Changes of Bone-Related Minerals during Denosumab Administration in Post-Menopausal Osteoporotic Patients
Abstract
:1. Introduction
2. Materials and Methods
Patient Characteristics
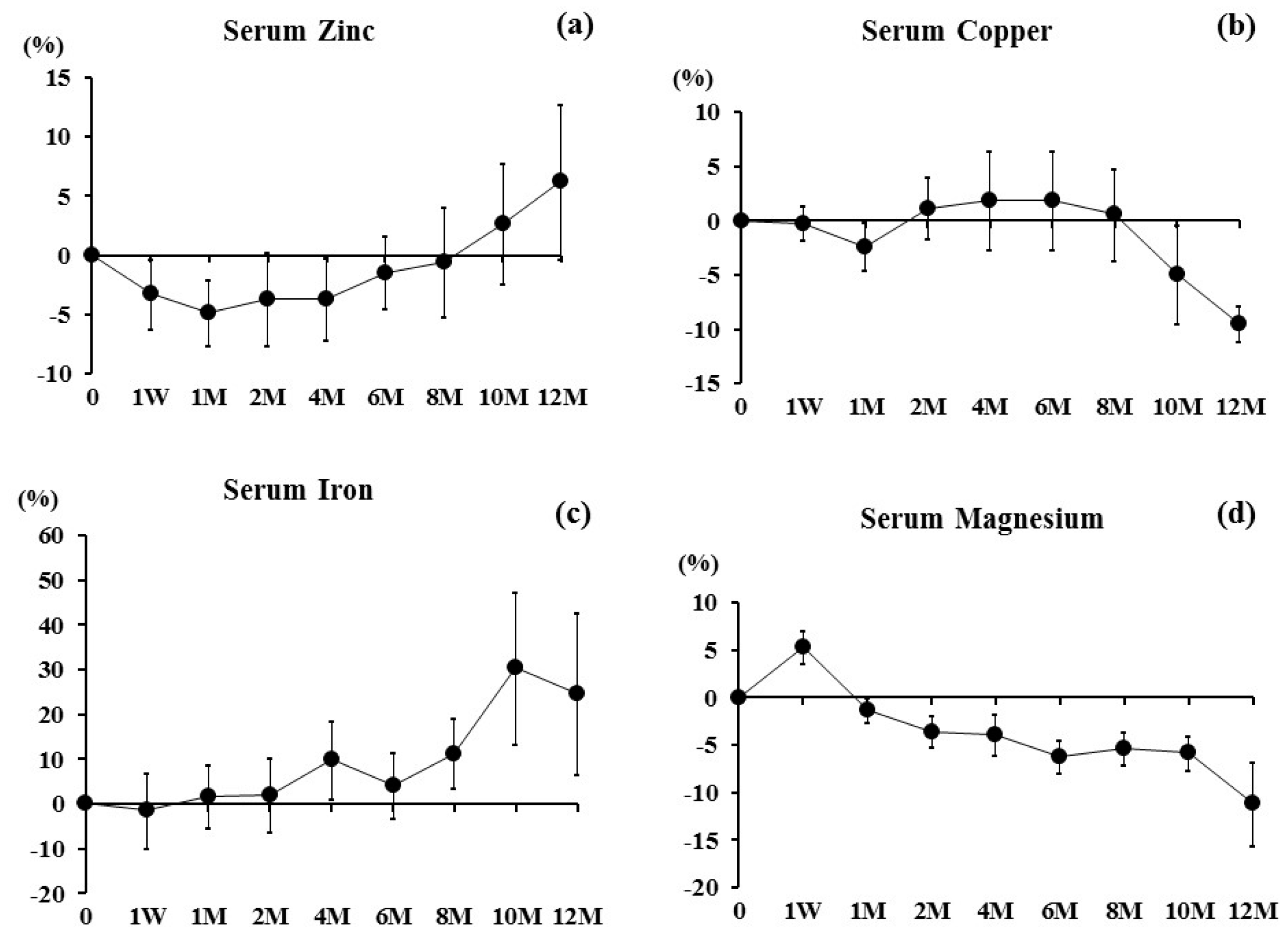
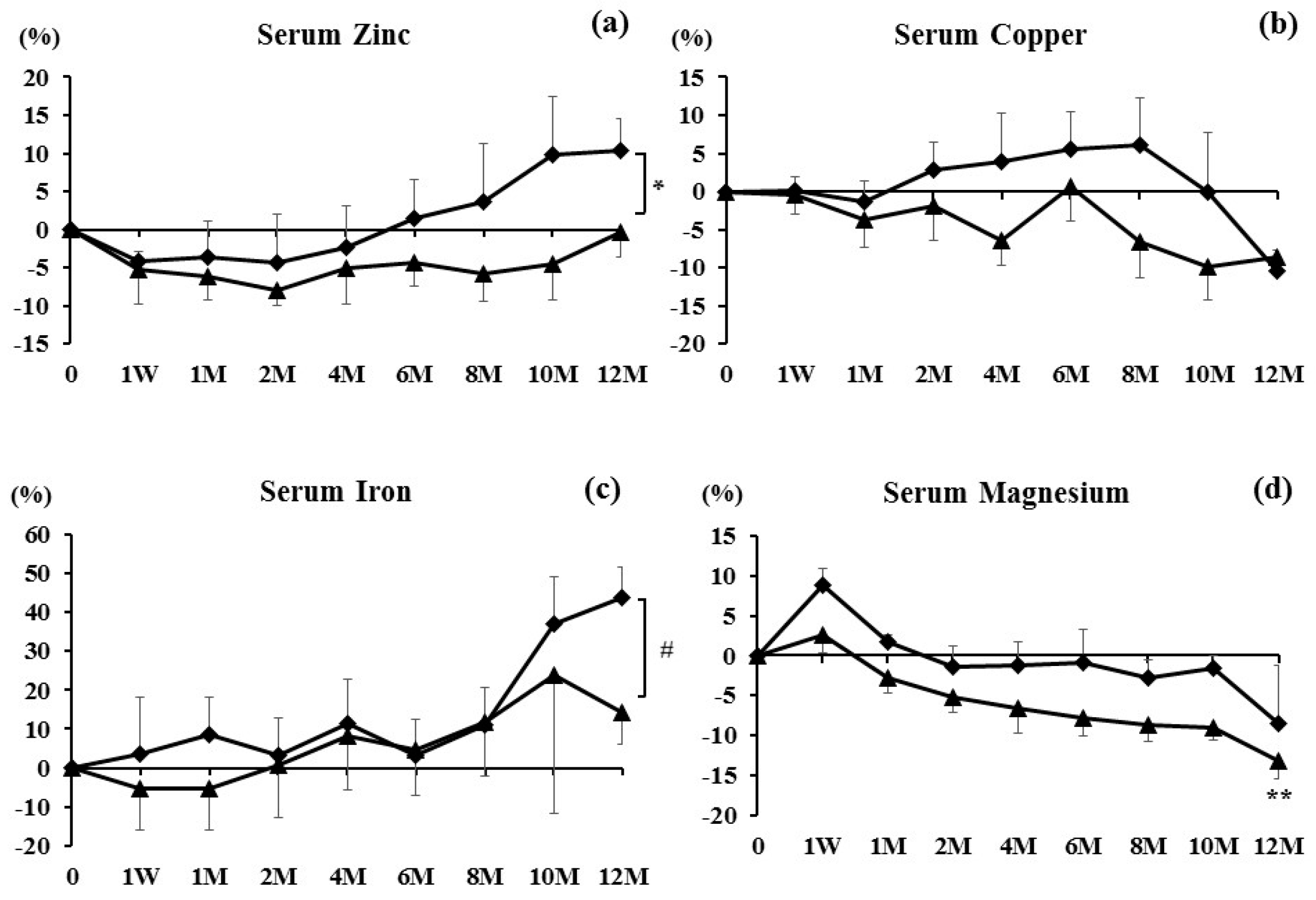
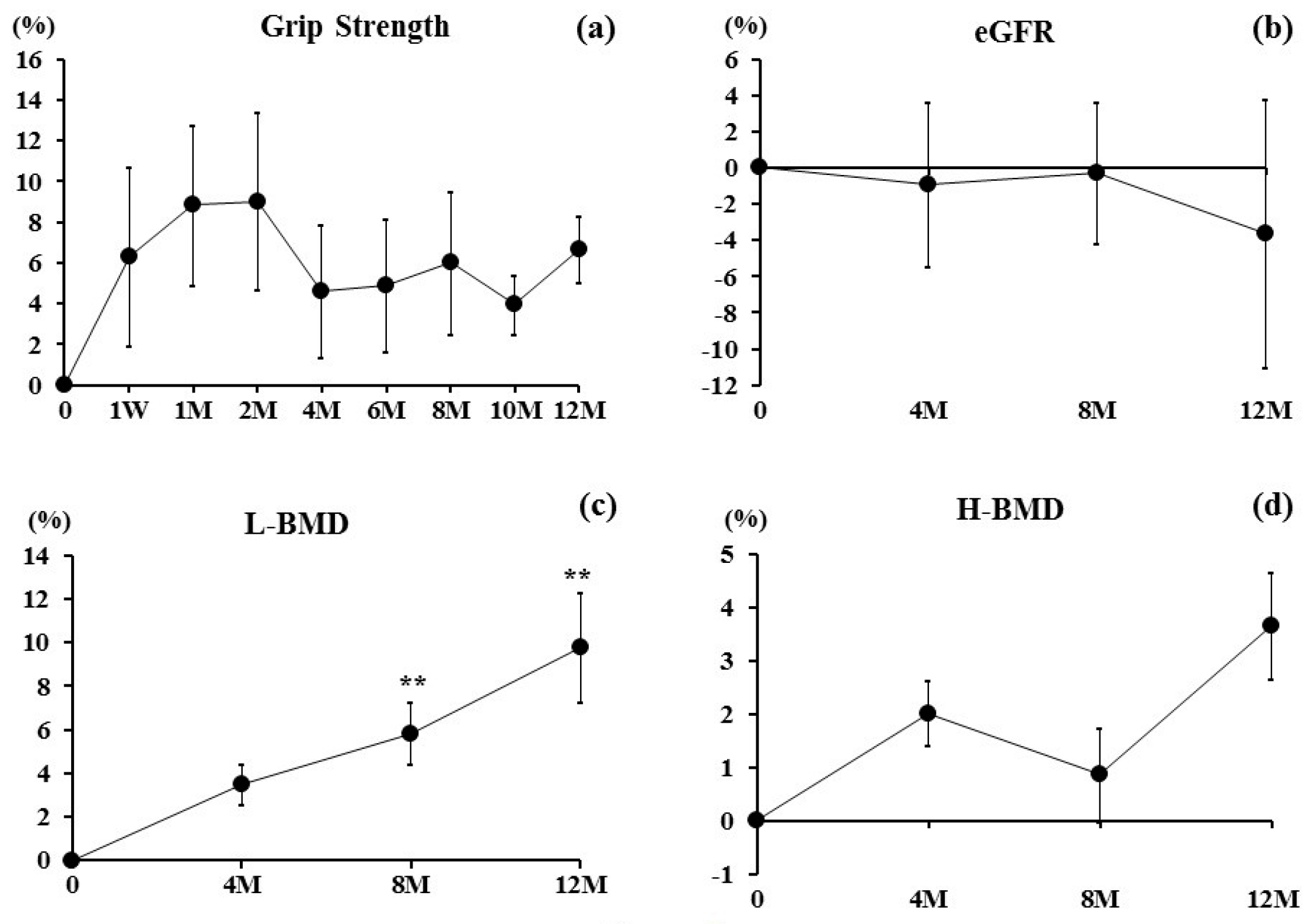
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Gür, A.; Colpan, L.; Nas, K.; Cevik, R.; Saraç, J.; Erdoğan, F.; Düz, M.Z. The role of trace minerals in the pathogenesis of postmenopausal osteoporosis and a new effect of calcitonin. J. Bone Miner Metab. 2002, 20, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.N.; Uihlein, A.V.; Lee, H.; Kumbhani, R.; Siwila-Sackman, E.; McKay, E.A.; Burnett-Bowie, S.A.; Neer, R.M.; Leder, B.Z. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: The DATA study randomised trial. Lancet 2013, 382, 50–56. [Google Scholar] [CrossRef]
- Leder, B.Z.; Tsai, J.N.; Uihlein, A.V.; Wallace, P.M.; Lee, H.; Neer, R.M.; Burnett-Bowie, S.A. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): Extension of a randomised controlled trial. Lancet 2015, 386, 1147–1455. [Google Scholar] [CrossRef]
- Diab, D.L.; Watts, N.B. Denosumab in osteoporosis. Expert Opin. Drug Saf. 2014, 13, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Okada, N.; Kawazoe, K.; Teraoka, K.; Kujime, T.; Abe, M.; Shinohara, M.; Minakuchi, K. Identification of the risk factors associated with hypocalcemia induced by denosumab. Biol. Pharm. Bull. 2013, 36, 1622–1626. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Matsumoto, T.; Hosoi, T.; Miki, T.; Gorai, I.; Yoshikawa, H.; Tanaka, Y.; Tanaka, S.; Fukunaga, M.; Sone, T.; et al. Three-year denosumab treatment in postmenopausal Japanese women and men with osteoporosis: results from a 1-year open-label extension of the Denosumab Fracture Intervention Randomized Placebo Controlled Trial (DIRECT). Osteoporos Int. 2015, 26, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, M.; Argun, M.; Kilic, E.; Saraymen, R.; Yazar, S. Magnesium, zinc and copper status in osteoporotic, osteopenic and normal post-menopausal women. J. Int. Med. Res. 2007, 35, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Rude, R.K.; Singer, F.R.; Gruber, H.E. Skeletal and hormonal effects of magnesium deficiency. J. Am. Coll. Nutr. 2009, 28, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Katsumata, S.; Matsuzaki, H.; Suzuki, K. Dietary zinc deficiency induces oxidative stress and promotes tumor necrosis factor-α- and interleukin-1β-induced RANKL expression in rat bone. J. Clin. Biochem. Nutr. 2016, 58, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Hill, T.; Meunier, N.; Andriollo-Sanchez, M.; Ciarapica, D.; Hininger-Favier, I.; Polito, A.; O’Connor, J.M.; Coudray, C.; Cashman, K.D. The relationship between the zinc nutritive status and biochemical markers of bone turnover in older European adults: The ZENITH study. Eur. J. Clin. Nutr. 2005, 59, S73–S78. [Google Scholar] [CrossRef] [PubMed]
- Opsahl, W.; Zeronian, H.; Ellison, M.; Lewis, D.; Rucker, R.B.; Riggins, R.S. Role of copper in collagen cross-linking and its influence on selected mechanical properties of chick bone and tendon. J. Nutr. 1982, 112, 708–716. [Google Scholar] [PubMed]
- Jonas, J.; Burns, J.; Abel, E.W. Impaired mechanical strength of bone in experimental copper deficiency. Ann. Nutr. Metab. 1993, 37, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Orimo, H.; Nakamura, T.; Hosoi, T.; Iki, M.; Uenishi, K.; Endo, N.; Ohta, H.; Shiraki, M.; Sugimoto, T.; Suzuki, T.; et al. Japanese 2011 guidelines for prevention and treatment of osteoporosis—Executive summary. Arch. Osteoporos. 2012, 7, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Gur, A.; Colpan, L.; Cevik, R.; Nas, K.; Sarac, A.J. Comparison of zinc excretion and biochemical markers of bone remodelling in the assessment of the effects of alendronate and calcitonin on bone in postmenopausal osteoporosis. Clin. Biochem. 2005, 38, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Relea, P.; Revilla, M.; Ripoll, E.; Arribas, I.; Villa, L.F.; Rico, H. Zinc, biochemical markers of nutrition, and type I osteoporosis. Age Ageing 1995, 24, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Steidl, L.; Ditmar, R. Blood zinc findings in osteoporosis. Acta Univ. Palacki. Olomuc. Fac. Med. 1990, 126, 129–138. [Google Scholar] [PubMed]
- Holloway, W.R.; Collier, F.M.; Herbst, R.E.; Hodge, J.M.; Nicholson, G.C. Osteoblast-mediated effects of zinc on isolated rat osteoclast: Inhibition of bone resorption and enhancement of osteoclast number. Bone 1996, 19, 137–142. [Google Scholar] [CrossRef]
- Togari, A.; Arakawa, S.; Arai, M.; Matsumoto, S. Alteration of in vitro bone metabolism and tooth formation by zinc. Gen. Pharmacol. 1993, 24, 1133–1140. [Google Scholar] [CrossRef]
- Kim, D.E.; Cho, S.H.; Park, H.M.; Chang, Y.K. Relationship between bone mineral density and dietary intake of β-carotene, vitamin C, zinc and vegetables in postmenopausal Korean women: A cross-sectional study. J. Int. Med. Res. 2016, 44, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Kawada, E.; Moridaira, K.; Itoh, K.; Hoshino, A.; Tamura, J.; Morita, T. In long-term bedridden elderly patients with dietary copper deficiency, biochemical markers of bone resorption are increased with copper supplementation during 12 weeks. Ann. Nutr. Metab. 2006, 50, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.; Talwar, D.; Morrison, I. The predictive value of low plasma copper and high plasma zinc in detecting zinc-induced copperdeficiency. Ann. Clin. Biochem. 2016, 53, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Odabasi, E.; Turan, M.; Aydin, A.; Akay, C.; Kutlu, M. Magnesium, zinc, copper, manganese, and selenium levels in postmenopausal women with osteoporosis. Can magnesium play a key role in osteoporosis? Ann. Acad. Med. Singap. 2008, 37, 564–567. [Google Scholar] [PubMed]
- Toxqui, L.; Vaquero, M.P. Chronic iron deficiency as an emerging risk factor for osteoporosis: A hypothesis. Nutrients 2015, 7, 2324–2344. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, D.M.; Stoecker, B.; Plattner, A.; Jennings, D.; Haub, M. Iron deficiency negatively affects vertebrae and femurs of rats independently of energy intake and body weight. J. Nutr. 2004, 134, 3061–3067. [Google Scholar] [PubMed]
- Katsumata, S.; Katsumata-Tsuboi, R.; Uehara, M.; Suzuki, K. Severe iron deficiency decreases both bone formation and bone resorption in rats. J. Nutr. 2009, 139, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Castro, J.; Lopez-Frias, M.R.; Campos, M.S.; Lopez-Frias, M.; Alferez, M.J.; Nestares, T.; Ojeda, M.L.; Lopez-Aliaga, I. Severe nutritional iron-deficiency anaemia has a negative effect on some bone turnover biomarkers in rats. Eur. J. Nutr. 2012, 51, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Luthringer, B.J.; Feyerabend, F.; Schilling, A.F.; Willumeit, R. Effects of extracellular magnesium on the differentiation and function of human osteoclasts. Acta Biomater. 2014, 10, 2843–2854. [Google Scholar] [CrossRef] [PubMed]
- Slovik, D.M.; Neer, R.M.; Potts, J.T., Jr. Short-term effects of synthetic human parathyroid hormone-(1--34) administration on bone mineral metabolism in osteoporotic patients. J. Clin. Invest. 1981, 68, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Ebina, K.; Kashii, M.; Hirao, M.; Hashimoto, J.; Noguchi, T.; Koizumi, K.; Kitaguchi, K.; Matsuoka, H.; Iwahashi, T.; Tsukamoto, Y.; et al. Comparison of the effects of denosumab between a native vitamin D combination and an active vitamin D combination in patients with postmenopausal osteoporosis. J. Bone Miner. Metable. 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Stubbs, B.; Solmi, M.; Noale, M.; Vaona, A.; Demurtas, J.; Maggi, S. Dietary magnesium intake and fracture risk: Data from a large prospective study. Br. J. Nutr. 2017, 117, 1570–1576. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Overall |
|---|---|
| Zinc (μg/dL) | 70.2 ± 3.2 |
| Copper (μg/dL) | 120.3 ± 4.2 |
| Iron (μg/dL) | 76.7 ± 5.5 |
| Magnesium (μg/dL) | 2.1 ± 0.05 |
| eGFR (mL/min/1.73 m2) | 67.1 ± 3.6 |
| Grip strength (kg) | 15.9 ± 1.3 |
| L-BMD (g/cm2) | 0.761 ± 0.04 |
| H-BMD (g/cm2) | 0.619 ± 0.02 |
| Characteristic | PTH Group (n = 11) | BP Group (n = 10) | p Value |
|---|---|---|---|
| Age | 74.1 ± 2.3 | 73.7 ± 2.9 | p = 0.9147 |
| BMI (kg/cm2) | 20.1 ± 0.4 | 20.3 ± 0.7 | p = 0.8476 |
| Zinc (μg/dL) | 70.7 ± 4.3 | 68.7 ± 4.9 | p = 0.6500 |
| Copper (μg/dL) | 124.0 ± 4.6 | 116.2 ± 7.2 | p = 0.3763 |
| Iron (μg/dL) | 78.8 ± 4.4 | 73.9 ± 6.4 | p = 0.5380 |
| Magnesium (μg/dL) | 2.1 ± 0.06 | 2.2 ± 0.07 | p = 0.9616 |
| L-BMD (g/cm2) | 0.760 ± 0.04 | 0.767 ± 0.03 | p = 0.8872 |
| H-BMD (g/cm2) | 0.620 ± 0.01 | 0.614 ± 0.02 | p = 0.7984 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, T.; Nakamura, Y.; Kato, H. Changes of Bone-Related Minerals during Denosumab Administration in Post-Menopausal Osteoporotic Patients. Nutrients 2017, 9, 871. https://doi.org/10.3390/nu9080871
Suzuki T, Nakamura Y, Kato H. Changes of Bone-Related Minerals during Denosumab Administration in Post-Menopausal Osteoporotic Patients. Nutrients. 2017; 9(8):871. https://doi.org/10.3390/nu9080871
Chicago/Turabian StyleSuzuki, Takako, Yukio Nakamura, and Hiroyuki Kato. 2017. "Changes of Bone-Related Minerals during Denosumab Administration in Post-Menopausal Osteoporotic Patients" Nutrients 9, no. 8: 871. https://doi.org/10.3390/nu9080871
APA StyleSuzuki, T., Nakamura, Y., & Kato, H. (2017). Changes of Bone-Related Minerals during Denosumab Administration in Post-Menopausal Osteoporotic Patients. Nutrients, 9(8), 871. https://doi.org/10.3390/nu9080871





