Natural Docosahexaenoic Acid in the Triglyceride Form Attenuates In Vitro Microglial Activation and Ameliorates Autoimmune Encephalomyelitis in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture and Treatment
2.3. Nitrite and Cytokine Quantification
2.4. Cell Viability: 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) Reduction Method
2.5. Mice
2.6. EAE Induction and Treatment
2.7. Mouse Splenocytes and CD4+ T-Cells Preparation
2.8. Proliferative Response of Lymphocytes
2.9. Determination of Tissue Fatty Acids Profile
2.10. Statistical Analysis
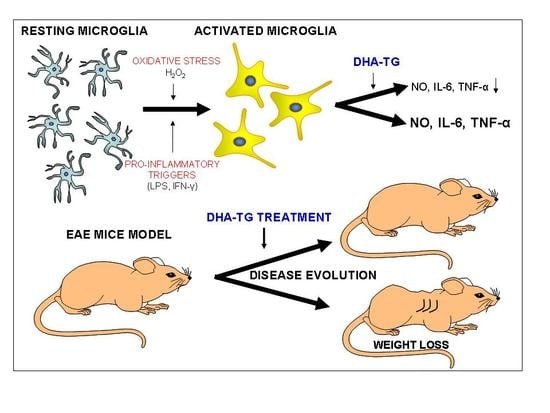
3. Results
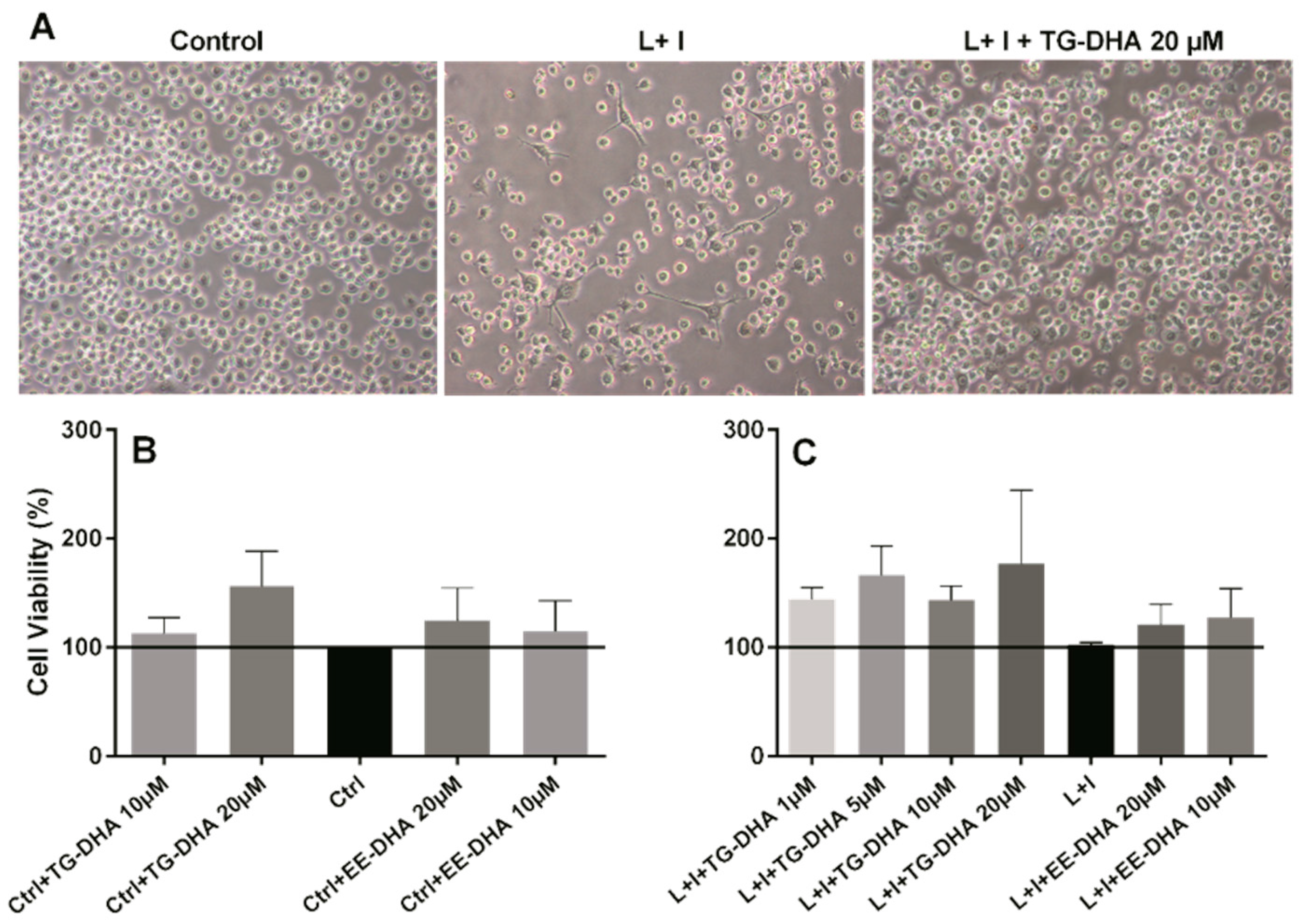
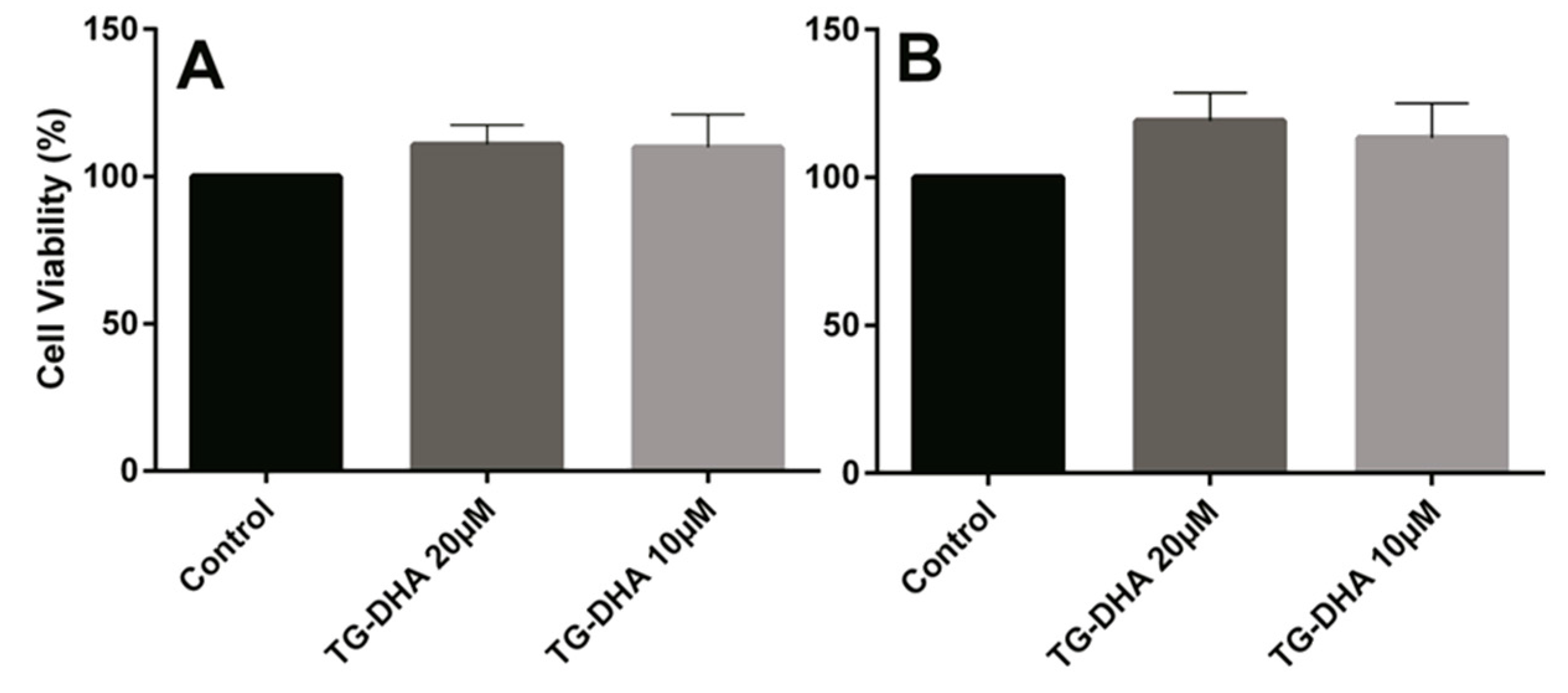
3.1. DHA Increased BV-2 Cells Viability
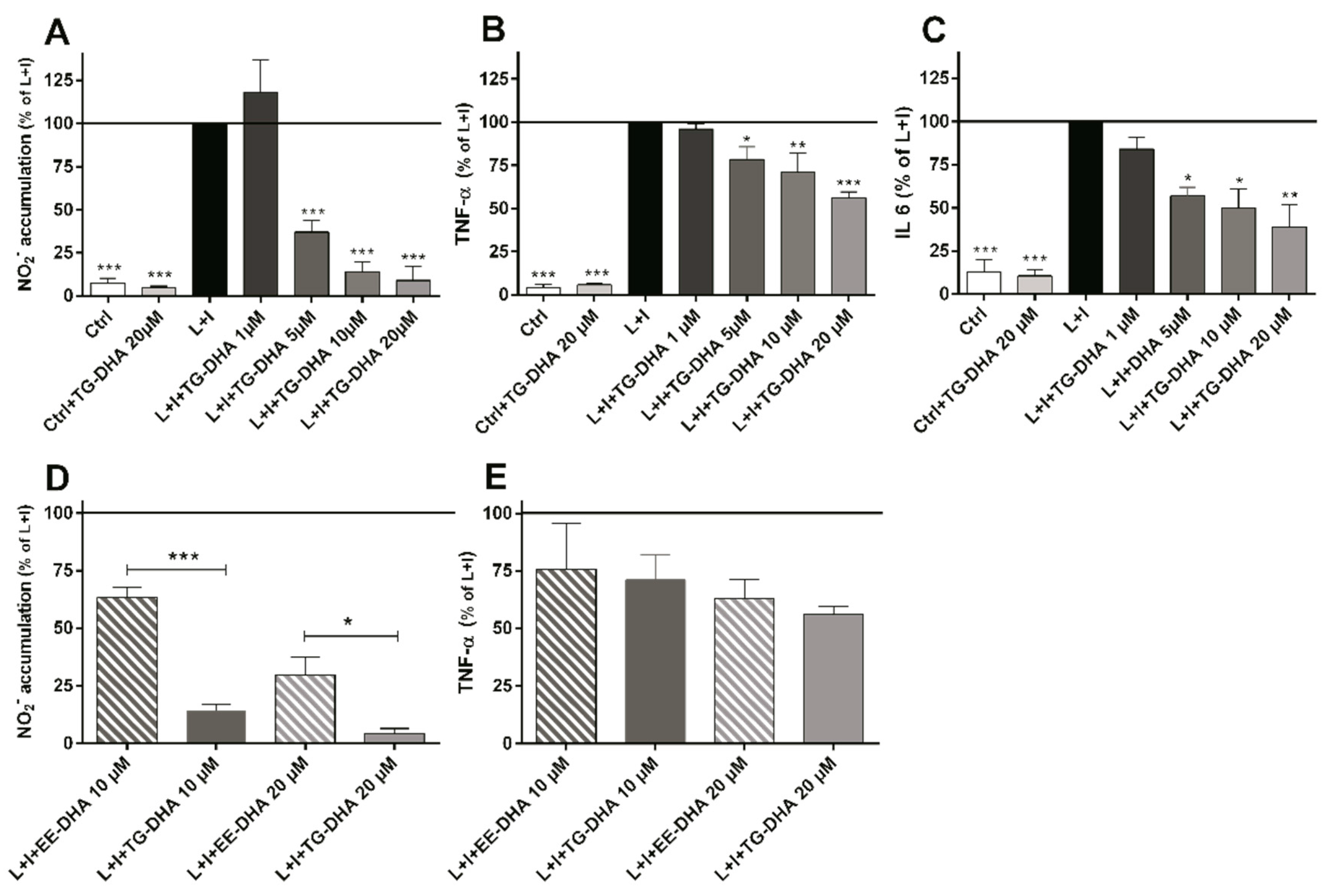
3.2. DHA Attenuated NO Production and Suppressed Induction of Inflammatory Cytokines in LPS-Stimulated BV-2 Microglia Cells
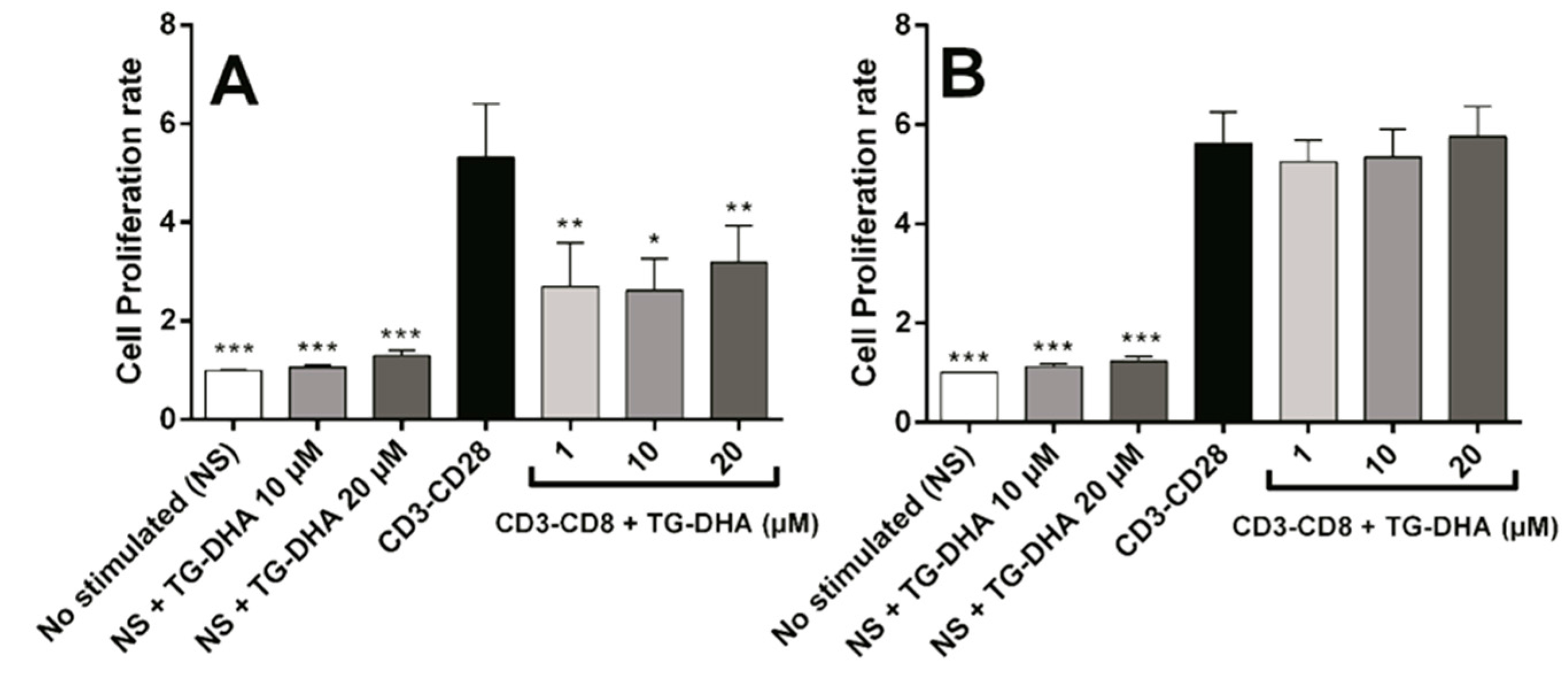
3.3. Effects of DHA on Splenocyte Viability and Proliferation after Lymphocyte Stimulation
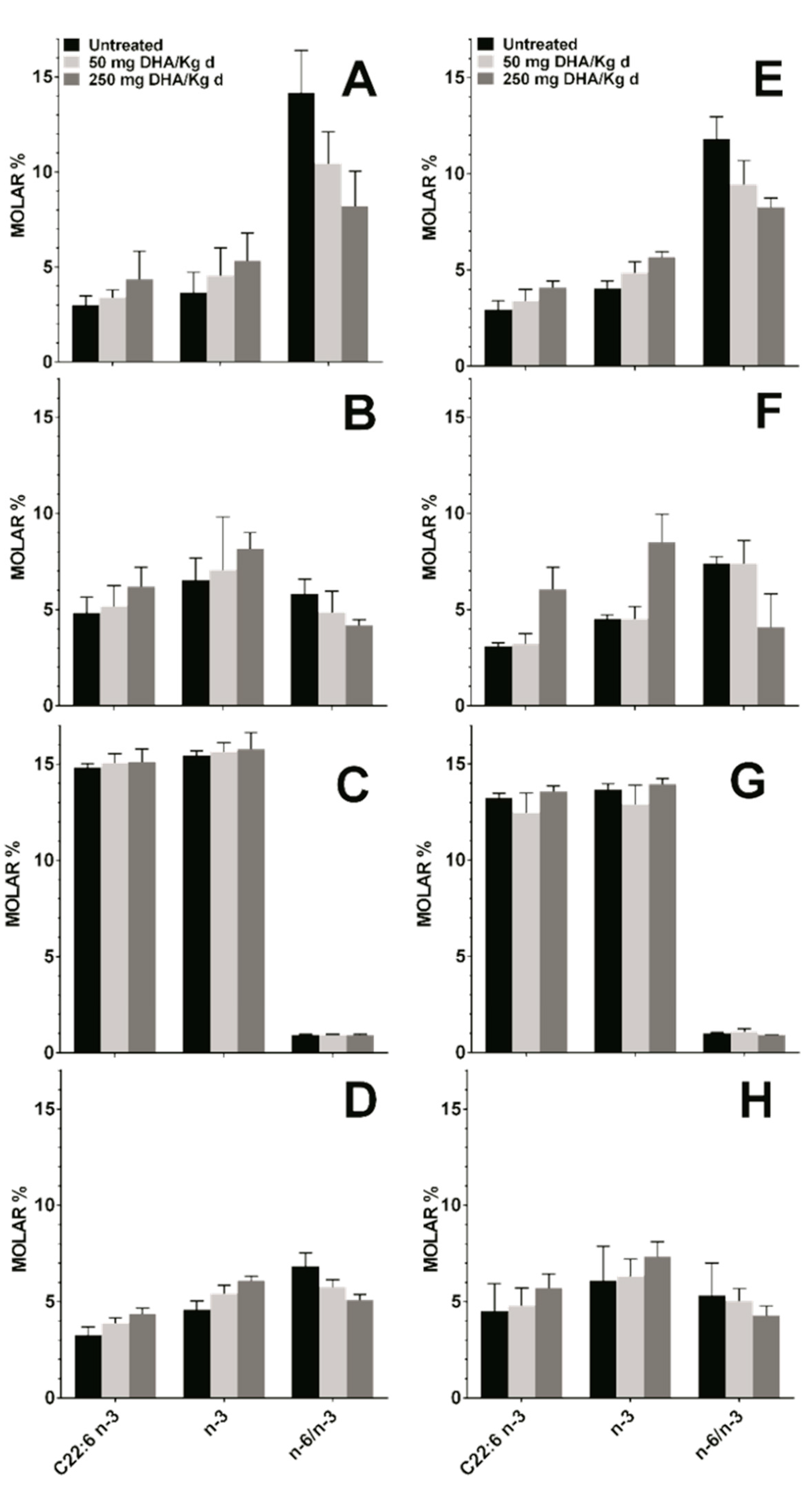
3.4. Effect DHA Dietary Supplementation on Fatty Acid Profile in Mice Tissues
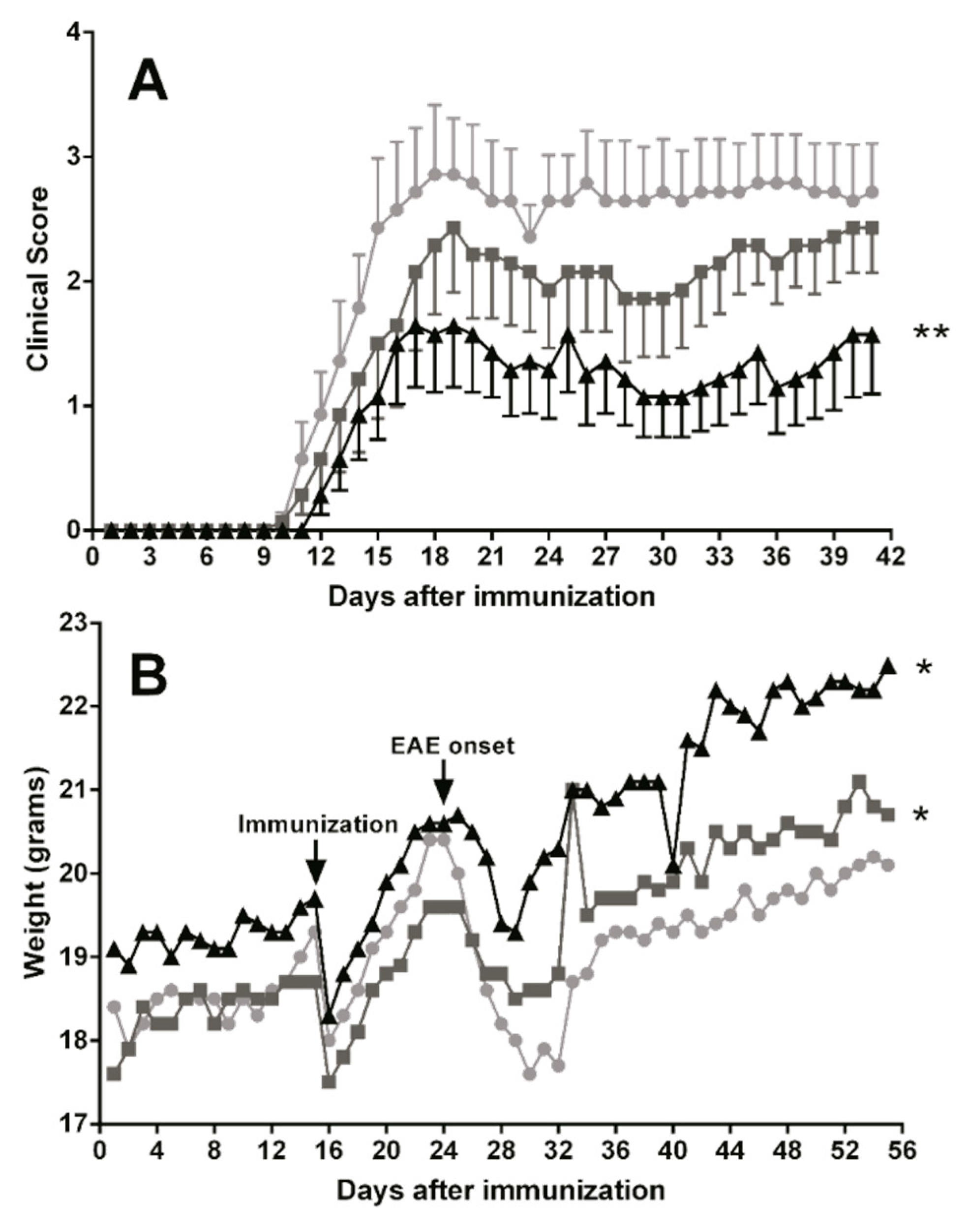
3.5. Dietary DHA Showed a Beneficial Effect on EAE Clinical Course
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Streit, W.J.; Mrak, R.E.; Griffin, W.S. Microglia and neuroinflammation: A pathological perspective. J. Neuroinflamm. 2004, 1, 14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matyszak, M.K.; Denis-Donini, S.; Citterio, S.; Longhi, R.; Granucci, F.; Ricciardi-Castagnoli, P. Microglia induce myelin basic protein-specific t cell anergy or t cell activation, according to their state of activation. Eur. J. Immunol. 1999, 29, 3063–3076. [Google Scholar] [CrossRef]
- Kong, W.; Yen, J.H.; Ganea, D. Docosahexaenoic acid prevents dendritic cell maturation, inhibits antigen-specific th1/th17 differentiation and suppresses experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2011, 25, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, F. Immune function of microglia. Glia 2001, 36, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Graeber, M.B.; Li, W.; Rodriguez, M.L. Role of microglia in cns inflammation. FEBS Lett. 2011, 585, 3798–3805. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.C.; Manzano, R.; Atencia-Cibreiro, G.; Olivan, S.; Munoz, M.J.; Zaragoza, P.; Cordero-Vazquez, P.; Esteban-Perez, J.; Garcia-Redondo, A.; Osta, R. Genetic biomarkers for als disease in transgenic sod1(g93a) mice. PLoS ONE 2012, 7, e32632. [Google Scholar] [CrossRef] [PubMed]
- Sanagi, T.; Yuasa, S.; Nakamura, Y.; Suzuki, E.; Aoki, M.; Warita, H.; Itoyama, Y.; Uchino, S.; Kohsaka, S.; Ohsawa, K. Appearance of phagocytic microglia adjacent to motoneurons in spinal cord tissue from a presymptomatic transgenic rat model of amyotrophic lateral sclerosis. J. Neurosci. Res. 2010, 88, 2736–2746. [Google Scholar] [CrossRef] [PubMed]
- Sherer, T.B.; Betarbet, R.; Kim, J.H.; Greenamyre, J.T. Selective microglial activation in the rat rotenone model of parkinson’s disease. Neurosci. Lett. 2003, 341, 87–90. [Google Scholar] [CrossRef]
- Tada, S.; Okuno, T.; Yasui, T.; Nakatsuji, Y.; Sugimoto, T.; Kikutani, H.; Sakoda, S. Deleterious effects of lymphocytes at the early stage of neurodegeneration in an animal model of amyotrophic lateral sclerosis. J. Neuroinflamm. 2011, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.J.; Talbot, K. Transgenics, toxicity and therapeutics in rodent models of mutant sod1-mediated familial als. Prog. Neurobiol. 2008, 85, 94–134. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.Y.; Tsao, Y.Y.; Leung, Y.M.; Su, K.P. Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in bv-2 microglia: Implications of antidepressant effects for omega-3 fatty acids. Neuropsychopharmacology 2010, 35, 2238–2248. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.P.; Richards, M.H.; Miller, S.D. Mouse models of multiple sclerosis: Experimental autoimmune encephalomyelitis and theiler’s virus-induced demyelinating disease. Methods Mol. Biol. 2012, 900, 381–401. [Google Scholar] [PubMed]
- Kroenke, M.A.; Carlson, T.J.; Andjelkovic, A.V.; Segal, B.M. Il-12- and il-23-modulated t cells induce distinct types of eae based on histology, cns chemokine profile, and response to cytokine inhibition. J. Exp. Med. 2008, 205, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Lees, J.R.; Golumbek, P.T.; Sim, J.; Dorsey, D.; Russell, J.H. Regional cns responses to ifn-gamma determine lesion localization patterns during eae pathogenesis. J. Exp. Med. 2008, 205, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Swanborg, R.H. Experimental autoimmune encephalomyelitis in rodents as a model for human demyelinating disease. Clin. Immunol. Immunopathol. 1995, 77, 4–13. [Google Scholar] [CrossRef]
- Yednock, T.A.; Cannon, C.; Fritz, L.C.; Sanchez-Madrid, F.; Steinman, L.; Karin, N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature 1992, 356, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, N.; Lassmann, S.; Li, Z.; Odoardi, F.; Ritter, T.; Ziemssen, T.; Klinkert, W.E.; Ellwart, J.W.; Bradl, M.; Krivacic, K.; et al. The activation status of neuroantigen-specific t cells in the target organ determines the clinical outcome of autoimmune encephalomyelitis. J. Exp. Med. 2004, 199, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Lull, M.E.; Block, M.L. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Cartier, N.; Lewis, C.A.; Zhang, R.; Rossi, F.M. The role of microglia in human disease: Therapeutic tool or target? Acta Neuropathol. 2014, 128, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Le, W. Differential roles of m1 and m2 microglia in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Suzumura, A. Neuron-microglia interaction in neuroinflammation. Curr. Protein Pept. Sci. 2013, 14, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Storer, P.D.; Xu, J.; Chavis, J.; Drew, P.D. Peroxisome proliferator-activated receptor-gamma agonists inhibit the activation of microglia and astrocytes: Implications for multiple sclerosis. J. Neuroimmunol. 2005, 161, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Kiaei, M. Peroxisome proliferator-activated receptor-gamma in amyotrophic lateral sclerosis and huntington’s disease. PPAR Res. 2008, 2008, 418765. [Google Scholar] [CrossRef] [PubMed]
- Kiaei, M.; Kipiani, K.; Chen, J.; Calingasan, N.Y.; Beal, M.F. Peroxisome proliferator-activated receptor-gamma agonist extends survival in transgenic mouse model of amyotrophic lateral sclerosis. Exp. Neurol. 2005, 191, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Gonzalez, F.; Rueda, F.; Petriz, J.; Domingo, P.; Villarroya, F.; Diaz-Delfin, J.; de Madariaga, M.A.; Domingo, J.C. Human dendritic cell activities are modulated by the omega-3 fatty acid, docosahexaenoic acid, mainly through ppar(gamma): Rxr heterodimers: Comparison with other polyunsaturated fatty acids. J. Leukoc. Biol. 2008, 84, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Pettit, L.K.; Varsanyi, C.; Tadros, J.; Vassiliou, E. Modulating the inflammatory properties of activated microglia with docosahexaenoic acid and aspirin. Lipids Health Dis. 2013, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Ajmone-Cat, M.A.; Salvatori, M.I.; De Simone, R.; Mancini, M.; Biagioni, S.; Bernardo, A.; Cacci, E.; Minghetti, L. Docosahexaenoic acid modulates inflammatory and antineurogenic functions of activated microglial cells. J. Neurosci. Res. 2012, 90, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Corsi, L.; Dongmo, B.M.; Avallone, R. Supplementation of omega 3 fatty acids improves oxidative stress in activated bv2 microglial cell line. Int. J. Food Sci. Nutr. 2015, 66, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G.; Molina, M.F.; Gordon, W.C. Docosahexaenoic acid signalolipidomics in nutrition: Significance in aging, neuroinflammation, macular degeneration, alzheimer’s, and other neurodegenerative diseases. Annu. Rev. Nutr. 2011, 31, 321–351. [Google Scholar] [CrossRef] [PubMed]
- Orr, S.K.; Trepanier, M.O.; Bazinet, R.P. N-3 polyunsaturated fatty acids in animal models with neuroinflammation. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Kohler, A.; Sarkkinen, E.; Tapola, N.; Niskanen, T.; Bruheim, I. Bioavailability of fatty acids from krill oil, krill meal and fish oil in healthy subjects-a randomized, single-dose, cross-over trial. Lipids Health Dis. 2015, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Dyerberg, J.; Madsen, P.; Moller, J.M.; Aardestrup, I.; Schmidt, E.B. Bioavailability of marine n-3 fatty acid formulations. Prostaglandins Leukot. Essent. Fat. Acids 2010, 83, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Virgili, N.; Espinosa-Parrilla, J.F.; Mancera, P.; Pasten-Zamorano, A.; Gimeno-Bayon, J.; Rodriguez, M.J.; Mahy, N.; Pugliese, M. Oral administration of the katp channel opener diazoxide ameliorates disease progression in a murine model of multiple sclerosis. J. Neuroinflamm. 2011, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Lien, E.L. Toxicology and safety of dha. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Fasano, E.; Serini, S.; Piccioni, E.; Toesca, A.; Monego, G.; Cittadini, A.R.; Ranelletti, F.O.; Calviello, G. DHA induces apoptosis by altering the expression and cellular location of grp78 in colon cancer cell lines. Biochim. Biophys. Acta 2012, 1822, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, D.J.; Abbey, M. Effects of n-3 fatty acids on growth and survival of j774 macrophages. Prostaglandins Leukot. Essent. Fat. Acids 2000, 62, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Paolisso, G.; Casamassimi, A.; Al-Omran, M.; Barbieri, M.; Sommese, L.; Infante, T.; Ignarro, L.J. Effects of nitric oxide on cell proliferation: Novel insights. J. Am. Coll. Cardiol. 2013, 62, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Arrington, J.L.; Chapkin, R.S.; Switzer, K.C.; Morris, J.S.; McMurray, D.N. Dietary n-3 polyunsaturated fatty acids modulate purified murine t-cell subset activation. Clin. Exp. Immunol. 2001, 125, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Manzoni Jacintho, T.; Gotho, H.; Gidlund, M.; Garcia Marques, C.; Torrinhas, R.; Mirtes Sales, M.; Linetzky Waitzberg, D. Anti-inflammatory effect of parenteral fish oil lipid emulsion on human activated mononuclear leukocytes. Nutr. Hosp. 2009, 24, 288–296. [Google Scholar] [PubMed]
- Schley, P.D.; Jijon, H.B.; Robinson, L.E.; Field, C.J. Mechanisms of omega-3 fatty acid-induced growth inhibition in mda-mb-231 human breast cancer cells. Breast Cancer Res. Treat. 2005, 92, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Crupi, R.; Marino, A.; Cuzzocrea, S. N-3 fatty acids: Role in neurogenesis and neuroplasticity. Curr. Med. Chem. 2013, 20, 2953–2963. [Google Scholar] [CrossRef] [PubMed]
- Kusunoki, C.; Yang, L.; Yoshizaki, T.; Nakagawa, F.; Ishikado, A.; Kondo, M.; Morino, K.; Sekine, O.; Ugi, S.; Nishio, Y.; et al. Omega-3 polyunsaturated fatty acid has an anti-oxidant effect via the nrf-2/ho-1 pathway in 3t3-l1 adipocytes. Biochem. Biophys. Res. Commun. 2013, 430, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.K.; Shahar, S.; Rajab, N.; Yusoff, N.A.; Jamal, R.A.; Then, S.M. The role of long chain omega-3 polyunsaturated fatty acids in reducing lipid peroxidation among elderly patients with mild cognitive impairment: A case-control study. J. Nutr. Biochem. 2013, 24, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Chang, C.D.; Chen, P.H.; Su, J.R.; Chen, C.C.; Chaung, H.C. Docosahexaenoic acid and phosphatidylserine supplementations improve antioxidant activities and cognitive functions of the developing brain on pentylenetetrazol-induced seizure model. Brain Res. 2012, 1451, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.; Huang, Y.; Kong, A.N. Synergistic anti-inflammatory effects of low doses of curcumin in combination with polyunsaturated fatty acids: Docosahexaenoic acid or eicosapentaenoic acid. Biochem. Pharmacol. 2010, 79, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G. Neuroprotectin d1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and alzheimer’s disease. J. Lipid Res. 2009, 50 (Suppl. S400–S405). [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Lim, J.W.; Kim, H. Inhibitory mechanism of omega-3 fatty acids in pancreatic inflammation and apoptosis. Ann. N. Y. Acad. Sci. 2009, 1171, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.A.; Harvey, K.A.; Ruzmetov, N.; Miller, S.J.; Zaloga, G.P. N-3 fatty acids prevent whereas trans-fatty acids induce vascular inflammation and sudden cardiac death. Br. J. Nutr. 2009, 102, 1811–1819. [Google Scholar] [CrossRef] [PubMed]
- Akhtar Khan, N. Polyunsaturated fatty acids in the modulation of t-cell signalling. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Liu, Y.; Liu, Y.; Zhang, J.; Kishimoto, C.; Ma, A.; Liu, Z. Peroxisome proliferator-activated receptor-gamma ligands ameliorate experimental autoimmune myocarditis associated with inhibition of self-sensitive t cells. J. Cardiovasc. Pharmacol. 2004, 43, 868–875. [Google Scholar] [CrossRef] [PubMed]
| % of Total Fatty Acids | ||||
|---|---|---|---|---|
| Total Fatty Acids | Plasma | RBC | ||
| Ctl (n = 7) | EAE (n = 7) | Ctl (n = 7) | EAE (n = 7) | |
| SFA | 31.70 ± 0.96 | 31.70 ± 0.49 | 47.53 ± 4.23 | 50.25 ± 6.89 |
| MUFA | 16.97 ± 1.19 | 16.23 ± 1.35 | 13.27 ± 0.57 | 14.54 ± 0.81 |
| PUFA | 51.33 ± 1.12 | 52.07 ± 1.36 | 39.20 ± 4.74 | 35.21 ± 7.60 |
| PUFA n-6 | 47.68 ± 1.36 | 47.60 ± 1.24 | 32.01 ± 4.43 | 29.93 ± 5.75 |
| PUFA n-3 | 3.65 ± 1.08 | 3.87 ± 0.91 | 6.55 ± 1.12 | 5.28 ± 1.93 |
| n-6/n-3 ratio | 14.17 ± 2.22 | 11.07 ± 2.25 | 5.83 ± 0.74 | 6.09 ± 1.29 |
| C22:6 n-3 | 3.01 ± 0.48 | 2.96 ± 0.79 | 4.83 ± 0.83 | 3.39 ± 1.08 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancera, P.; Wappenhans, B.; Cordobilla, B.; Virgili, N.; Pugliese, M.; Rueda, F.; Espinosa-Parrilla, J.F.; Domingo, J.C. Natural Docosahexaenoic Acid in the Triglyceride Form Attenuates In Vitro Microglial Activation and Ameliorates Autoimmune Encephalomyelitis in Mice. Nutrients 2017, 9, 681. https://doi.org/10.3390/nu9070681
Mancera P, Wappenhans B, Cordobilla B, Virgili N, Pugliese M, Rueda F, Espinosa-Parrilla JF, Domingo JC. Natural Docosahexaenoic Acid in the Triglyceride Form Attenuates In Vitro Microglial Activation and Ameliorates Autoimmune Encephalomyelitis in Mice. Nutrients. 2017; 9(7):681. https://doi.org/10.3390/nu9070681
Chicago/Turabian StyleMancera, Pilar, Blanca Wappenhans, Begoña Cordobilla, Noemí Virgili, Marco Pugliese, Fèlix Rueda, Juan F. Espinosa-Parrilla, and Joan C. Domingo. 2017. "Natural Docosahexaenoic Acid in the Triglyceride Form Attenuates In Vitro Microglial Activation and Ameliorates Autoimmune Encephalomyelitis in Mice" Nutrients 9, no. 7: 681. https://doi.org/10.3390/nu9070681
APA StyleMancera, P., Wappenhans, B., Cordobilla, B., Virgili, N., Pugliese, M., Rueda, F., Espinosa-Parrilla, J. F., & Domingo, J. C. (2017). Natural Docosahexaenoic Acid in the Triglyceride Form Attenuates In Vitro Microglial Activation and Ameliorates Autoimmune Encephalomyelitis in Mice. Nutrients, 9(7), 681. https://doi.org/10.3390/nu9070681