.A.; Rendina, A.R.; Williams, S.P.; Moore, M.L.; Wang, L.; Krueger, J.A.; Plant, R.N.; Totoritis, R.D.; Zhang, G.; Briand, J.; et al. A human fatty acid synthase inhibitor binds beta-ketoacyl reductase in the keto-substrate site. Nat. Chem. Biol. 2014, 10, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Piemontese, L. Plant food supplements with antioxidant properties for the treatment of chronic and neurodegenerative diseases: Benefits or risks? J. Diet Suppl. 2017, 14, 478–484. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herranz-López, M.; Olivares-Vicente, M.; Encinar, J.A.; Barrajón-Catalán, E.; Segura-Carretero, A.; Joven, J.; Micol, V. Multi-Targeted Molecular Effects of Hibiscus sabdariffa Polyphenols: An Opportunity for a Global Approach to Obesity. Nutrients 2017, 9, 907. https://doi.org/10.3390/nu9080907
Herranz-López M, Olivares-Vicente M, Encinar JA, Barrajón-Catalán E, Segura-Carretero A, Joven J, Micol V. Multi-Targeted Molecular Effects of Hibiscus sabdariffa Polyphenols: An Opportunity for a Global Approach to Obesity. Nutrients. 2017; 9(8):907. https://doi.org/10.3390/nu9080907
Chicago/Turabian StyleHerranz-López, María, Mariló Olivares-Vicente, José Antonio Encinar, Enrique Barrajón-Catalán, Antonio Segura-Carretero, Jorge Joven, and Vicente Micol. 2017. "Multi-Targeted Molecular Effects of Hibiscus sabdariffa Polyphenols: An Opportunity for a Global Approach to Obesity" Nutrients 9, no. 8: 907. https://doi.org/10.3390/nu9080907
APA StyleHerranz-López, M., Olivares-Vicente, M., Encinar, J. A., Barrajón-Catalán, E., Segura-Carretero, A., Joven, J., & Micol, V. (2017). Multi-Targeted Molecular Effects of Hibiscus sabdariffa Polyphenols: An Opportunity for a Global Approach to Obesity. Nutrients, 9(8), 907. https://doi.org/10.3390/nu9080907









