The Effect of Buffering High Acid Load Meal with Sodium Bicarbonate on Postprandial Glucose Metabolism in Humans—A Randomized Placebo-Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design and Randomization
2.3. Meal Study Procedures
2.4. Blood Pressure and Arterial Stiffness
2.5. Sample Collection and Analysis
2.6. Hunger and Satiety Assessment
2.7. Statistical Analysis
3. Results
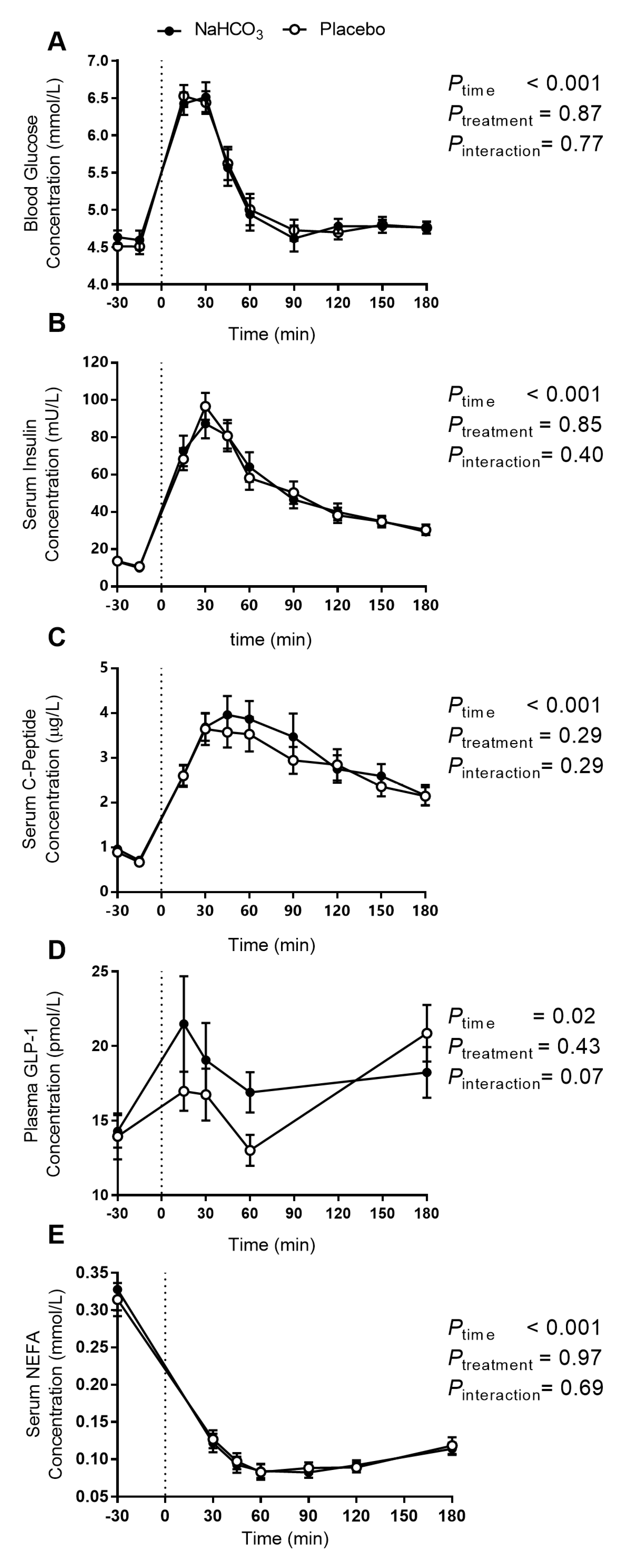
3.1. Postprandial Acidity Markers with Sodium Bicarbonate or Placebo
3.2. Postprandial Glucose Regulation with Sodium Bicarbonate or Placebo
3.3. Satiety and Hunger with Sodium Bicarbonate or Placebo
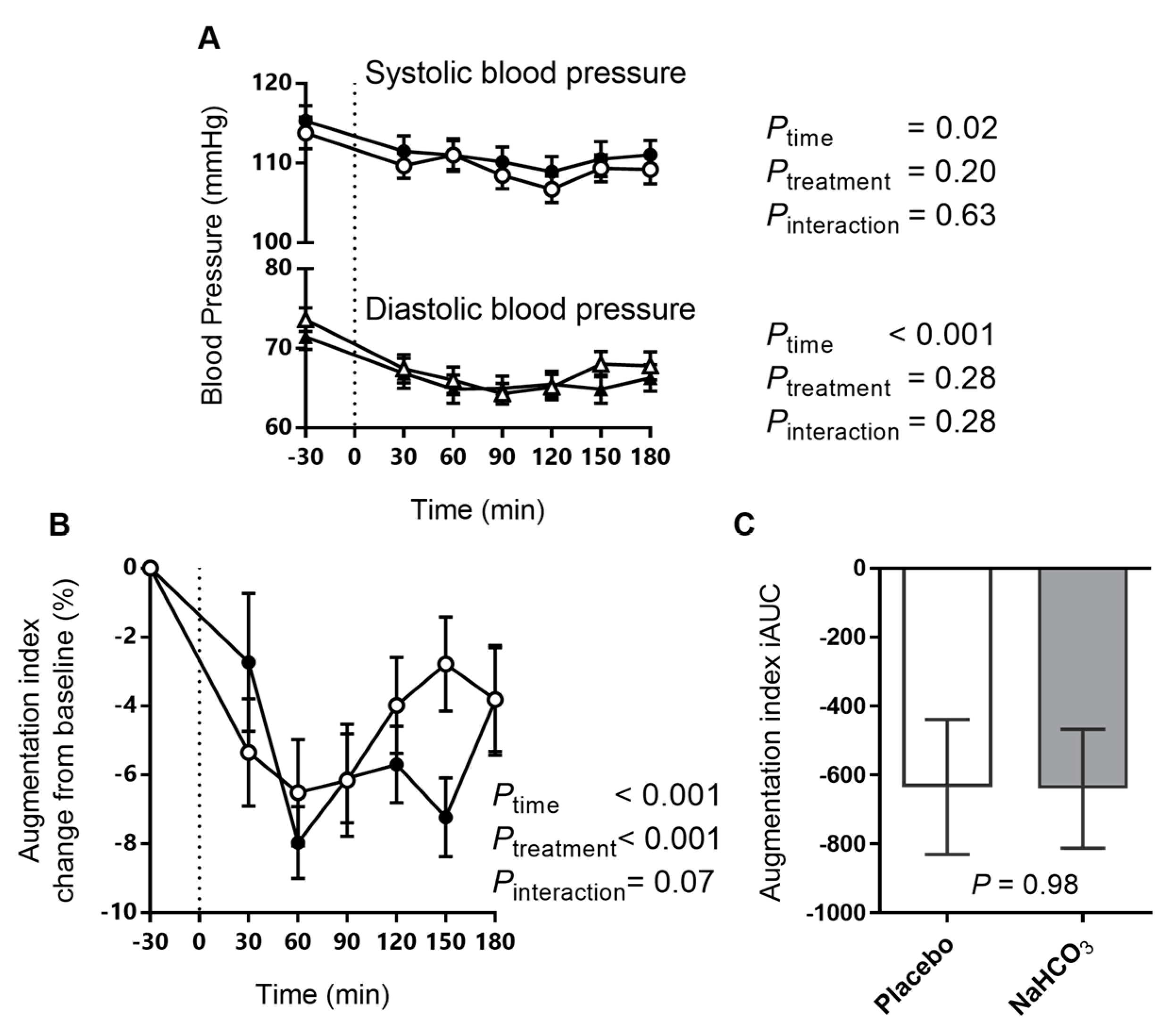
3.4. Arterial Stiffness and Blood Pressure with Sodium Bicarbonate or Placebo
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Koeppen, B.M. The kidney and acid-base regulation. Adv. Physiol. Educ. 2009, 33, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Rimm, E.; Smith-Warner, S.A.; Feskanich, D.; Stampfer, M.J.; Ascherio, A.; Sampson, L.; Willett, W.C. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am. J. Clin. Nutr. 1999, 69, 243–249. [Google Scholar] [PubMed]
- Williams, R.S.; Kozan, P.; Samocha-Bonet, D. The role of dietary acid load and mild metabolic acidosis in insulin resistance in humans. Biochimie 2016, 124, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Fagherazzi, G.; Vilier, A.; Bonnet, F.; Lajous, M.; Balkau, B.; Boutron-Rualt, M.C.; Clavel-Chapelon, F. Dietary acid load and risk of type 2 diabetes: The E3N-EPIC cohort study. Diabetologia 2014, 57, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Eguchi, M.; Kuwahara, K.; Kochi, T.; Ito, R.; Kurotani, K.; Tsuruoka, H.; Nanri, A.; Kabe, I.; Mizoue, T. High dietary acid load is associated with insulin resistance: The furukawa nutrition and health study. Clin. Nutr. 2016, 35, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Kiefte-de Jong, J.C.; Li, Y.; Chen, M.; Curhan, G.C.; Mattei, J.; Malik, V.S.; Forman, J.P.; Franco, O.H.; Hu, F.B. Diet-dependent acid load and type 2 diabetes: Pooled results from three prospective cohort studies. Diabetologia 2017, 60, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jia, T.; Huang, X.; Riserus, U.; Cederholm, T.; Arnlov, J.; Sjogren, P.; Lindholm, B.; Carrero, J.J. Dietary acid load, insulin sensitivity and risk of type 2 diabetes in community-dwelling older men. Diabetologia 2014, 57, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, H.O.; Zelnick, L.; Ruzinski, J.; Curtin, L.; Utzschneider, K.M.; Kestenbaum, B.; Himmelfarb, J.; de Boer, I.H. Dietary acid load is associated with serum bicarbonate but not insulin sensitivity in chronic kidney disease. J. Ren. Nutr. 2016, 26, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Farwell, W.R.; Taylor, E.N. Serum bicarbonate, anion gap and insulin resistance in the national health and nutrition examination survey. Diabet. Med. 2008, 25, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Mandel, E.I.; Curhan, G.C.; Hu, F.B.; Taylor, E.N. Plasma bicarbonate and risk of type 2 diabetes mellitus. Can. Med. Assoc. J. 2012, 184, E719–E725. [Google Scholar] [CrossRef] [PubMed]
- Haldane, J.B.S.; Wigglesworth, V.B.; Woodrow, C.E. Effect of Reaction Changes on Human Carbohydrate and Oxygen Metabolism. Proc. R. Soc. Lond. Ser. B Containing Pap. Biol. Character 1924, 96, 15–28. [Google Scholar] [CrossRef]
- Defronzo, R.A.; Beckles, A.D. Glucose intolerance following chronic metabolic acidosis in man. Am. J. Physiol. 1978, 236, E328–E334. [Google Scholar]
- Reaich, D.; Graham, K.A.; Channon, S.M.; Hetherington, C.; Scrimgeour, C.M.; Wilkinson, R.; Goodship, T.H. Insulin-mediated changes in PD and glucose uptake after correction of acidosis in humans with CRF. Am. J. Physiol. 1995, 268, E121–E126. [Google Scholar] [PubMed]
- Bellasi, A.; Di Micco, L.; Santoro, D.; Marzocco, S.; De Simone, E.; Cozzolino, M.; Di Lullo, L.; Guastaferro, P.; Di Iorio, B. UBI Study Investigators. Correction of metabolic acidosis improves insulin resistance in chronic kidney disease. BMC Nephrol. 2016, 17, 158. [Google Scholar] [CrossRef] [PubMed]
- Berkemeyer, S. Acid-base balance and weight gain: Are there crucial links via protein and organic acids in understanding obesity? Med. Hypotheses 2009, 73, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; Consort Group. Consort 2010 statement: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2011, 9, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, J.R.; Samaras, K.; Chisholm, D.J.; Campbell, L.V. Effect of postprandial insulinemia and insulin resistance on measurement of arterial stiffness (augmentation index). Int. J. Cardiol. 2007, 114, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.D.; McGrath, B.P.; Dart, A.M. Use of radial artery applanation tonometry and a generalized transfer function to determine aortic pressure augmentation in subjects with treated hypertension. J. Am. Coll. Cardiol. 1998, 32, 1214–1220. [Google Scholar] [CrossRef]
- Pruessner, J.C.; Kirschbaum, C.; Meinlschmid, G.; Hellhammer, D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003, 28, 916–931. [Google Scholar] [CrossRef]
- Remer, T. Influence of diet on acid-base balance. Semin. Dial. 2000, 13, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.L.; McLay-Cooke, R.T.; Brown, R.C.; Gray, A.R.; Fairbairn, K.A. Increased blood ph but not performance with sodium bicarbonate supplementation in elite rugby union players. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.; Robinson, A.L.; Sparks, S.A.; Bridge, C.A.; Bentley, D.J.; McNaughton, L.R. The effects of novel ingestion of sodium bicarbonate on repeated sprint ability. J. Strength Cond. Res. 2016, 30, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Buclin, T.; Cosma, M.; Appenzeller, M.; Jacquet, A.F.; Decosterd, L.A.; Biollaz, J.; Burckhardt, P. Diet acids and alkalis influence calcium retention in bone. Osteoporos. Int. 2001, 12, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Schwalfenberg, G.K. The alkaline diet: Is there evidence that an alkaline pH diet benefits health? J. Environ. Public Health 2012, 2012, 727630. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Huang, T. Systematic review of the association between dietary acid load, alkaline water and cancer. BMJ Open 2016, 6, e010438. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Lyon, A.W.; Eliasziw, M.; Tough, S.C.; Hanley, D.A. Meta-analysis of the effect of the acid-ash hypothesis of osteoporosis on calcium balance. J. Bone Miner. Res. 2009, 24, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Kurotani, K.; Kashino, I.; Goto, A.; Mizoue, T.; Noda, M.; Sawada, N.; Tsugane, S. Japan Public Health Center-based Prospective Study Group. High dietary acid load score is associated with increased risk of type 2 diabetes in Japanese men: The Japan public health center-based prospective study. J. Nutr. 2016, 146, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Gabutti, L.; Bianchi, G.; Soldini, D.; Marone, C.; Burnier, M. Haemodynamic consequences of changing bicarbonate and calcium concentrations in haemodialysis fluids. Nephrol. Dial. Transplant. 2009, 24, 973–981. [Google Scholar] [CrossRef] [PubMed]
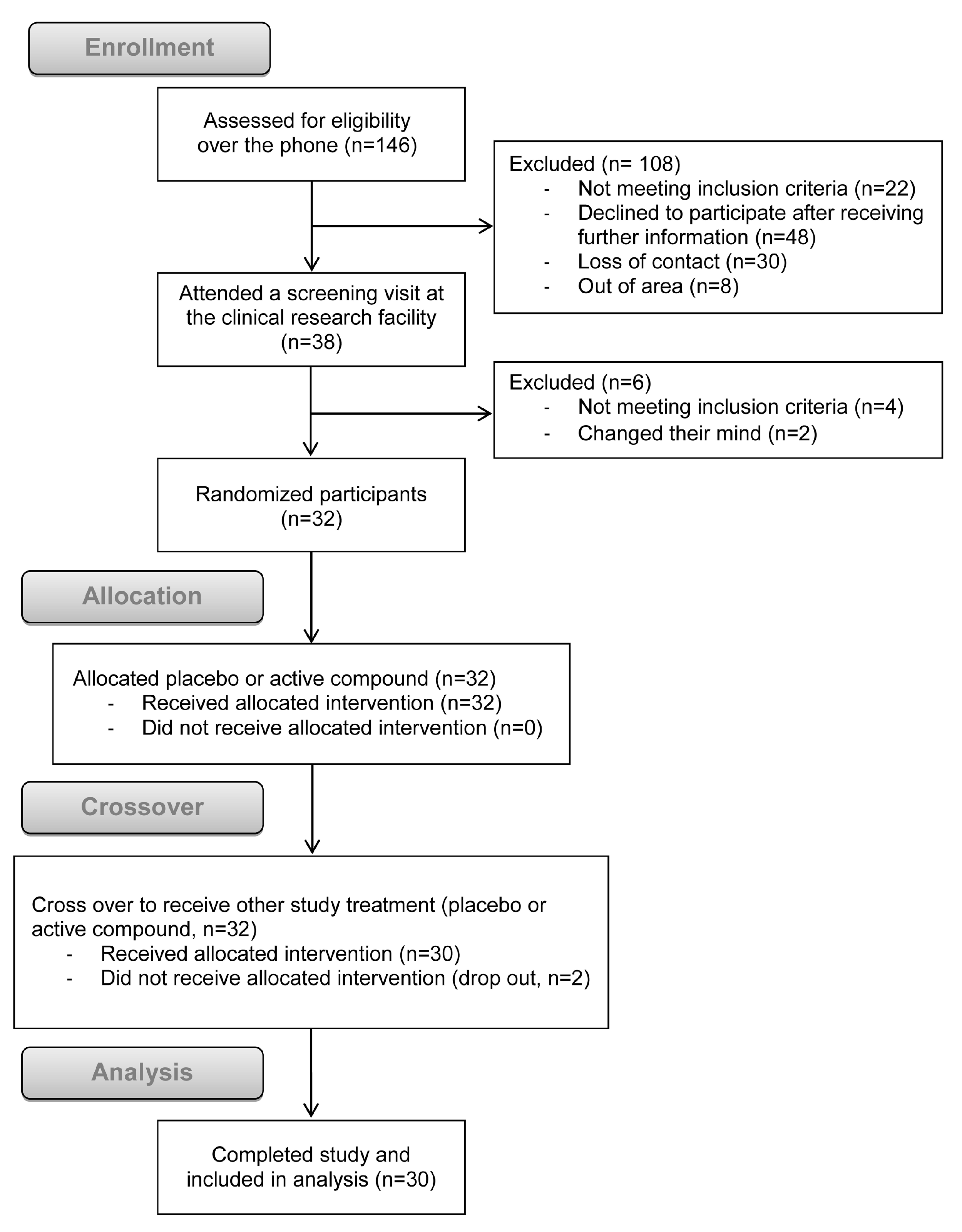
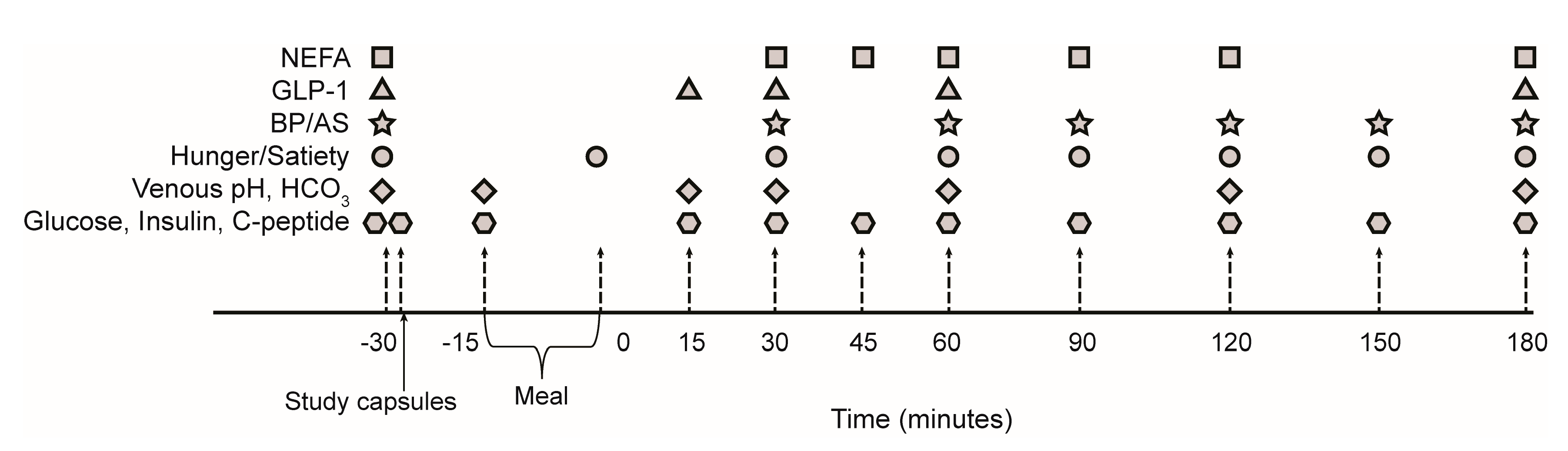


| Constituent | Average Per Meal |
|---|---|
| Energy (kJ) | 3567 |
| Protein (g) | 42 |
| Fat (g) | 39 |
| Saturated fat (g) | 18 |
| Total carbohydrates (g) | 79 |
| Sugar (g) | 31 |
| Sodium (g) | 1.3 |
| a PRAL (mEq) | 18.3 |
| N (Men) | 30 (20) |
| Age (years) | 31.5 (24.0–44.3) |
| Waist circumference (cm) | 83.0 (77.0–91.0) |
| Weight (kg) | 74.4 ± 14.9 |
| BMI (kg/m2) | 24.0 (22.1–25.9) |
| Family history of type 2 diabetes (n) | 10 |
| Systolic blood pressure (mmHg) | 120 ± 12 |
| Diastolic blood pressure (mmHg) | 77 ± 7 |
| a Fasting blood glucose (mmol/L) | 4.6 ± 0.4 |
| a Fasting serum insulin (mU/L) | 10.8 (8.0–17.3) |
| a HOMA-IR | 2.3 (1.7–3.6) |
| Serum creatinine (μmol/L) | 81.1 ± 10.1 |
| a Fasting venous pH | 7.39 ± 0.02 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozan, P.; Blythe, J.C.; Greenfield, J.R.; Samocha-Bonet, D. The Effect of Buffering High Acid Load Meal with Sodium Bicarbonate on Postprandial Glucose Metabolism in Humans—A Randomized Placebo-Controlled Study. Nutrients 2017, 9, 861. https://doi.org/10.3390/nu9080861
Kozan P, Blythe JC, Greenfield JR, Samocha-Bonet D. The Effect of Buffering High Acid Load Meal with Sodium Bicarbonate on Postprandial Glucose Metabolism in Humans—A Randomized Placebo-Controlled Study. Nutrients. 2017; 9(8):861. https://doi.org/10.3390/nu9080861
Chicago/Turabian StyleKozan, Pinar, Jackson C. Blythe, Jerry R. Greenfield, and Dorit Samocha-Bonet. 2017. "The Effect of Buffering High Acid Load Meal with Sodium Bicarbonate on Postprandial Glucose Metabolism in Humans—A Randomized Placebo-Controlled Study" Nutrients 9, no. 8: 861. https://doi.org/10.3390/nu9080861
APA StyleKozan, P., Blythe, J. C., Greenfield, J. R., & Samocha-Bonet, D. (2017). The Effect of Buffering High Acid Load Meal with Sodium Bicarbonate on Postprandial Glucose Metabolism in Humans—A Randomized Placebo-Controlled Study. Nutrients, 9(8), 861. https://doi.org/10.3390/nu9080861






