Abstract
Rice bran (RB) is a major by-product of rice polishing and a rich source of bioactive compounds. Here, we investigated the anti-colitis effect of diet supplementation with fermented rice bran (FRB) in a murine model of ulcerative colitis. FRB was prepared by dual fermentation of RB using fungi and lactic acid bacteria. Colitis was induced in C57Bl/6N male mice (n = 8/group) by dextran sodium sulfate (DSS). Body weight change, disease activity index (DAI), histopathology score, tissue myeloperoxidase (MPO) activity, cytokine and chemokine transcript levels, and the production of short-chain fatty acids (SCFAs) and mucin in the colonic tissue were monitored. Based on histopathology scores, DSS induced severe mucosal inflammation, with an increased loss of crypts, and inflammatory cell infiltration in the control and RB groups, but not in the FRB group. MPO activity, thiobarbituric acid-reactive substance levels, and pro-inflammatory cytokine transcript (Tnf-α, Il-1β, Il-6, and Il-17) levels were significantly higher in the control and RB groups than in the FRB group. Thus, dietary FRB attenuated intestinal inflammation owing to elevated SCFAs and tryptamine production, which might regulate tight junction barrier integrity and intestinal homeostasis. These results suggest that FRB could comprise an effective potential preventive agent for ulcerative colitis.
1. Introduction
Inflammatory bowel disease (IBD), a chronic and relapsing inflammation of the gastrointestinal tract, encompasses two types of intestinal inflammation: ulcerative colitis (UC) and Crohn’s disease [1]. UC is a complex and debilitating disorder that is characterized by the presence of discontinuous lesions in the rectal and colonic mucosa [2]. While the incidence rate of UC is high in Western countries, its occurrence in East Asian countries, such as Korea and Japan, has also increased in recent years on account of an increasing intake of a Westernized diet, characterized by high protein and fat content, as well as excessive sugar intake, with lower fiber consumption [3,4].
Although the etiology and pathogenesis of UC remain obscure, increasing evidence suggests that the dysregulation of mucosal immunological function is responsible for the initiation and propagation of this disease. UC is predominantly triggered by the epithelial invasion of intestinal microbiota due to the loss of epithelial layer integrity [5]. The resulting overproduction of pro-inflammatory cytokines, e.g., tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and IL-6, induces colonic tissue damage and ulceration of the colon [6]. The efficacy of conventional treatments varies and the commonly used drugs have long-term side effects [7]. Thus, an effective preventive agent should be investigated, that would reduce inflammation for extended periods of time. Prebiotics are safe, indigestible food constituents, benefitting the host by selectively enhancing the growth of commensal microbiota in the gastrointestinal tract, that have been used for the treatment of IBD [8].
Rice bran (RB) is a rich source of bioactive components, e.g., dietary fiber, vitamins, free amino acids, and antioxidants, which have a potential to promote gastrointestinal health [9]. RB is presently available in most regions of the world as a by-product of rice polishing. The usage of RB for human consumption is limited, however, and RB is discarded or used as animal feed [10]. Such RB treatments as fermentation, enzymatic processing, or fractionalization have been employed to increase its quality or render it edible for humans, in the form of a dietary supplement. Processed RB or fermented rice bran (FRB) are foodstuffs that are more enriched in such ingredients as protein, fiber, and phenolic compounds, than the usual raw bran [11]. Therefore, it is extremely important to investigate the basic physiochemical and functional properties of FRB and to identify the mechanisms of action for its application as a nutraceutical.
Several studies have investigated the anti-colitis effects of processed RB in animal models, focusing on rice bran oil [12], brown rice fermented by Aspergillus oryzae [5,13], enzyme-treated rice fiber [8], and RB fermented by Saccharomyces cerevisiae and Lactobacillus plantarum [3]. Processed RB is enriched for different ingredients to varying degrees because of different fermentation processes and preparation methods. Among these enriched ingredients, an essential amino acid, tryptophan, is considered an effective candidate agent against UC [7,14]. In addition, in the gut, tryptophan metabolites of the microbiota, e.g., indole-3-aldehyde, kynurenine, indole-3-acetic acid, and tryptamine, can act as ligands for aryl hydrocarbon receptor (Ahr), a transcription factor that regulates IL-22 gene expression, controls autoimmunity processes, and promotes rapid recovery from colitis [15].
FRB may also stimulate the microbial production of short-chain fatty acids (SCFAs), especially acetic acid (AA), propionic acid (PA), butyric acid (BA), and lactic acid (LA), which are strongly associated with the colonic health in human [16]. SCFAs are produced by the microbiota in the course of breaking down complex carbohydrates, such as fiber, and are a primary source of energy for the enteric epithelium [16]. The intestinal microbiota has a profound impact on the host immune system; dysbiosis of bacterial populations and suppression of SCFA production has been implicated in UC [17]. Increased SCFA levels promote colonic epithelial cell proliferation, stimulate mucin production, and epithelial cell integrity. In particular, BA has been shown to induce colonic regulatory T cells and limit the innate immune cell-driven inflammation; it also has the potential to eliminate mutated epithelial cells through the induction of apoptosis [17]. These activities contribute to the maintenance of colonic homeostasis by regulating the intestinal barrier integrity [18]. The intestinal barrier integrity is crucial for maintaining the beneficial relationship between the host and the intestinal microbes; as it not only constitutes a physical barrier, preventing the entry of invading microorganisms, but also provide a defense mechanism by sensing pathogenic microorganisms or their toxins [19].
Intercellular tight junction (TJ) complexes consist of multiple proteins, including occludin (OCLN) and claudin (CLDN), which mainly determine the intestinal barrier integrity [20]. TJs are located at the apical ends of the lateral membrane of the epithelial cells; the loss of TJ barrier integrity is associated with the initiation and development of UC [20,21]. Another characteristic of human UC is a pronounced depletion of mucin-producing goblet cells and the mucus layer, correlating with an increased microbiota-induced colonic inflammation and disease pathology [22].
Dextran sodium sulfate (DSS) is commonly used in rodent models to chemically induce the intestinal inflammation [15]. DSS administration leads to weight loss, bloody diarrhea, and immune cell infiltration, and to increased production of inflammatory mediators, similarly to human colitis [6].
The purpose of the present study was to investigate the effects of dietary FRB supplementation on UC pathology in a murine model of DSS-induced UC. We used a unique FRB, in which RB was fermented by both Aspergillus kawachii and Lactobacillus sp., notably enriched with free tryptophan and tryptamine. We hypothesized that FRB supplementation would mitigate the symptoms of colitis and its clinical severity by enhancing the indole derivatives and colonic SCFAs. To evaluate the protective effect of FRB, we also determined the serum pro-inflammatory cytokines and colonocyte pro-inflammatory gene expression, which include genes responsible for tight junction’s barrier integrity, mucin production, and inflammatory cell infiltration in the inflamed sites. Our results indicate a protective effect of dietary FRB in DSS-induced UC in the mouse model, linked to modulation of colonic SCFAs and pro-inflammatory gene expression.
2. Materials and Methods
2.1. Materials
The components of AIN-93M standard diet were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan) and Oriental Yeast Co., Ltd. (Tokyo, Japan). DSS (MW > 40 kDa) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Serum TNF-α and IL-6 levels were determined by using commercially-available mouse enzyme-linked immunosorbent assay (ELISA) kits provided by Diaclone SAS (Besancon CEDEX, France) and R&D Inc. (Minneapolis, MN, USA), respectively. Unless otherwise specified, all chemicals and solvents used were of analytical grade. RB and FRB were kindly provided by Sunbran Company (Tendo, Japan). We used commercial microorganisms for FRB production. A. kawachii was obtained from Akita Konno (Akita, Japan), and Lactobacillus brevis, Lactobacillus rhamnosus, and Enterococcus faecium from Koei-science (Wako, Japan).
2.2. Preparation of FRB
FRB was prepared by dual fermentation using fungi and lactic acid bacteria. RB was initially steamed and cooled to about 30 °C, after which a spore solution of A. kawachii (106 spores/g of rice bran) was mixed and incubated at 30 °C in a fermentation chamber for 44 h. The solid-state culture obtained was named rice bran koji. A mixture of rice bran koji and rice powder (2:1) was saccharified using a four-fold amount of water at 56 °C for 12 h, heated at 85 °C for 15 min, and then cooled to about 30 °C and the solution was inoculated with a mixture of lactic acid bacteria (L. brevis, L. rhamnosus, and E. faecium) at a concentration of 0.01% (w/w). The solution was then incubated at 37 °C overnight and then heated at 85 °C for 15 min to obtain the FRB. The FRB solution was then filtered, lyophilized, and kept at −30 °C until use. The major differences in RB and FRB micronutrient contents are described elsewhere [11]. Details of the experimental diets (10% (w/w) of RB and FRB) are given in Table 1.

Table 1.
Diet composition (g/1000 g).
2.3. Animals and Treatments
Animal Care Committee of Tohoku University approved the experimental plan of the present study. All experiments were conducted according to the guidelines issued by this committee, and in accordance with the Japanese governmental legislation (2005).
Male C57BL/6N mice (age: 10–12 weeks) were used in the study. They were littermates, and were maintained with free access to a commercial diet (F2, Funabashi Farm, Funabashi, Japan) prior to the study. The mice were housed in a pathogen-free mouse colony (temperature: 23 ± 3 °C; relative humidity: 55% ± 10%; light cycle: 12 h/d), with free access to food and drinking water. Mice were provided a control diet (AIN93M standard diet for rodents) or the control diet supplemented with 10% (w/w) of RB and FRB, starting 4 d before the initial DSS administration (n = 8/group). After 4 d, fresh mouse feces were collected to determine the tryptophan, tryptamine, and SCFAs levels. Acute colitis was then induced in all groups by supplementing the drinking water with 3% (w/v) DSS for 12 consecutive days. Body weight, food intake, water intake, stool consistency, and rectal bleeding were monitored daily. The mice were sacrificed on the following day and the severity of colitis examined. Disease activity index (DAI) was determined by combining the stool consistency and stool blood scores [23].
2.4. Determination of Myeloperoxidase (MPO) Activity and Thiobarbituric Acid-Reactive Substance (TBARS) Levels
MPO activity, an enzymatic marker generally used for the quantification of the extent of inflammatory cell infiltration in the colonic tissue, was determined according to the manufacturer’s instruction (Biovision, Milpitas, CA, USA). Briefly, colonic samples were thawed and homogenized on ice in 20 volumes of phosphate-buffered saline (PBS) supplemented with 0.1% NP40, and processed following the manufacturer’s recommendations. MPO activity was expressed as mU/mg of tissue. Lipid peroxidation was measured based on the formation of TBARS [24].
2.5. Histopathology
For histopathological analysis, the distal colon was fixed in a 10% formalin solution, incubated in 70% ethanol, at 4 °C, and embedded in paraffin; the fixed tissues were sectioned (4-μm thickness), stained with hematoxylin and eosin (H and E) [15], and examined by light microscopy at 100× magnification. The histological score was calculated depending on the severity of epithelial cell damage, crypt damage, and the degree of infiltration of inflammatory cells [15].
2.6. Tryptophan, Tryptamine, and SCFA Analyses
Tryptophan and tryptamine content in the feces and serum of RB and FRB group animals were determined by fluorescence high-performance liquid chromatography (HPLC) [25]. SCFA levels were quantified using a previously published protocol, with slight modification [26]. Briefly, fresh fecal samples (100 mg) were weighed and suspended in 2 mL of 10 mM NaOH solution containing crotonic acid (CA) internal standard (50 µL of 250 µg/mL), homogenized for 1 min using a homogenizer, and centrifuged for 20 min at 1500× g at 4 °C. Supernatants were collected and fat-soluble compounds were removed by chloroform extraction. Prior to analysis, the samples were further diluted (10×) in 20 mM NaH2PO4 (pH 2.7). The separations were performed under isocratic conditions (mobile phase: 20 mM NaH2PO4) on an Atlantis C18 column (4.6 × 50 mm, 5 μm, Waters, Milford, MA, USA) at 30 °C, and with a flow rate of 0.5 mL/min. The HPLC run time for each sample was 35 min, and the injection volume was 20 μL, with UV detection at 214 nm. The analytical curves were constructed by calculating the regression line, and the linearity was defined by the coefficient of correlation (R2). All analytical curves were linear within the studied range, with R2 > 0.99. The limits of detection for AA, BA, LA, PA, and CA were 31.25 μM, 62.50 μM, 15.62 μM, 156.0 μM, and 2.5 μM, respectively. The recovery percentages of AA, BA, LA, PA, and CA were 103.13%, 91.61%, 96.26%, 92.25%, and 99.10%, respectively, indicating that the extraction procedure and HPLC-UV detection method were sufficiently precise, accurate, and sensitive for the quantification.
2.7. RNA Extraction and mRNA Quantification
RNA was isolated from the colonic tissue using the Isogen reagent (NIPPON GENE, Tokyo, Japan), followed by RNA cleanup using the RNeasy mini kit (QIAGEN, Tokyo, Japan) with optional DNase I treatment according to the manufacturer’s instructions. RNA purity was determined spectrophotometrically by measuring the absorbance at 260 nm in relation to that at 280 nm. RNA concentration was adjusted to 1 μg/μL with RNase-free water; for cDNA synthesis, the samples were pre-incubated with oligo-dT primer and dNTP (GE Healthcare) at 65 °C for 5 min; next, SuperScript III Reverse Transcriptase and RNaseOUT RNase inhibitor (Invitrogen, Carlsbad, CA, USA) were added, and the incubation was continued at 50 °C for 60 min. An aliquot of synthesized cDNA was used as the template in quantitative polymerase chain reaction (PCR) using an Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The target cDNAs were amplified using gene-specific primers and SYBR Premix Ex Taq (Takara Bio, Otsu, Japan). The relative mRNA levels were normalized to the amount of eukaryotic translation elongation factor 1α1 mRNA (Eef1a1) [15]. The following primers were used: Tnf-α: 5′-GACGTGGAACTGGCAGAAGAG-3′ (forward) and 5′-TCTGGAAGCCCCCCATCT-3′ (reverse) [NM_013693.3]; Il-6: 5′-AGAGGAGACTTCACAGAGGATACC-3′ (forward) and 5′-AATCAGAATTGCCATTGCACAAC-3′ (reverse) [NM_001314054.1]; Il-1β: 5′-CTGTGTCTTTCCCGTGGACC-3′ (forward) and 5′-CAGCTCATATGGGTCCGACA-3′ (reverse) [NM_008361.4]; Ccl2: 5′-GTTGGCTCAGCCAGATGCA-3′ (forward) and 5′-AGCCTACTCATTGGGATCATCTTG-3′ (reverse) [NM_011333.3]; Cxcl1: 5′-TTGTGCGAAAAGAAGTGCAG-3′ (forward) and 5′-TACAAACACAGCCTCCCACA-3′ (reverse) [NM_008176.3]; Cxcl2: 5′-CCAACCACCAGGCTACAGG-3′ (forward) and 5′-GCGTCACACTCAAGCTCTG-3′ (reverse) [NM_009140.2]; Cxcr3: 5′-GCTGCTGTCCAGTGGGTTTT-3′ (forward) and 5′-AGTTGATGTTGAACAAGGCGC-3′ (reverse) [NM_009910.3]; Ocln: 5′-CTTCTGCTTCATCGCTTCC-3′ (forward) and 5′-CTTGCCCTTTCCTGCTTTC-3′ (reverse) [NM_008756.2]; Cldn-1: 5′-TGAGCCTAGAAAAGAGCC-3′ (forward) and 5′-GCCACTAATATCGCCAGACC-3′ (reverse) [NM_016674.4]; Cldn-4: 5′-CCTCTGGATGAACTGCGTGGTG-3′ (forward) and 5′-GTCGCGGATGACGTTGTGAG-3′ (reverse) [NM_009903.2]; Muc2: 5′-GCTGACGAGTGGTTGGTGAATG-3′ (forward) and 5′-GATGAGGTGGCAGACAGGAGAC-3′ (reverse) [NM_023566.3]; Il-17: 5′-CTCCAGAAGGCCCTCAGACTAC-3′ (forward) and 5′-GCT TTCCCTCCGCATTGACACAG-3′ (reverse) [NM_010552.3]; Eef1a1: 5′-GATGGCCCCAAATTCTTGAAG-3′ (forward) and 5′-GGACCATGTCAATGGCAG-3′ (reverse) [M81088].
2.8. Statistical Analysis
Numerical data are expressed as the mean ± standard error of the mean (SEM). Statistical evaluation was done by one-way ANOVA followed by Dunnet’s test (Table 2, Figure 1, Figure 2, Figure 3 and Figure 4) and Tukey multiple comparison test (Figure 5). The analysis was performed using SigmaPlot v. 12.5 (San Jose, CA, USA). A p-value < 0.05 was considered to indicate statistically significant differences.

Table 2.
Concentration of short-chain fatty acids (SCFAs; μmol/g) in the feces and colon tissue.
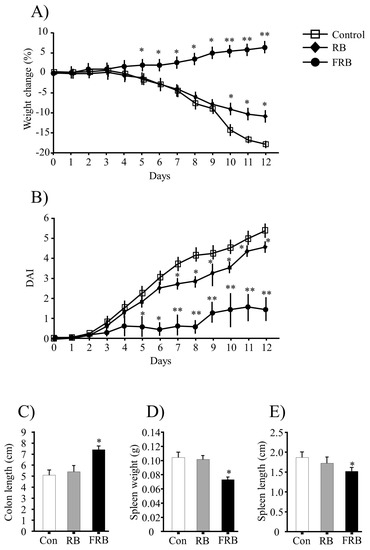
Figure 1.
General evaluation of colitis. (A) Body weight change (%); (B) disease activity index (DAI); (C) colon length; (D) spleen weight; and (E) spleen length determination. Body weight loss was calculated as the percent difference between the original body weight (day 0) and the body weight on any particular day. The DAI was calculated based on the diarrheal score (0, normal; 1, mild soft; 2, very soft; and 3, watery stool) and the presence or absence of fecal blood (0, normal; 2, brown; 3, reddish; and 4, bloody stool). The values are expressed as the mean ± SEM (n = 8); one-way ANOVA was done followed by Dunnett’s test. * p < 0.05, ** p < 0.01, compared with the control (Con) group. RB, rice bran; FRB, fermented rice bran.
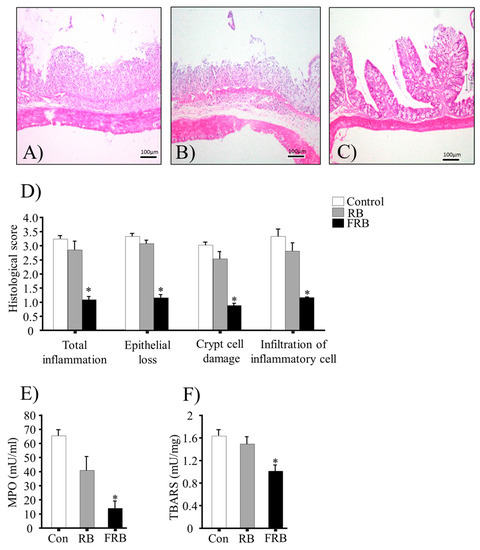
Figure 2.
Dietary fermented rice bran (FRB) diet is associated with lower histopathology scores and inflammatory marker levels in dextran sodium sulfate (DSS)-induced colitis in the mouse model. Representative images (out of n = 4 in each group) of hematoxylin and eosin (H and E)-stained colonic tissues in (A) control; (B) rice bran (RB); and (C) FRB group animals; (D) histological scores. Histological scores were determined with following parameters: epithelial loss (0, none evident; 1, mild; 2, moderate; 3, severe; and 4, massive), crypt cell damage (0, none evident; 1, mild; 2, moderate; 3, severe; and 4, massive), and infiltration of the inflammatory cells in the mucosa (0, none evident; 1, mild; 2, moderate; 3, severe; and 4, massive). Scores were averaged to determine the total histological score in all parameters (five slides/sample); (E) Colonic myeloperoxidase (MPO) activity and (F) thiobarbituric acid-reactive substance (TBARS) values. The values are expressed as the mean ± SEM (n = 4); one-way ANOVA was done followed by Dunnett’s test. * p < 0.05, compared with the control (Con) group.
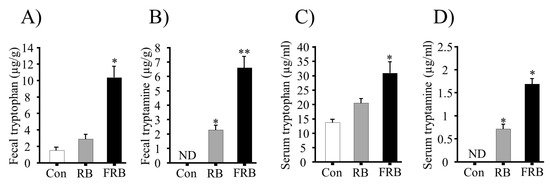
Figure 3.
The involvement of fermented rice bran (FRB) in tryptophan and tryptamine production. (A) Fecal tryptophan; (B) fecal tryptamine; (C) serum tryptophan; and (D) serum tryptamine levels. The values are expressed as the mean ± SEM (n = 4); one-way ANOVA was done followed by Dunnett’s test. * p < 0.05, compared with the control (Con) group. RB, rice bran.
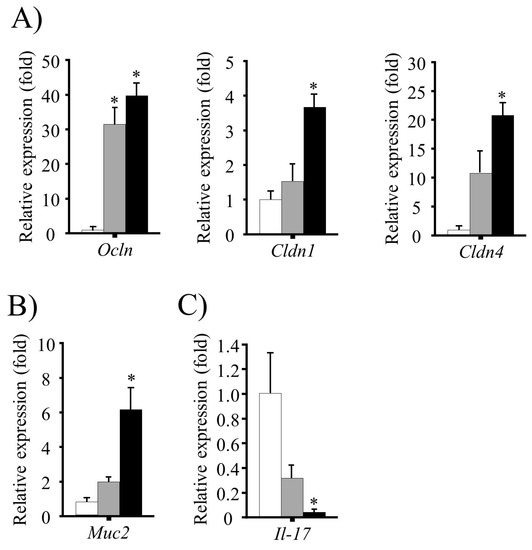
Figure 4.
The role of fermented rice bran (FRB) in the intestinal barrier integrity. Colonic expression of genes encoding (A) tight junction proteins; (B) secretory mucin 2; and (C) Il-17. The mRNA levels were normalized to the expression of Eef1a1 gene. The values are expressed as the mean ± SEM (n = 4); one-way ANOVA was done followed by Dunnett’s test. * p < 0.05, compared with the control (Con) group. RB, rice bran.
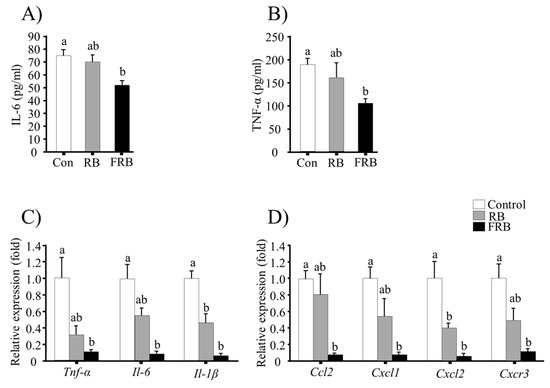
Figure 5.
Evaluation of the anti-inflammatory effects of fermented rice bran (FRB). Serum levels of pro-inflammatory cytokines (A) TNF-α and (B) IL-6; (C) relative expression of colonic pro-inflammatory cytokine genes; and (D) relative expression of chemokine and chemokine receptor genes in the colon. The mRNA levels were normalized to the expression of Eef1a1 gene. The values are expressed as the mean ± SEM (n = 4); One-way ANOVA was done followed by Tukey’s multiple comparison test. Different letters indicate significant difference. Con, control; RB, rice bran.
3. Results
3.1. General Evaluation of Colitis
During the DSS treatment, significant body weight loss was observed in the animals from the control and RB groups but not in the FRB group. The percentage weight change in different groups is shown in Figure 1A. Following DSS challenge, the control and RB mice started to show clinical signs of illness earlier and the disease was more severe than in FRB mice. Watery stool and rectal bleeding were more severe in the control and RB groups (Figure 1B). Consistently with these observations, the DAI scores were higher in the RB group (p > 0.05, RB vs. control) than in the FRB group (p < 0.05, FRB vs. control) (Figure 1B).
After 12 d of DSS treatment, the mice were sacrificed and the colon lengths, and spleen lengths and weights were determined. The colon was significantly shorter in the control and RB groups (p > 0.05), than in the FRB group (p < 0.05 vs. control), as shown in Figure 1C. The spleen weights and lengths were significantly higher in the control and RB groups than in the FRB group (Figure 1D,E). Shortening of the colon in mice is correlated with histological changes, such as crypt cell damage, and colon length is often used as a morphological marker of the degree of inflammation [27].
3.2. Effect of FRB Diet on the Colonic Histological Score, MPO Activity, and TBARS Levels
Representative H and E-stained colon sections are shown in Figure 2A–C, and the histological scores (total inflammation, epithelial loss, crypt cell damage, and infiltration of inflammatory cells) are provided in Figure 2D. The results indicated acute inflammation in the colonic tissue of mice, with markedly higher histological scores in the control and RB group (p > 0.05, RB vs. control) than in the FRB group (p < 0.05, FRB vs. control). After the DSS challenge, MPO activity and TBARS values in the colon were higher in the control and RB groups than in the FRB group, as shown in Figure 2E,F.
3.3. FRB Diet Suppresses the Expression of Pro-Inflammatory Cytokines and Chemokines
Serum levels of the pro-inflammatory cytokines TNF-α and IL-6 were higher in the control and RB groups than in the FRB group (Figure 5A,B). The changes in Tnf-α, Il-6, and Il-1β mRNA levels in the colon are shown in Figure 5C; the expression of these pro-inflammatory cytokines was significantly higher in the control and RB groups (p > 0.05, RB vs. control) than in the FRB group (p < 0.05, FRB vs. control). Furthermore, FRB diet was associated with a significant reduction (p < 0.05, FRB vs. control) in mRNA levels of the chemokine genes Ccl2 and Cxcl1 and the chemokine receptor gene Cxcr3 in comparison to those in the RB groups (p > 0.05, RB vs. control), as shown in Figure 5D. However, significant statistical differences were observed in Cxcl2 mRNA levels in both RB and FRB groups compared to the control group (Figure 5D).
3.4. Effect of FRB Diet on Tryptophan, Tryptamine, and SCFA Production
Tryptophan levels were 5.5 mg/100 g and 31.3 mg/100 g, with tryptamine levels of 3.78 mg/100 g and 32.49 mg/100 g, in the RB and FRB, respectively. The high tryptophan and tryptamine content in the FRB supplementation in the diet increased the fecal tryptophan and tryptamine levels before DSS administration, as well as serum tryptophan and tryptamine levels after DSS administration (Figure 3A–D). Our analysis also revealed that the FRB diet increased the production of fecal SCFAs before DSS administration (Day 4), and colonic SCFAs after DSS administration (Day 12), as shown in Table 2.
3.5. Effect of FRB Diet on Intestinal Barrier Function and Mucin Production
The expression of genes encoding the TJ proteins OCLN, CLDN1 and 4, and the secretory mucin MUC2, were determined by reverse-transcription PCR. The mRNA levels of Ocln were higher in both FRB and RB groups (p < 0.05 vs. control), whereas Cldn1 and Cldn4 levels were higher in the FRB group only (p < 0.05 vs. control) as shown in Figure 4A. In addition, Muc2 mRNA levels were found to be elevated in the FRB group (p < 0.05 vs. control), but not in the RB group (p > 0.05 vs. control) as shown in Figure 4B. In contrast, mRNA levels of the pro-inflammatory cytokine gene Il-17 were lower in the FRB group than in the control and RB groups (Figure 4C).
4. Discussion
Dietary components have been recently revealed to exert remarkable beneficial effects on host physiology, and are currently targeted, or have high potential to be targeted, as treatments for human disease [28]. UC is a gastrointestinal disease with rapidly increasing world prevalence. It is important to identify the dietary compounds and its mechanisms to ameliorate the progression of inflammation during UC. In the current study, we demonstrated that FRB completely protects the animals against DSS-induced UC, by improving body weight and stool consistency, as well as decreasing intestinal bleeding, compared to the control and RB diets. Histological analysis revealed lower crypt damage and inflammatory cell infiltration in FRB group animals than in RB and control group animals.
Pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, amplify the inflammatory cascade of inflammatory mediators, destructive enzymes, and free radicals that cause tissue damage [29]. We observed elevated levels of TNF-α and IL-6 in the sera of RB and control group animals but not in FRB group animals. Furthermore, dietary FRB significantly inhibited the transcripts of these cytokines in the colonic tissue, similarly to another study [12].
Chemokines act as chemoattractants for neutrophils during acute inflammation and prolonged inflammation [30]. In the current study, we detected a significantly higher colonic expression of Ccl2, Cxcl1, Cxcl2, and Cxcr3 genes in the control and RB groups (p < 0.05, RB vs. control) compared to the FRB group, which supports the protective effect of FRB in colitis.
MPO is an important bactericidal marker in UC; elevated MPO levels are responsible for greater neutrophil influx [15]. In the current study, MPO activity was significantly lower in the FRB group (p < 0.05 vs. control) than in the RB group (p > 0.05 vs. control). This indicated that the DSS-induced neutrophil infiltration in the colonic mucosa was suppressed by FRB supplementation.
The severity of chronic gut inflammation may result from a sustained overproduction of reactive oxygen metabolites and acute lipid peroxidation [31]. In the current study, we also observed a significant elevation of TBARS levels in the control and RB groups, which indicate severe tissue damage in the RB group (p > 0.05 vs. control), but not in the FRB group (p < 0.05 vs. control).
Although the fermentation process may enrich the protein and dietary fiber content in FRB [11], it is not known which specific FRB component contributes to the reduction of colitis. Here, during fermentation, FRB becomes enriched almost 10 times in tryptamine, which may act as an Ahr ligand [15]. Ahr has been highlighted as an immunological regulator in DSS-induced inflammation by promoting the differentiation of Th17, or regulatory T cells, and IL-22 production by Th22 T cells, which in turn regulate the epithelial barrier function and intestinal homeostasis [32]. The serum tryptophan levels were higher in the FRB group (p = 0.0101 vs. control), but not in the RB group (p > 0.05 vs. control). Low tryptophan levels are responsible for severe IBD complications and, thus, tryptophan containing diet supplementation comprises a novel therapeutic strategy for IBD treatment [7,14,15].
Although high levels of dietary fiber and prebiotics increase the intestinal SCFA concentration [33], few studies investigated the stimulation of SCFA production during UC by FRB. In the current study, fecal and colonic SCFA levels were significantly higher in the FRB group than in control and RB groups, as shown in Table 2. The differences in SCFA levels, especially BA and LA levels, clearly reflect the UC-ameliorating potency of FRB compared to RB. Generally, the absorption of SCFAs by the colonic cell is rapid, and the colon absorbs more than 95% of the SCFAs produced [34]. Cell-surface G-protein coupled receptors (GPRs), such as GPR41 and GPR43, are activated by SCFAs and exhibit anti-inflammatory effects to minimize inflammation. SCFAs, in particular BA, are known to induce apoptosis of T cells by inhibiting histone deacetylase, thus eliminating the source of inflammation in the colon [35,36]. The amount and relative abundance of SCFA in the colon may be considered as biomarkers of a healthy status [37]. Prebiotic substrates that selectively promote the growth of beneficial microbiota also induce changes in SCFA production in patients with irritable bowel syndrome [38]. Thus, FRB supplementation may alter the gut microbiota.
AA, PA, and BA enhance the mRNA levels of intestinal TJs genes, the major determinants of intestinal barrier integrity, and play an important role in intestinal defense, as well as in the maintenance of intestinal homeostasis [18,21]. Thus, the increased SCFAs levels by FRB supplementation may contribute to promote symbiotic intestinal environment, and prevent the development and progression of UC.
TJ proteins, especially OCLN, and CLDN1 and 4, are important for epithelial barrier integrity and seem to play a pivotal role in intestinal homeostasis [39]. Our data suggest that FRB supplementation significantly enhances the expression of genes encoding TJ proteins in the inflamed colonic tissue. Protection of the epithelial TJ barrier integrity by FRB involves the suppression of a chronic and robust activation of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6. We also observed that the expression of a gene encoding another pro-inflammatory cytokine, IL-17, which is responsible for augmenting autoantibody production in UC [40], was lower in the FRB group than in the RB and control groups. It is tempting to speculate that the modulation of epithelial-immune interactions by dietary FRB supplementation might present a novel preventive strategy for UC.
The mucus layer protects the gastrointestinal mucosa from mechanical, chemical, and microbial challenge [41]. Mucin 2 (encoded by Muc2) is the most prominent mucin secreted by the goblet cells [41]. High Muc2 mRNA expression was observed only in the FRB group. Thus, in the control and RB groups, reduced Muc2 expression in the intestine leads to abnormal tissue morphology, marked by an increased thickness of the gut mucosa, increased inflammatory cell infiltration, and robust pathogenesis of UC.
RB fermentation by A. kawachii increases the flavonoid content in FRB [42]; flavonoids are not only responsible for the smell and flavor, but also regulate innate immunity, inhibit the production of pro-inflammatory cytokines and, thus, reduce the severity of experimental colitis [43]. An overview of the mechanisms of action of FRB underlying the protection against DSS-induced colitis is shown in Figure 6.
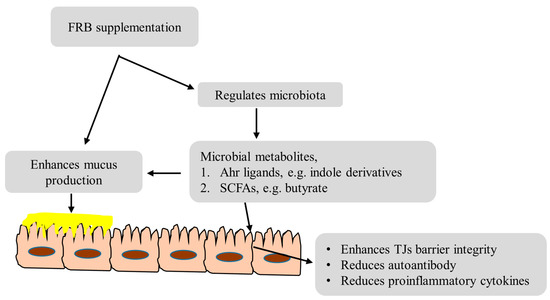
Figure 6.
Prominent examples of FRB contributing to protect from DSS-induced colitis.
In the present study, the effect of whole RB on UC is unclear. RB is rich in bioactive components, such as ferulic acid [12]. Ferulic acid has both antioxidant and anti-inflammatory properties and its anti-colitis effect in a DSS model has been reported [12]. Here, we have analyzed TBARS levels and it was found to be elevated in the RB group compared to that in the FRB group (Figure 2F). Thus, a dual fermentation process may augment the antioxidant properties in FRB.
In the future, more evidence on different FRB fractions and active components should be collected to clarify their mechanism of action. Metabolomics analysis might also reveal multiple potential protective mechanisms of FRB, including immunomodulation to prevent UC. We anticipate that FRB will emerge as an excellent source of nutraceuticals, as well as a functional food.
5. Conclusions
Dual fermentation of RB enriches its functional value. The nutritional benefit of RB fermentation is widely recognized, although the details remain unclear. A promising food component, FRB can be consumed by a large number of people, with health-promoting effects. The current study demonstrates the potential of FRB consumption as a dietary supplement for preventing intestinal inflammatory disorders.
Acknowledgments
This work was partially supported by grants from the Bilateral Joint Research Projects of the Japan Society for the Promotion of Science, Kobayashi International Scholarship Foundation, and the Joint Projects of Rice Bran of Sunstar Inc. We are grateful to the Sunbran Company for providing the test bran samples used in this study.
Author Contributions
J.I., A., S.B., T.G., M.K. and H.S. conceived and designed the experiments; J.I. and H.S. analyzed the data and wrote the paper; and J.I., K.W., H.A., T.K., A.O. and M.A. performed the experiments. All authors read and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Ritchie, L.E.; Taddeo, S.S.; Weeks, B.R.; Carroll, R.J.; Dykes, L.; Rooney, L.W.; Turner, N.D. Impact of Novel Sorghum Bran Diets on DSS-Induced Colitis. Nutrients 2017, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.C.; Lyra, A.C.; Rocha, R.; Santana, G.O. Epidemiology, demographic characteristics and prognostic predictors of ulcerative colitis. World J. Gastroenterol. 2014, 28, 9458–9467. [Google Scholar]
- Kondo, S.; Kuda, T.; Nemoto, M.; Usami, Y.; Takahashi, H.; Kimura, B. Protective effects of rice bran fermented by Saccharomyces cerevisiae Misaki-1 and Lactobacillus plantarum Sanriki-SU8 in dextran sodium sulphate-induced inflammatory bowel disease model mice. Food Biosci. 2016, 16, 44–49. [Google Scholar] [CrossRef]
- Ruemmele, F.M. Role of Diet in Inflammatory Bowel Disease. Ann. Nutr. Metab. 2016, 68, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Ogasa, S.; Kuwahara, T.; Bando, Y.; Hagiwara, M.; Arimochi, H.; Nakanishi, S.; Iwasaki, T.; Ohnishi, Y. Inhibitory effects of fermented brown rice on induction of acute colitis by dextran sulfate sodium in rats. Dig. Dis. Sci. 2008, 53, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Tian, T.; Feng, X.; Ye, S.; Wang, H.; Wu, W.; Qiu, Y.; Yu, C.; He, Y.; Zeng, J.; et al. An adenosine A3 receptor agonist inhibits DSS-induced colitis in mice through modulation of the NF-κB signaling pathway. Sci. Rep. 2015, 5, 9047. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Kovacs-Nolan, J.A.; Yang, C.; Archbold, T.; Fan, M.Z.; Mine, Y. l-Tryptophan exhibits therapeutic function in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J. Nutr. Biochem. 2010, 21, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, Y.; Andoh, A.; Fujiwara, D.; Ohmae, H.; Araki, Y.; Fujiyama, Y.; Mitsuyama, K.; Kanauchi, O. New prebiotics from rice bran ameliorate inflammation in murine colitis models through the modulation of intestinal homeostasis and the mucosal immune system. Scand. J. Gastroenterol. 2011, 46, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Paik, D.J.; Kwon, D.Y.; Park, Y. Dietary supplementation with rice bran fermented with Lentinus edodes increases interferon-γ activity without causing adverse effects: A randomized, double-blind, placebo-controlled, parallel-group study. Nutr. J. 2014, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Goodyear, A.; Kumar, A.; Ehrhart, E.J.; Swanson, K.S.; Grusak, M.A.; Leach, J.E.; Dow, S.W.; McClung, A.; Ryan, E.P. Dietary rice bran supplementation prevents Salmonella colonization differentially across varieties and by priming intestinal immunity. J. Funct. Foods 2015, 18, 653–664. [Google Scholar] [CrossRef]
- Alauddin, M.; Shirakawa, H.; Koseki, T.; Kijima, N.; Ardiansyah; Budijanto, S.; Islam, J.; Goto, T.; Komai, M. Fermented rice bran supplementation mitigates metabolic syndrome in stroke-prone spontaneously hypertensive rats. BMC Complement. Altern. Med. 2016, 16, 442. [Google Scholar]
- Islam, M.S.; Murata, T.; Fujisawa, M.; Nagasaka, R.; Ushio, H.; Bari, A.M.; Hori, M.; Ozaki, H. Anti-inflammatory effects of phytosteryl ferulates in colitis induced by dextran sulphate sodium in mice. Br. J. Pharmacol. 2008, 154, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, M.; Shiomi, S.; Ushikubo, S.; Inai, R.; Matsuo, T. Effect of a fermented brown rice extract on the gastrointestinal function in methotrexate-treated rats. Biosci. Biotechnol. Biochem. 2013, 77, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Hisamatsu, T.; Okamoto, S.; Hashimoto, M.; Muramatsu, T.; Andou, A.; Uo, M.; Kitazume, M.T.; Matsuoka, K.; Yajima, T.; Inoue, N.; et al. Novel, objective, multivariate biomarkers composed of plasma amino acid profiles for the diagnosis and assessment of inflammatory bowel disease. PLoS ONE 2012, 7, e31131. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.; Sato, S.; Watanabe, K.; Watanabe, T.; Ardiansyah; Hirahara, K.; Aoyama, Y.; Tomita, S.; Aso, H.; Komai, M.; et al. Dietary tryptophan alleviates dextran sodium sulfate-induced colitis through aryl hydrocarbon receptor in mice. J. Nutr. Biochem. 2017, 42, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Van Eijk, H.M.; Bloemen, J.G.; Dejong, C.H. Application of liquid chromatography-mass spectrometry to measure short chain fatty acids in blood. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yoshida, S.; Hara, H. Physiological concentrations of short chain fatty acids immediately suppress colonic epithelial permeability. Br. J. Nutr. 2008, 100, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest. Res. 2015, 13, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.V.; Suzuki, T. Dietary fermentable fiber reduces intestinal barrier defects and inflammation in colitic mice. J. Nutr. 2016, 146, 1970–1979. [Google Scholar] [CrossRef] [PubMed]
- Strugala, V.; Dettmar, P.W.; Pearson, J.P. Thickness and continuity of the adherent colonic mucus barrier in active and quiescent ulcerative colitis and Crohn’s disease. Int. J. Clin. Pract. 2008, 62, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Kataoka, T.; Yamato, K.; Taguchi, T.; Yamaoka, K. Suppression of dextran sulfate sodium-induced colitis in mice by radon inhalation. Mediat. Inflamm. 2012, 2012, 239617. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Islam, J.; Shirakawa, H.; Nguyen, T.K.; Aso, H.; Komai, M. Simultaneous analysis of serotonin, tryptophan and tryptamine levels in common fresh fruits and vegetables in Japan using fluorescence HPLC. Food Biosci. 2016, 13, 56–59. [Google Scholar] [CrossRef]
- Hoshi, S.; Sakata, T.; Mikuni, K.; Hashimoto, H.; Kimura, S. Galactosylsucrose and xylosylfructoside alter digestive tract size and concentrations of cecal organic acids in rats fed diets containing cholesterol and cholic acid. J. Nutr. 1994, 124, 52–60. [Google Scholar] [PubMed]
- Tanaka, K.; Namba, T.; Arai, J.; Fujimoto, M.; Adachi, H.; Sobue, G.; Takeuchi, K.; Nakai, A.; Mizushima, T. Genetic evidence for a protective role for heat shock factor 1 and heat shock protein 70 against colitis. J. Biol. Chem. 2007, 282, 23240–23252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Davies, S.S. Microbial metabolism of dietary components to bioactive metabolites: Opportunities for new therapeutic interventions. Genome Med. 2016, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, I.; Vavassori, P.; Biancone, L.; Monteleone, G.; Pallone, F. Immunoregulation in the gut: Success and failures in human disease. Gut 2002, 50, III60–III64. [Google Scholar] [CrossRef] [PubMed]
- Bordon, Y.; Hansell, C.A.; Sester, D.P.; Clarke, M.; Mowat, A.M.; Nibbs, R.J. The atypical chemokine receptor D6 contributes to the development of experimental colitis. J. Immunol. 2009, 182, 5032–5040. [Google Scholar] [CrossRef] [PubMed]
- Medicherla, K.; Sahu, B.D.; Kuncha, M.; Kumar, J.M.; Sudhakar, G.; Sistla, R. Oral Administration of Geraniol Ameliorates Acute Experimental Murine Colitis by Inhibiting pro-Inflammatory Cytokines and NF-κB Signaling. Food Funct. 2015, 6, 2984–2995. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [PubMed]
- Steliou, K.; Boosalis, M.S.; Perrine, S.P.; Sangerman, J.; Faller, D.V. Butyrate histone deacetylase inhibitors. BioRes. Open Access 2012, 1, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.A.; Singh, N.; Martin, P.M.; Thangaraju, M.; Ganapathy, V.; Waller, J.L.; Shi, H.; Robertson, K.D.; Munn, D.H.; Liu, K. Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1405–G1415. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Tabarkiewicz, J.; Pogoda, K.; Karczmarczyk, A.; Pozarowski, P.; Giannopoulos, K. The Role of IL-17 and Th17 Lymphocytes in Autoimmune Diseases. Arch. Immunol. Ther. Exp. 2015, 63, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, L.E.; Koetsier, M.A.; van Deventer, S.J.; van Tol, E.A. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut 2003, 52, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Watanabe, T.; Yamori, M.; Takebe, M.; Wakatsuki, Y. Isoflavones regulate innate immunity and inhibit experimental colitis. J. Gastroenterol. Hepatol. 2009, 24, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Herath, H.M.; Takano-Ishikawa, Y.; Yamaki, K. Inhibitory effect of some flavonoids on tumor necrosis factor-alpha production in lipopolysaccharide-stimulated mouse macrophage cell line J774.1. J. Med. Food 2003, 6, 365–370. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).