Docosahexaenoic Acid Inhibits Cerulein-Induced Acute Pancreatitis in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
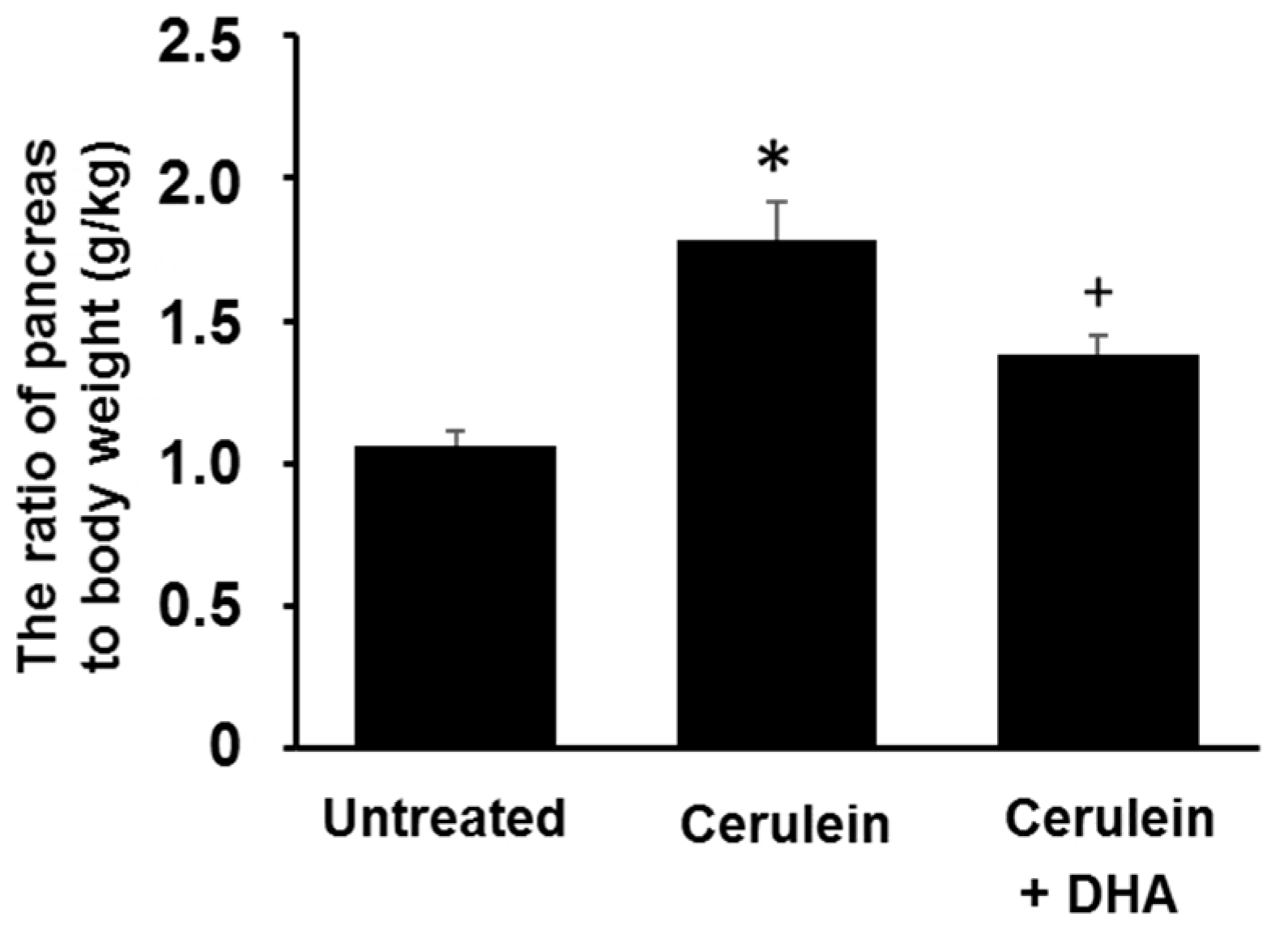
2.3. Measurement of Pancreatic Edema
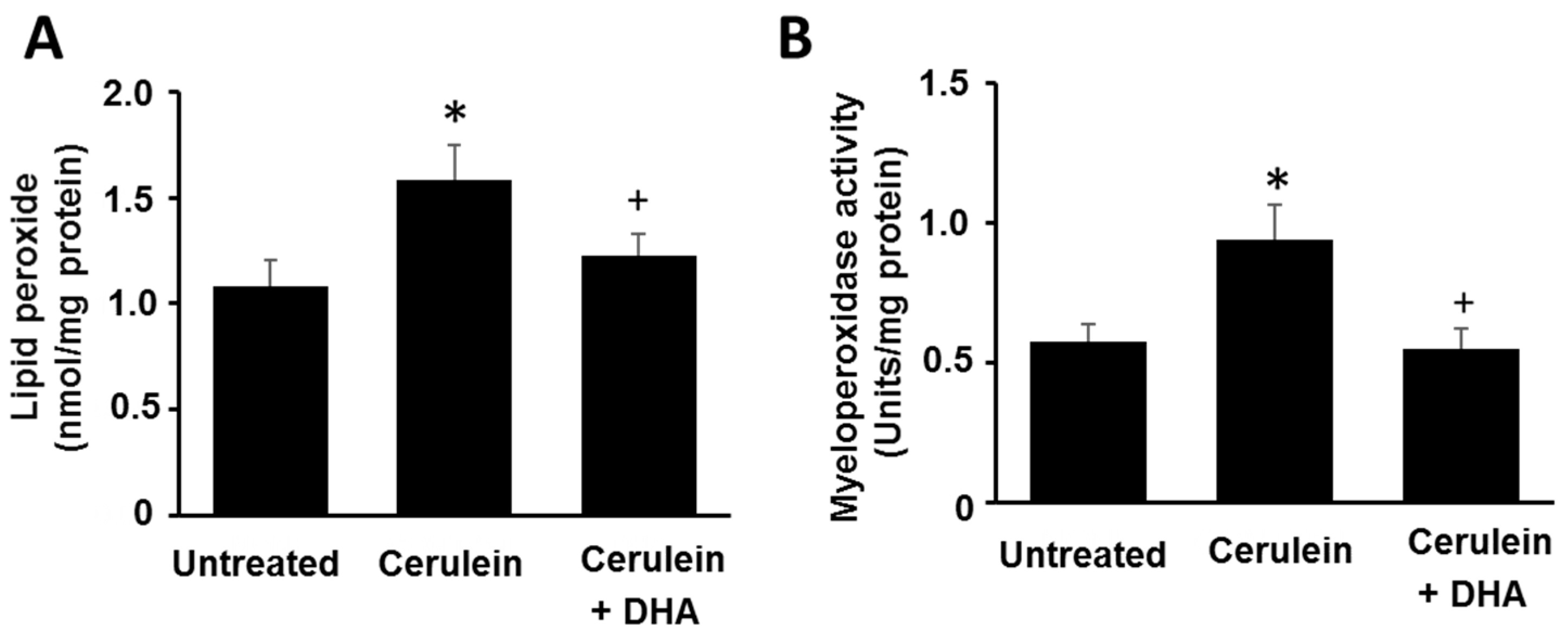
2.4. Determination of LPO and MPO Activity
2.5. Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.6. Western Blot Analysis
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Electrophoretic Mobility Shift Assay (EMSA)
2.9. Immunohistochemical Analysis
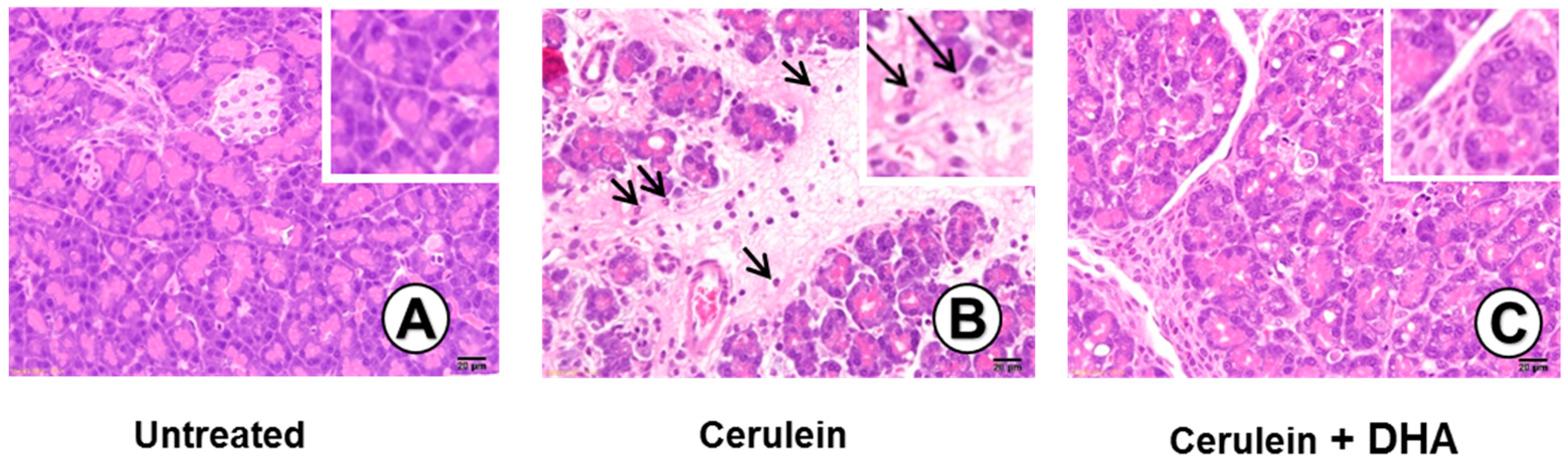
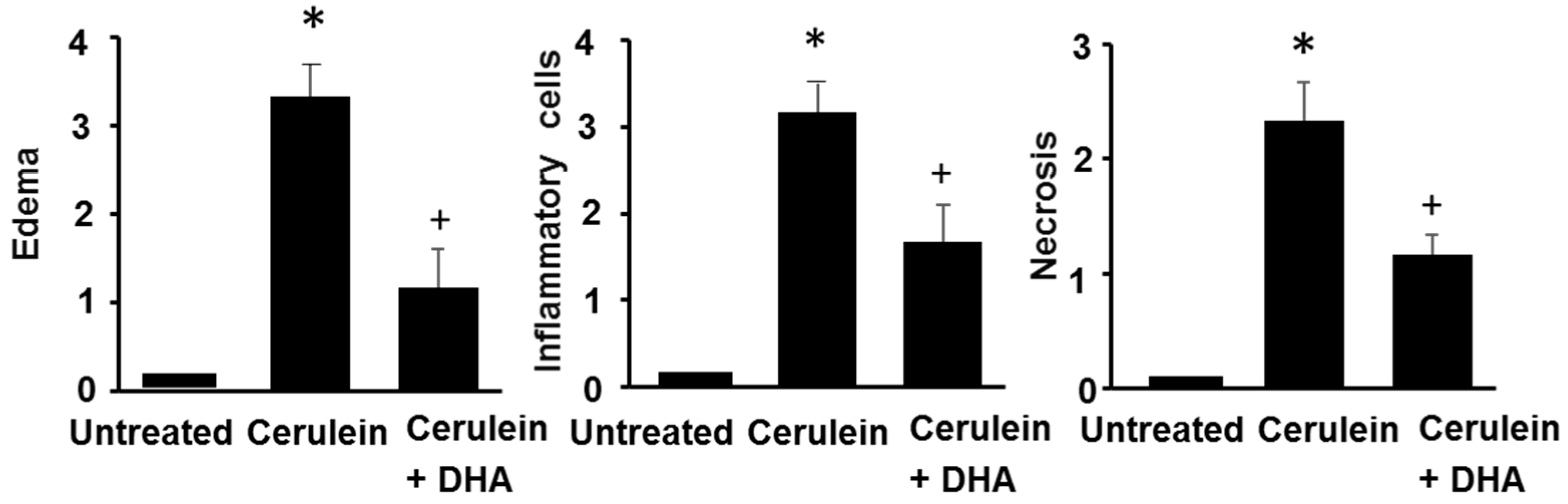
2.10. Histological Observation
2.11. Statistical Analysis
3. Results
3.1. DHA Reduced Cerulein-Induced Pancreatic Edema in Rats
3.2. DHA Reduced the Abundance of LPO and Activity of MPO in Pancreas of Cerulein-Stimulated Rats
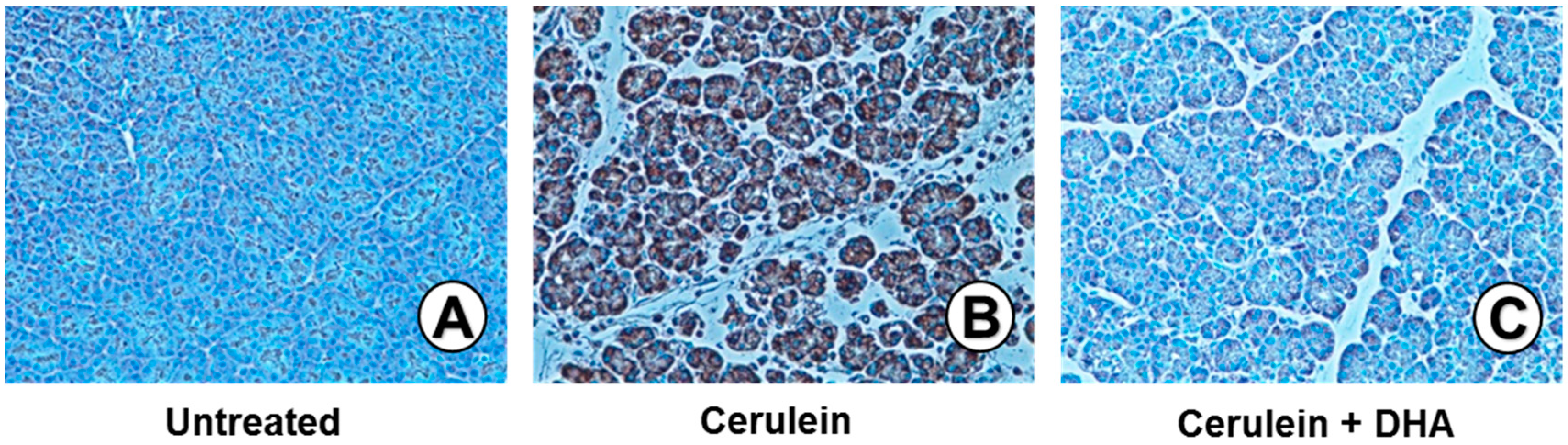
3.3. DHA Inhibited Cerulein-Induced Histopathologic Changes in Rat Pancreas
3.4. DHA Reduced IL-6 Expression in the Pancreas of Cerulein-Induced Rats
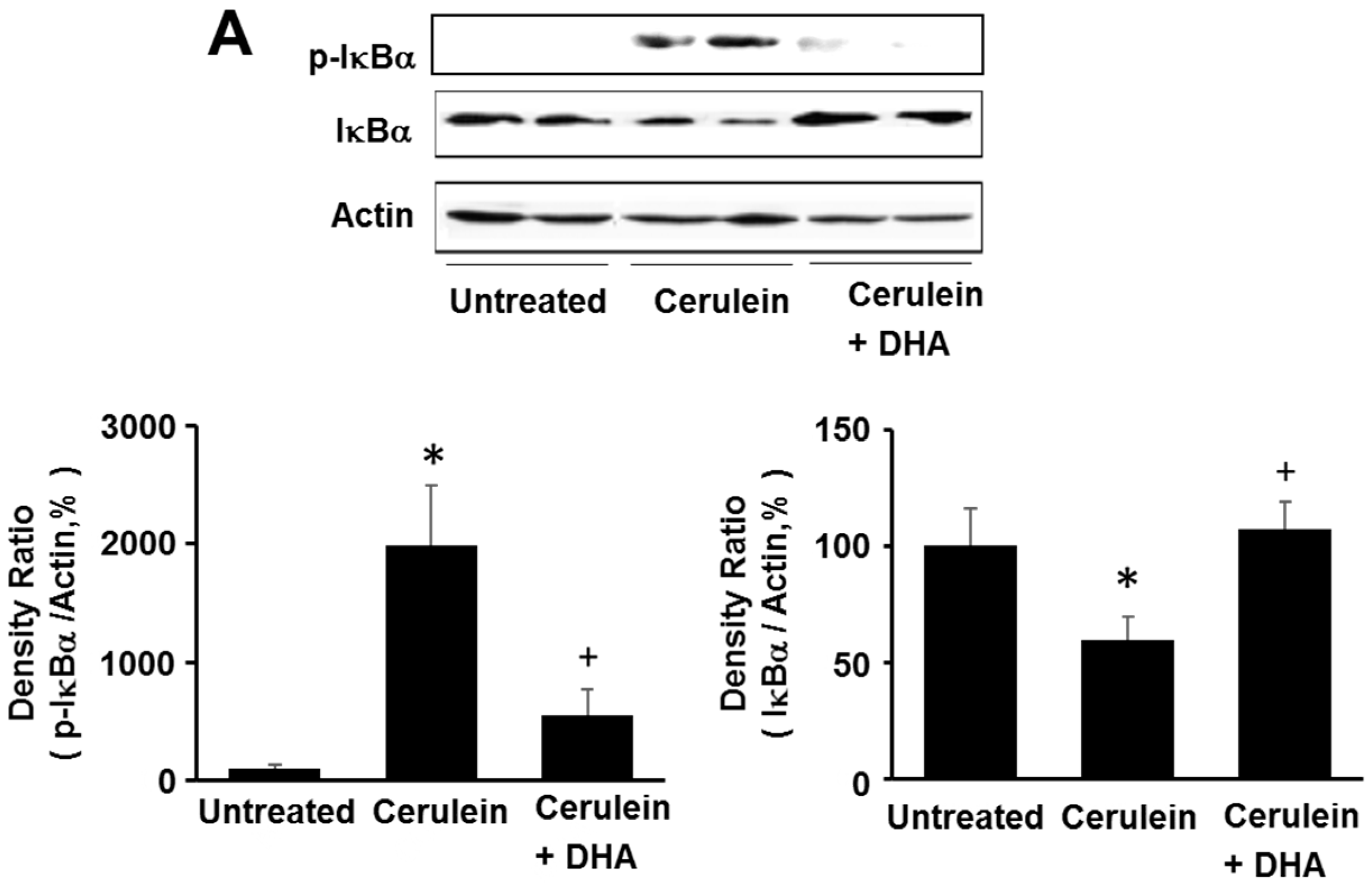
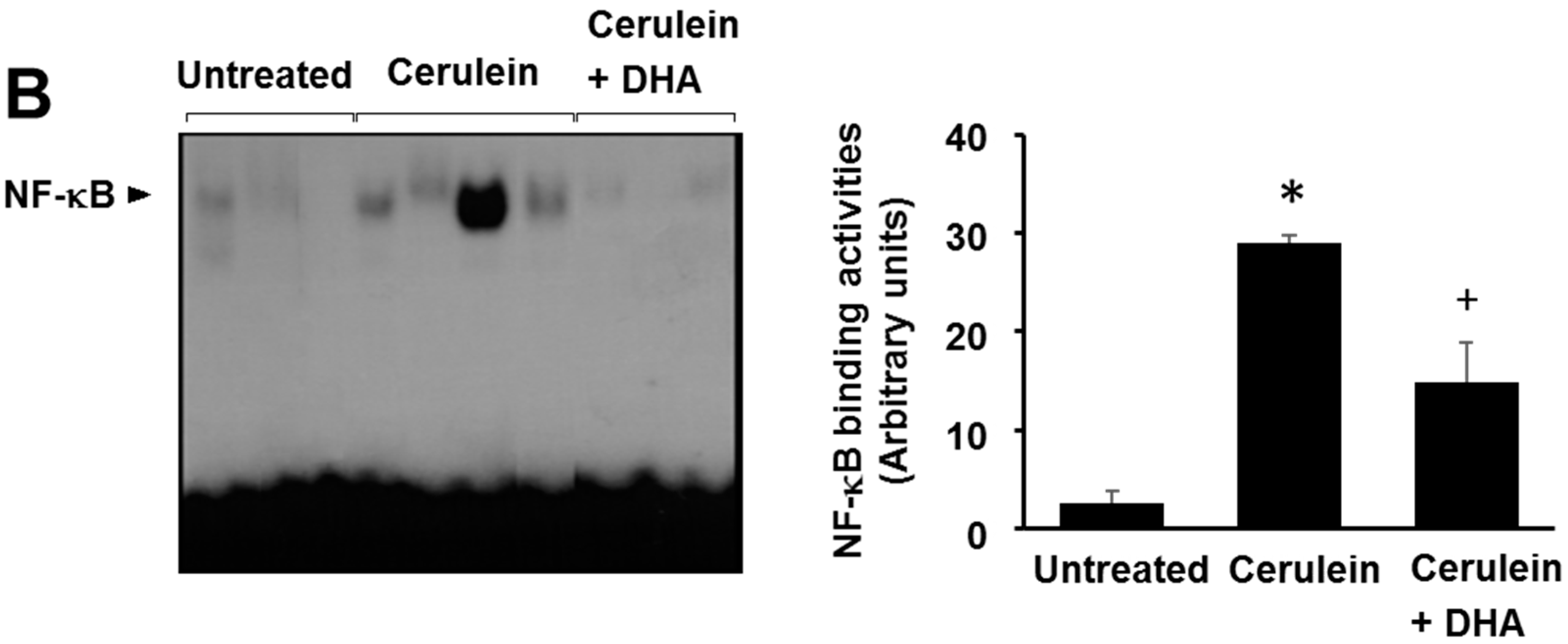
3.5. DHA Inhibited Cerulein-Induced Phosphorylation and Degradation of IκBα and Activation of NF-kB in the Rat Pancreas
3.6. DHA Suppressed Cerulein-Induced Activation of PKCδ in Rat Pancreas
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bhatia, M.; Wong, F.L.; Cao, Y.; Lau, H.Y.; Huang, J.; Puneet, P.; Chevali, L. Pathophysiology of acute pancreatitis. Pancreatology 2005, 5, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Saluja, A.K.; Steer, M.L. Pathophysiology of pancreatitis. Digestion 1999, 60, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Frossard, J.L.; Hadengue, A.; Pastor, C.M. New serum markers for the detection of severe acute pancreatitis in humans. Am. J. Respir. Crit. Care Med. 2001, 164, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, B.; Saluja, A.K.; Lerch, M.M.; Bhagat, L.; Bhatia, M.; Lee, H.S.; Frossard, J.L.; Adler, G.; Steer, M.L. Intra-acinar cell activation of trypsinogen during caerulein-induced pancreatitis in rats. Am. J. Physiol. 1998, 275, 352–362. [Google Scholar]
- Lerch, M.M.; Adler, G. Experimental animal models of acute pancreatitis. Int. J. Pancreatol. 1994, 15, 159–170. [Google Scholar] [PubMed]
- Kim, H. Cerulein pancreatitis: Oxidative stress, inflammation, and apoptosis. Gut Liver 2008, 2, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Schoenberg, M.H.; Büchler, M.; Gaspar, M.; Stinner, A.; Younes, M.; Melzner, I.; Bültmann, B.; Beger, H.G. Oxygen free radicals in acute pancreatitis of the rat. Gut 1990, 31, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Gough, D.B.; Boyle, B.; Joyce, W.P.; Delaney, C.P.; McGeeney, K.F.; Gorey, T.F.; Fitzpatrick, J.M. Free radical inhibition and serial chemiluminescence in evolving experimental pancreatitis. Br. J. Surg. 1990, 77, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Niki, E.; Eguchi, J.; Kamiya, Y.; Shimasaki, H. Oxidation of biological membranes and its inhibition. Free radical chain oxidation of erythrocyte ghost membranes by oxygen. Biochim. Biophys. Acta 1985, 819, 29–36. [Google Scholar] [CrossRef]
- Koster, J.F.; Slee, R.G. Lipid peroxidation of human erythrocyte ghosts induced by organic hydroperoxides. Biochim. Biophys. Acta 1983, 752, 233–239. [Google Scholar] [CrossRef]
- De Groot, H.; Littauer, A. Hypoxia, reactive oxygen, and cell injury. Free Radic. Biol. Med. 1989, 6, 541–551. [Google Scholar] [CrossRef]
- Farber, J.L.; Kyle, M.E.; Coleman, J.B. Mechanisms of cell injury by activated oxygen species. Lab. Investig. 1990, 62, 670. [Google Scholar] [PubMed]
- Gómez-Cambronero, L.; Camps, B.; de La Asunción, J.G.; Cerdá, M.; Pellín, A.; Pallardó, F.V.; Calvete, J.; Sweiry, J.H.; Mann, G.E.; Viña, J.; et al. Pentoxifylline ameliorates cerulein-induced pancreatitis in rats: Role of glutathione and nitric oxide. J. Pharmacol. Exp. Ther. 2000, 293, 670–676. [Google Scholar] [PubMed]
- Alsfasser, G.; Gock, M.; Herzog, L.; Gebhard, M.M.; Herfarth, C.; Klar, E.; Schmidt, J. Glutathione depletion with l-buthionine-(S,R)-sulfoximine demonstrates deleterious effects in acute pancreatitis of the rat. Dig. Dis. Sci. 2002, 47, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Curran, F.J.; Sattar, N.; Talwar, D.; Baxter, J.N.; Imrie, C.W. Relationship of carotenoid and vitamins A and E with the acute inflammatory response in acute pancreatitis. Br. J. Surg. 2000, 87, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.J.; Mitros, F.A.; Oberley, L.W. Expression of antioxidant enzymes in diseases of the human pancreas: Another link between chronic pancreatitis and pancreatic cancer. Pancreas 2003, 26, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Schölmerich, J. Interleukins in acute pancreatitis. Scand. J. Gastroenterol. Suppl. 1996, 219, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Leser, H.G.; Gross, V.; Scheibenbogen, C.; Heinisch, A.; Salm, R.; Lausen, M.; Rückauer, K.; Andreesen, R.; Farthmann, E.H.; Schölmerich, J. Elevation of serum interleukin-6 concentration precedes acute-phase response and reflects severity in acute pancreatitis. Gastroenterology 1991, 101, 782–785. [Google Scholar] [CrossRef]
- Yu, J.H.; Lim, J.W.; Kim, H.; Kim, K.H. NADPH oxidase mediates interleukin-6 expression in cerulein-stimulated pancreatic acinar cells. Int. J. Biochem. Cell Biol. 2005, 37, 1458–1469. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Kim, K.H.; Kim, H. SOCS 3 and PPAR-lignads inhibits the expression of IL-6 and TGF-β by regulating JAK2/STAT3 signaling in pancreas. Int. J. Biochem. Cell Biol. 2008, 40, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, E.C.; Newton, A.C.; Mochly-Rosen, D.; Fields, A.P.; Reyland, M.E.; Insel, P.A.; Messing, R.O. Protein kinase C isozymes and the regulation of diverse cell responses. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L429–L438. [Google Scholar] [PubMed]
- Ron, D.; Kazanietz, M.G. New insights into the regulation of protein kinase C and novel phorbol ester receptors. FASEB J. 1999, 13, 1658–1676. [Google Scholar] [PubMed]
- Bastani, B.; Yang, L.; Baldassare, J.J.; Pollo, D.A.; Gardner, J.D. Cellular distribution of isoforms of protein kinase C (PKC) in pancreatic acini. Biochim. Biophys. Acta 1995, 1269, 307–315. [Google Scholar] [CrossRef]
- Pollo, D.A.; Baldassare, J.J.; Honda, T.; Henderson, P.A.; Talkad, V.D.; Gardner, J.D. Effects of cholecystokinin (CCK) and other secretagogues on isoforms of protein kinase C (PKC) in pancreatic acini. Biochim. Biophys. Acta 1994, 1224, 127–138. [Google Scholar] [CrossRef]
- Satoh, A.; Gukovskaya, A.S.; Nieto, J.M.; Cheng, J.H.; Gukovsky, I.; Reeve, J.R., Jr.; Shimosegawa, T.; Pandol, S.J. PKC-delta and -epsilon regulate NF-kappaB activation induced by cholecystokinin and TNF-alpha in pancreatic acinar cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Moyad, M.A. An introduction to dietary/supplemental omega-3 fatty acids for general health and prevention: Part II. Urol. Oncol. 2005, 23, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Lim, J.W.; Kim, H. Inhibitory mechanism of omega-3 fatty acids in pancreatic inflammation and apoptosis. Ann. N. Y. Acad. Sci. 2009, 1171, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Malleo, G.; Mazzon, E.; Genovese, T.; Di Paola, R.; Muià, C.; Centorrino, T.; Siriwardena, A.K.; Cuzzocrea, S. Etanercept attenuates the development of cerulein-induced acute pancreatitis in mice: A comparison with TNF-alpha genetic deletion. Shock 2007, 27, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb, A.; Chahed, S.; Bachaalany, S.; Griffey, S.; Hammock, B.D.; Haj, F.G. Soluble epoxide hydrolase pharmacological inhibition ameliorates experimental acute pancreatitis in mice. Mol. Pharmacol. 2015, 88, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Qui, B.; Mei, Q.B.; Ma, J.J.; Korsten, M.A. Susceptibility to cerulein-induced pancreatitis in inducible nitric oxide synthase-deficient mice. Pancreas 2001, 23, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Rossi, A.; Serraino, I.; Di Paola, R.; Dugo, L.; Genovese, T.; Britti, D.; Sciarra, G.; De Sarro, A.; Caputi, A.P.; et al. 5-Lipoxygenase knockout mice exhibit a resistance to acute pancreatitis induced by cerulein. Immunology 2003, 110, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Krawisz, J.E.; Sharon, P.; Stenson, W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 1984, 87, 1344–1350. [Google Scholar] [PubMed]
- Chen, P.; Sun, B.; Chen, H.; Wang, G.; Pan, S.; Kong, R.; Bai, X.; Wang, S. Effects of carbon monoxide releasing molecule-liberated CO on severe acute pancreatitis in rats. Cytokine 2010, 49, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Rattner, D.W.; Lewandrowski, K.; Compton, C.C.; Mandavilli, U.; Knoefel, W.T.; Warshaw, A.L. A better model of acute pancreatitis for evaluating therapy. Ann. Surg. 1992, 215, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Zar, J.H. Biostatistical Analysis, 2nd ed.; Prentice Hall: New Jersey, NY, USA, 1984. [Google Scholar]
- Siriwardhana, N.; Kalupahana, N.S.; Moustaid-Moussa, N. Health benefits of n-3 polyunsaturated fatty acids: eicosapentaenoic acid and docosahexaenoic acid. Adv. Food Nutr. Res. 2012, 65, 211–222. [Google Scholar] [PubMed]
- Riediger, N.D.; Othman, R.A.; Suh, M.; Moghadasian, M.H. A systemic review of the roles of n-3 fatty acids in health and disease. J. Am. Diet Assoc. 2009, 109, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Chung, H.Y. Antioxidative and anti-inflammatory actions of docosahexaenoic acid and eicosapentaenoic acid in renal epithelial cells and macrophages. J. Med. Food 2007, 10, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Véricel, E.; Polette, A.; Bacot, S.; Calzada, C.; Lagarde, M. Pro- and antioxidant activities of docosahexaenoic acid on human blood platelets. J. Thromb. Haemost. 2005, 1, 566–572. [Google Scholar] [CrossRef]
- Song, E.A.; Lim, J.W.; Kim, H. Docosahexaenoic acid inhibits IL-6 expression via PPARγ-mediated expression of catalase in cerulein-stimulated pancreatic acinar cells. Int. J. Biochem. Cell Biol. 2017, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Das, S. Identification of cytotoxic mediators and their putative role in the signaling pathways during docosahexaenoic acid (DHA)-induced apoptosis of cancer cells. Apoptosis 2016, 21, 1408–1421. [Google Scholar] [CrossRef] [PubMed]
- Merendino, N.; Loppi, B.; D’Aquino, M.; Molinari, R.; Pessina, G.; Romano, C.; Velotti, F. Docosahexaenoic acid induces apoptosis in the human PaCa-44 pancreatic cancer cell line by active reduced glutathione extrusion and lipid peroxidation. Nutr. Cancer 2005, 52, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Trépanier, M.O.; Lim, J.; Lai, T.K.; Cho, H.J.; Domenichiello, A.F.; Chen, C.T.; Taha, A.Y.; Bazinet, R.P.; Burnham, W.M. Intraperitoneal administration of docosahexaenoic acid for 14 days increases serum unesterified DHA and seizure latency in the maximal pentylenetetrazol model. Epilepsy Behav. 2014, 33, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, A.; Sugimoto, T.; Onuki, Y.; Shinoda, H.; Mihara, T.; Hori, M.; Inomata, T. The 5-HT3 receptor antagonist ondansetron attenuates pancreatic injury in cerulein-induced acute pancreatitis model. Inflammation 2017, 40, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Li, L.; Liu, H.; Hu, L.; Dai, Y.; Chen, J.; Xu, S.; Chen, W.; Xu, X.; et al. Propylene glycol alginate sodium sulfate alleviates cerulein-induced acute pancreatitis by modulating the MEK/ERK pathway in mice. Mar. Drugs 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Kosekli, M.A.; Sungurtekin, U.; Cobankara, V.; Ozmen, O.; Sahinduran, S.; Yilmaz, M. Effects of certolizumab on cerulein-induced acute pancreatitis in rats. Pancreas 2016, 45, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Hirota, M.; Nozawa, F.; Okabe, A.; Shibata, M.; Beppu, T.; Shimada, S.; Egami, H.; Yamaguchi, Y.; Ikei, S.; Okajima, T.; et al. Relationship between plasma cytokine concentration and multiple organ failure in patients with acute pancreatitis. Pancreas 2000, 21, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xu, B.; Xia, T.; He, W.; Gao, P.; Guo, L.; Wang, Z.; Niu, Q.; Wang, A. Relationship between intracellular Ca2⁺ and ROS during fluoride-induced injury in SH-SY5Y cells. Environ. Toxicol. 2013, 28, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Lim, J.W.; Kim, K.H.; Morio, T.; Kim, H. NADPH oxidase and apoptosis in cerulein-stimulated pancreatic acinar AR42J cells. Free Radic. Biol. Med. 2005, 39, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Gukovskaya, A.S.; Hosseini, S.; Satoh, A.; Cheng, J.H.; Nam, K.J.; Gukovsky, I.; Pandol, S.J. Ethanol differentially regulates NF-kappaB activation in pancreatic acinar cells through calcium and protein kinase C pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G204–G213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Han, B.; Logsdon, C.D. CCK stimulates mob-1 expression and NF-kappaB activation via protein kinase C and intracellular Ca2+. Am. J. Physiol. Cell Physiol. 2000, 278, C344–C351. [Google Scholar] [PubMed]
- Yuan, J.; Lugea, A.; Zheng, L.; Gukovsky, I.; Edderkaoui, M.; Rozengurt, E.; Pandol, S.J. Protein kinase D1 mediates NF-kappaB activation induced by cholecystokinin and cholinergic signaling in pancreatic acinar cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G1190–G1201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, X.; Westlund, K.N. Restoration of spontaneous exploratory behaviors with an intrathecal NMDA receptor antagonist or a PKC inhibitor in rats with acute pancreatitis. Pharmacol. Biochem. Behav. 2004, 77, 145–153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jakkampudi, A.; Jangala, R.; Reddy, B.R.; Mitnala, S.; Nageshwar Reddy, D.; Talukdar, R. NF-κB in acute pancreatitis: Mechanisms and therapeutic potential. Pancreatology 2016, 16, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Lim, J.W.; Kim, J.M.; Kim, H. Anti-inflammatory mechanism of polyunsaturated fatty acids in Helicobacter pylori-infected gastric epithelial cells. Mediat. Inflamm. 2014, 128919. [Google Scholar] [CrossRef]
- Lian, S.; Xia, Y.; Nguyen, T.T.; Ung, T.T.; Yoon, H.J.; Kim, N.H.; Kim, K.K.; Jung, Y.D. Docosahexaenoic Acid Inhibits Tumor Promoter-Induced Urokinase-Type Plasminogen Activator Receptor by Suppressing PKCδ- and MAPKs-Mediated Pathways in ECV304 Human Endothelial Cells. PLoS ONE 2016, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.A.; Li, C.; Ha, T.; Kao, R.L.; Browder, W. Therapeutic modification of nuclear factor kappa B binding activity and tumor necrosis factor-alpha gene expression during acute biliary pancreatitis. Am. Surg. 1997, 63, 1036–1043. [Google Scholar] [PubMed]
- Grady, T.; Liang, P.; Ernst, S.A.; Logsdon, C.D. Chemokine gene expression in rat pancreatic acinar cells is an early event associated with acute pancreatitis. Gastroenterology 1997, 113, 1966–1975. [Google Scholar] [CrossRef]
- Gukovsky, I.; Gukovskaya, A.S.; Blinman, T.A.; Zaninovic, V.; Pandol, S.J. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am. J. Physiol. 1998, 275, G1402–G1414. [Google Scholar] [PubMed]
- Satoh, A.; Shimosegawa, T.; Fujita, M.; Kimura, K.; Masamune, A.; Koizumi, M.; Toyota, T. Inhibition of nuclear factor-kappaB activation improves the survival of rats with taurocholate pancreatitis. Gut 1999, 44, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ji, B.; Han, B.; Ernst, S.A.; Simeone, D.; Logsdon, C.D. NF-kappaB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology 2002, 122, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Baumann, B.; Wagner, M.; Aleksic, T.; von Wichert, G.; Weber, C.K.; Adler, G.; Wirth, T. Constitutive IKK2 activation in acinar cells is sufficient to induce pancreatitis in vivo. J. Clin. Investig. 2007, 117, 1502–1513. [Google Scholar] [CrossRef] [PubMed]
- Algül, H.; Treiber, M.; Lesina, M.J.; Nakhai, H.; Saur, D.; Geisler, F.; Pfeifer, A.; Paxian, S.; Schmid, R.M. Pancreas-specific RelA/p65 truncation increases susceptibility of acini to inflammation-associated cell death following cerulein pancreatitis. J. Clin. Investig. 2007, 117, 1490–1501. [Google Scholar] [CrossRef] [PubMed]

| Target Gene | Primer | Sequence (5′-3′) |
|---|---|---|
| GAPDH | Forward primer | GAAGGTGAAGGTCGGAGT |
| Reverse primer | GAAGATGGTGATGGGATTC | |
| IL-6 | Forward primer | GAGAGGAGACTTCACAGAGGATACCAC |
| Reverse primer | ACCACAGTGAGGAATGTCCACAA |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.K.; Lee, S.; Lim, J.W.; Kim, H. Docosahexaenoic Acid Inhibits Cerulein-Induced Acute Pancreatitis in Rats. Nutrients 2017, 9, 744. https://doi.org/10.3390/nu9070744
Jeong YK, Lee S, Lim JW, Kim H. Docosahexaenoic Acid Inhibits Cerulein-Induced Acute Pancreatitis in Rats. Nutrients. 2017; 9(7):744. https://doi.org/10.3390/nu9070744
Chicago/Turabian StyleJeong, Yoo Kyung, Sle Lee, Joo Weon Lim, and Hyeyoung Kim. 2017. "Docosahexaenoic Acid Inhibits Cerulein-Induced Acute Pancreatitis in Rats" Nutrients 9, no. 7: 744. https://doi.org/10.3390/nu9070744
APA StyleJeong, Y. K., Lee, S., Lim, J. W., & Kim, H. (2017). Docosahexaenoic Acid Inhibits Cerulein-Induced Acute Pancreatitis in Rats. Nutrients, 9(7), 744. https://doi.org/10.3390/nu9070744






